Hogweed Seed Oil: Physico–Chemical Characterization, LC-MS Profile, and Neuroprotective Activity of Heracleum dissectum Nanosuspension
Abstract
:1. Introduction
2. Materials and Methods
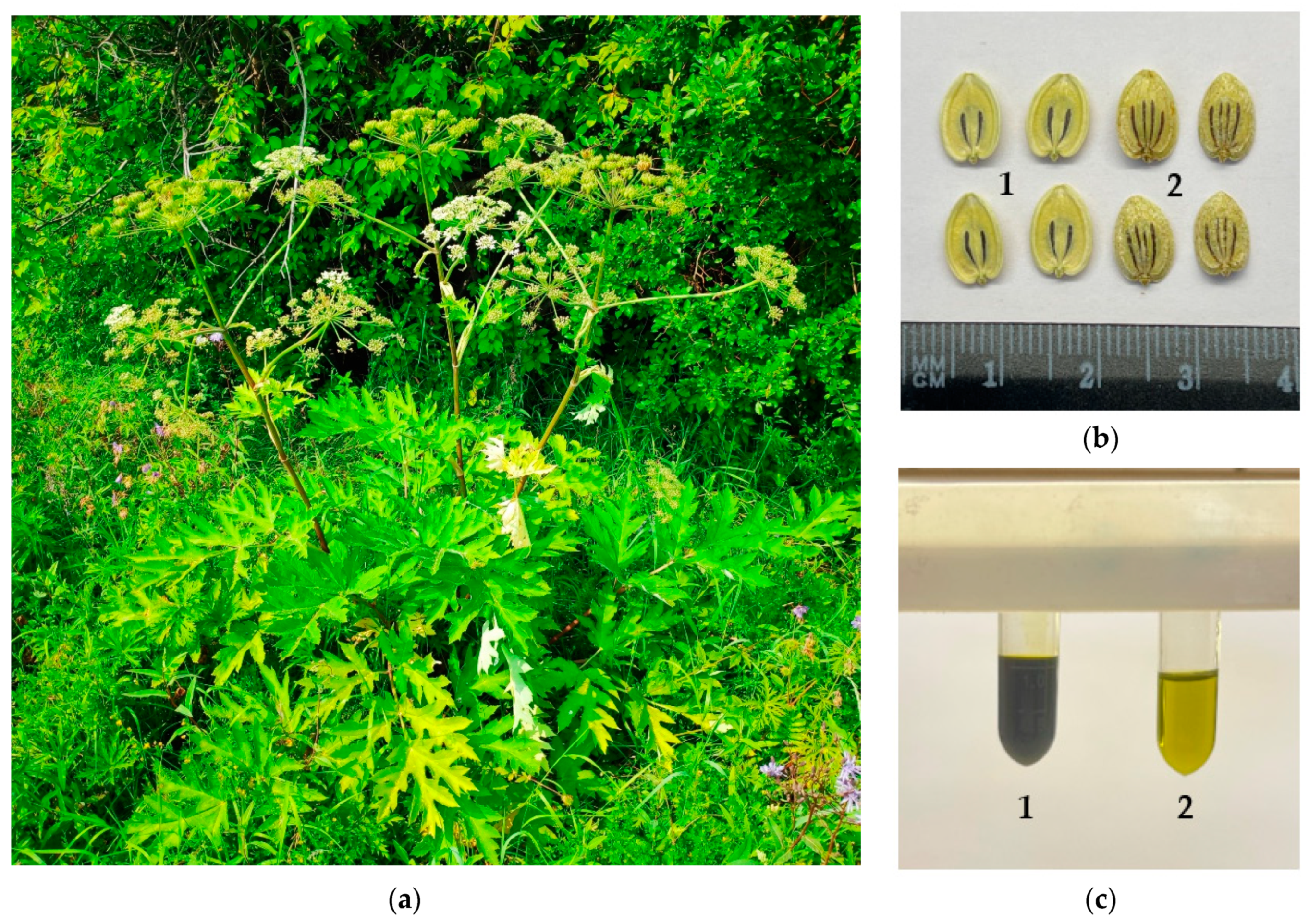
2.1. Plant Material and Chemicals
2.2. Seed Oil Preparation
2.3. Seed Oil Physico–Chemical Analysis
2.4. Ultraviolet Spectroscopy of Seed Oil
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) of Seed Oil
2.6. High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-PDA-ESI-TQ-MS) Metabolite Profiling of Seed Oil
2.7. HPLC-ESI-TQ-MS Quantification of Coumarins in Seed Oil
2.8. Heracleum dissectum Seed Oil Storage Experiment
2.9. Nanosuspension of H. dissectum Seed Oil Preparation
2.10. Characterization of H. dissectum Seed Oil Nanosuspension
2.11. Neuroprotective Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physico–Chemical and Spectral Characteristics of Heracleum dissectum Seed Oil
3.2. Coumarin Profile of Heracleum dissectum Seed Oil


| No. | t, min | UV, λmax, nm | ESI-MS, m/z (I, %) | Compound [Ref.] | IL * | ||
|---|---|---|---|---|---|---|---|
| [M + H]+ | [M + Na]+ | [M + K]+ | |||||
| 1 | 6.22 | 211, 229, 344 | 209 (11) | 231 (100) | 247 (54) | Isofraxetin [84] | 1 |
| 2 | 6.93 | 230, 259, 302, 345 | 179 (9) | 201 (100) | 217 (63) | Esculetin [85] | 1 |
| 3 | 8.19 | 216, 324 | 163 (22) | 185 (100) | 201 (58) | Umbelliferone [86] | 1 |
| 4 | 9.92 | 228, 230, 300, 342 | 193 (4) | 215 (100) | 231 (69) | Scopoletin [84] | 1 |
| 5 | 10.05 | 230, 261, 345 | 209 (10) | 231 (100) | 247 (82) | Fraxetin [85] | 1 |
| 6 | 10.72 | 217, 248, 265, 300 | 305 (8) | 327 (100) | 343 (42) | Heraclenol (prangenin hydrate, komaline) [84] | 1 |
| 7 | 11.29 | 222, 250, 265, 308 | 305 (11) | 327 (100) | 343 (38) | Oxypeucedanin hydrate (prangol) [84] | 1 |
| 8 | 11.62 | 222, 249, 267, 311 | 335 (26) | 357 (100) | 373 (73) | Byakangelicin [84] | 1 |
| 9 | 11.88 | 272, 310 | 147 (4) | 169 (100) | 185 (56) | Coumarin [84] | 1 |
| 10 | 12.28 | 214, 300, 318 | 177 (11) | 199 (100) | 215 (36) | Herniarin [84] | 1 |
| 11 | 12.82 | 244, 292, 330 | 187 (12) | 209 (100) | 225 (49) | Psoralen [87] | 1 |
| 12 | 13.11 | 200, 216, 258, 302 | 187 (3) | 209 (100) | 225 (72) | Angelicin [87] | 1 |
| 13 | 13.72 | 217, 247, 265, 301 | 217 (8) | 239 (100) | 255 (53) | Xanthotoxin [87] | 1 |
| 14 | 14.15 | 222, 248, 268, 312 | 217 (5) | 239 (100) | 255 (42) | Bergapten [87] | 1 |
| 15 | 14.63 | 215, 250, 265, 305 | 287 (18) | 309 (100) | 325 (56) | Heraclenin (prangenine) [84] | 1 |
| 16 | 14.92 | 217, 247, 265, 300 | 247 (26) | 269 (100) | 285 (73) | Pimpinellin [87] | 1 |
| 17 | 15.34 | 221, 247, 267, 310 | 317 (5) | 339 (100) | 355 (42) | Byakangelicol [84] | 1 |
| 18 | 15.37 | 220, 251, 266, 304 | 289 (2) | 311 (100) | 327 (39) | Pranferol [85] | 1 |
| 19 | 16.03 | 220, 248, 268, 311 | 271 (15) | 293 (100) | 309 (73) | Alloimperatorin (prangenidin) [84] | 1 |
| 20 | 16.14 | 220, 251, 265, 304 | 287 (12) | 309 (100) | 325 (31) | Isooxypeucedanin [84] | 1 |
| 21 | 16.42 | 220, 248, 267, 312 | 319 (9) | 341 (100) | 357 (39) | Heracol [84] | 2 |
| 22 | 16.82 | 217, 248, 266, 301 | 287 (5) | 309 (100) | 325 (63) | Oxypeucedanin (prangolarlin) [84] | 1 |
| 23 | 17.22 | 217, 248, 267, 300 | 287 (3) | 309 (100) | 325 (40) | Oxypeucedanin isomer [84] | 2 |
| 24 | 17.73 | 217, 248, 265, 300 | 271 (11) | 293 (100) | 309 (70) | Imperatorin (ammidin, marmelosin) [84] | 1 |
| 25 | 18.51 | 221, 248, 268, 311 | 301 (26) | 323 (100) | 339 (72) | Phellopterin [85] | 1 |
| 26 | 19.12 | 220, 250, 265, 305 | 271 (7) | 293 (100) | 309 (65) | Isoimperatorin [87] | 1 |
| 27 | 20.27 | 221, 249, 267, 312 | 301 (14) | 323 (100) | 339 (51) | Cnidilin (isophellopterin) [87] | 1 |
| 28 | 21.18 | 224, 326 | 383 (6) | 405 (100) | 421 (63) | Farnesiferol C [85] | 1 |
| 29 | 21.80 | 220, 248, 267, 312 | 355 (8) | 377 (100) | 393 (52) | Cnidicin [84] | 1 |
| 30 | 22.09 | 216, 247, 265, 301 | 299 (10) | 321 (100) | 337 (45) | Auraptene isomer [84] | 2 |
| 31 | 22.53 | 220, 247, 267, 311 | 339 (7) | 361 (100) | 377 (83) | Bergamottin isomer [84] | 2 |
| 32 | 22.82 | 216, 247, 265, 301 | 339 (12) | 361 (100) | 377 (46) | 8-Geranyloxypsoralen [84] | 1 |
| 33 | 23.22 | 220, 325 | 299 (8) | 321 (100) | 337 (40) | Auraptene [84] | 1 |
| 34 | 25.71 | 220, 247, 267, 311 | 339 (5) | 361 (100) | 377 (80) | Bergamottin [84] | 1 |
| 35 | 26.64 | 220, 324 | 299 (12) | 321 (100) | 337 (63) | Ostruthin [85] | 1 |
| 36 | 27.28 | 219, 267, 325 | 313 (10) | 335 (100) | 351 (61) | 5-Geranyloxy-7-methoxycoumarin [84] | 1 |
| 37 | 27.91 | 220, 324 | 299 (14) | 321 (100) | 337 (60) | Ostruthin isomer [85] | 2 |
| 38 | 28.47 | 219, 267, 325 | 313 (11) | 335 (100) | 351 (53) | 5-Geranyloxy-7-methoxycoumarin isomer [84] | 2 |
3.3. Quantification of Six Coumarins in Heracleum dissectum Seed Oil before and after Storage
3.4. Nanosuspension of Heracleum dissectum Seed Oil and Its Neuroprotective Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiviya, P.; Gunawardena, N.; Gamage, A.; Madhujith, T.; Merah, O. Apiaceae family as a valuable source of biocidal components and their potential uses in agriculture. Horticulturae 2022, 8, 614. [Google Scholar] [CrossRef]
- Aćimović, M.G. Nutraceutical potential of Apiaceae. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–31. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Pimenov, M.G.; Leonov, M.V.; van Wyk, B.-E.; Tilney, P.M. Asia, the continent with the highest Umbelliferae biodiversity. S. Afr. J. Bot. 2004, 70, 417–419. [Google Scholar] [CrossRef]
- Plants of the World Online. Heracleum L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30054087-2 (accessed on 14 April 2023).
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The genus Heracleum: A comprehensive Review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [CrossRef]
- Yu, Y.; Downie, S.R.; He, X.; Deng, X.; Yan, L. Phylogeny and biogeography of Chinese Heracleum (Apiaceae tribe Tordylieae) with comments on their fruit morphology. Plant Syst. Evol. 2011, 296, 179–203. [Google Scholar] [CrossRef]
- Pimenov, M.G.; Ostroumova, T.A. Umbelliferae of Russia; KMK Scientific Press Ltd.: Moscow, Russia, 2012; pp. 320–321. [Google Scholar]
- Batorova, S.M.; Yakovlev, G.P.; Aseeva, T.A. Reference-Book of Traditional Tibetan Medicine Herbs; Nauka: Novosibirsk, Russia, 2013; pp. 79–81. [Google Scholar]
- Aseeva, T.A. Adaptogens in Tibetan Medicine; Nauka: Novosibirsk, Russia, 2019; pp. 63–65. [Google Scholar]
- Aseeva, T.A. Tibetan Medicine of Buryats; SO RAN: Novosibirsk, Russia, 2008; pp. 193–195. [Google Scholar]
- Ivanov, B.I. (Ed.) Atlas of Medicinal Plants of Yakutia; YaNTs SO RAN: Yakutsk, Russia, 2005; Volume 2, pp. 15–16. [Google Scholar]
- Gao, Y.; Liu, Y.; Wang, Z.; Zhang, H. Chemical constituents of Heracleum dissectum and their cytotoxic activity. Phytochem. Lett. 2014, 10, 276–280. [Google Scholar] [CrossRef]
- Zhang, H.; Mi, J.; Peng, Y.; Wang, Z.; Liu, Y.; Gao, Y. A new semiterpenoid glycoside and a new benzofuran derivative glycoside from the roots of Heracleum dissectum. Phytochem. Lett. 2017, 21, 256–259. [Google Scholar] [CrossRef]
- Gao, Y.; Mi, J.; Zhang, C.; Zhang, X.; Peng, Y.; Bao, H.; Zhang, H. Three new polyacetylene glycosides from the roots of Heracleum dissectum and their triglyceride accumulating activities in 3T3-L1 cells. Chem. Biodivers. 2019, 16, e1800424. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, S.; Chen, L.; Yang, M.; Wang, B.; Zhang, X.; Zhang, H. A new coumarin isolated from the roots of Heracleum dissectum Ledeb. Nat. Prod. Res. 2022, 36, 3241–3246. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Mi, J.; Wang, X.-R.; Huo, Y.-Y.; Peng, Y.-J.; Zhang, H.-M.; Zhang, H.-L. A new cinnamic acid glycoside from roots of Heracleum dissectum. Nat. Prod. Res. 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Wu, X.-Y.; Mi, J.; Peng, Y.-J.; Wang, Z.-G.; Liu, Y.; Gao, Y. A new anti-inflammatory alkaloid from roots of Heracleum dissectum. Chem. Biodivers. 2017, 14, e1700184. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Peng, Y.; Zhang, H.; Wang, X.; Huo, Y.; Wang, Z.; Zhang, H. A new benzofuran derivative glycoside and a new coumarin glycoside from roots of Heracleum dissectum Ledeb. Med. Chem. Res. 2018, 27, 470–475. [Google Scholar] [CrossRef]
- Benešová, V. Plant substances. XX. Constituents of Heracleum dissectum L. root. Collect. Czechoslov. Chem. Commun. 1962, 27, 2714–2716. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Wang, X.; Mi, J.; Huo, Y.; Wang, Z.; Gao, Y. Antidiabetic activity and chemical constituents of the aerial parts of Heracleum dissectum Ledeb. Food Chem. 2017, 214, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Belenovskaya, L.M.; Sinitskii, V.S.; Tumbaa, K. 7-Isopentenyloxycoumarin from Heracleum dissectum. Chem. Nat. Compd. 1977, 13, 478. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Y.; Peng, Y.; Zhang, X.; Zhang, H. A new coumarin from the roots of Heracleum dissectum. Nat. Prod. Commun. 2019, 14, 111–112. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Ochir, G.; Motl, O.; Argyriadou, N.; Dunkel, H. Composition of the essential oil from Heracleum dissectum. J. Nat. Prod. 1985, 48, 851–853. [Google Scholar] [CrossRef]
- Montanarella, L.; Bos, R.; Fischer, F. The essential oil in lamina and petiole of Heracleum dissectum leaves. Planta Med. 1986, 52, 332–334. [Google Scholar] [CrossRef]
- Di Stasi, L.C. Natural coumarin derivatives activating Nrf2 signaling pathway as lead compounds for the design and synthesis of intestinal anti-inflammatory drugs. Pharmaceuticals 2023, 16, 511. [Google Scholar] [CrossRef]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Zyryanov, G.V.; Chupakhin, O.N.; Tsurkan, M.V. Plant coumarins with anti-HIV activity: Isolation and mechanisms of action. Int. J. Mol. Sci. 2023, 24, 2839. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patel, H.M.; Patil, V.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 2022, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic effects of coumarins against cancer: From chemistry to medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as fungal metabolites with potential medicinal properties. Antibiotics 2022, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging nanopharmaceuticals and nanonutraceuticals in cancer management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to improve the solubility of curcumin from turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Singh, S.; Grewal, S.; Sharma, N.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Mohan, S.; Bungau, S.G.; Bumbu, A. Unveiling the pharmacological and nanotechnological facets of daidzein: Present state-of-the-art and future perspectives. Molecules 2023, 28, 1765. [Google Scholar] [CrossRef]
- Yekefallah, M.; Raofie, F. Preparation of potent antioxidant nanosuspensions from olive leaves by rapid expansion of supercritical solution into aqueous solutions (RESSAS). Ind. Crops Prod. 2020, 155, 112756. [Google Scholar] [CrossRef]
- Touqeer, S.I.; Jahan, N.; Abbas, N.; Ali, A. Formulation and process optimization of Rauvolfia serpentina nanosuspension by HPMC and in vitro evaluation of ACE inhibitory potential. J. Funct. Biomater. 2022, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Bajaber, M.A.; Hussain, G.; Farooq, T.; Noreen, R.; Ibrahim, M.; Umbreen, H.; Batool, S.; Rehman, K.; Hameed, A.; Farid, M.F.; et al. Nanosuspension of Foeniculum vulgare promotes accelerated sensory and motor function recovery after sciatic nerve injury. Metabolites 2023, 13, 391. [Google Scholar] [CrossRef]
- Fountain, C.W.; Jennings, J.; McKie, C.K.; Oakman, P.; Fetterolf, M.L. Viscosity of common seed and vegetable oils. J. Chem. Educ. 1997, 74, 224–227. [Google Scholar] [CrossRef]
- Noureddini, H.; Teoh, B.C.; Davis Clements, L. Densities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1184–1188. [Google Scholar] [CrossRef]
- AOCS. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2013. [Google Scholar]
- ASTM D664–18e2; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Deman, J.M.; Deman, L.; Blackman, B. Melting-point determination of fat products. J. Am. Oil Chem. Soc. 1983, 60, 91–94. [Google Scholar] [CrossRef]
- Bulda, O.V.; Rassadina, V.V.; Alekseichuk, H.N.; Laman, N.A. Spectrophotometric measurement of carotenes, xanthophylls, and chlorophylls in extracts from plant seeds. Russ. J. Plant Physiol. 2008, 55, 544–551. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Fedorov, I.A.; Kashchenko, N.I.; Chirikova, N.K.; Vennos, C. Khellactone derivatives and other phenolics of Phlojodicarpus sibiricus (Apiaceae): HPLC-DAD-ESI-QQQ-MS/MS and HPLC-UV profile, and antiobesity potential of dihydrosamidin. Molecules 2019, 24, 2286. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules 2017, 22, 16. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Gornostai, T.G.; Selyutina, I.Y.; Zilfikarov, I.N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. Int. J. Molec. Sci. 2017, 18, 2579. [Google Scholar] [CrossRef]
- Rabiej, D.; Szydłowska-Czerniak, A. Fluorescence and UV-VIS spectroscopy to determine the quality changes of rapeseed oil fortified with new antioxidant after storage under various conditions. Food Anal. Methods 2020, 13, 1973–1982. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.W.; Morton, D. Essential oil quality and purity evaluation via FT-IR spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- LabSolutions. Available online: https://www.shimadzu.eu/labsolutions-0 (accessed on 12 April 2023).
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Zilfikarov, I.N.; Penzina, T.A. Use of microcolumn HPLC for analysis of aloenin in Aloe arborescens raw material and related drugs. Pharm. Chem. J. 2013, 47, 494–497. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Khandy, M.T.; Chirikova, N.K. Oriental strawberry metabolites: LC–MS profiling, antioxidant potential, and postharvest changes of Fragaria orientalis fruits. Horticulturae 2022, 8, 975. [Google Scholar] [CrossRef]
- Han, X.; Wang, M.; Ma, Z.; Xue, P.; Wang, Y. A new approach to produce drug nanosuspensions CO2-assisted effervescence to produce drug nanosuspensions. Colloids Surf. B Biointerfaces 2016, 143, 107–110. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Zhu, J.; Wang, J.; Xu, Y.; Luan, H.; Wang, H. Optimization of extended-release ZL-004 nanosuspensions for in vivo pharmacokinetic study to enhance low solubility and compliance. Molecules 2019, 24, 7. [Google Scholar] [CrossRef]
- Shamilov, A.A.; Olennikov, D.N.; Pozdnyakov, D.I.; Bubenchikova, V.N.; Garsiya, E.R.; Larskii, M.V. Caucasian blueberry: Comparative study of phenolic compounds and neuroprotective and antioxidant potential of Vaccinium myrtillus and Vaccinium arctostaphylos leaves. Life 2022, 12, 2079. [Google Scholar] [CrossRef]
- Wang, N.; Chen, X.; Geng, D.; Huang, H.; Zhou, H. Ginkgo biloba leaf extract improves the cognitive abilities of rats with D-galactose induced dementia. J. Biomed. Res. 2013, 27, 29–36. [Google Scholar] [CrossRef]
- Voronkov, A.V.; Dyakova, I.N.; Pozdnyakov, D.I. The influence of natural compounds of polyphenolic structure on the vasodilating function of the vascular endothelium of the rat brain in conditions of its focal ischemia. Eksperimental’naia I Klin. Farmakol. 2016, 79, 7–9. [Google Scholar]
- Özcan, M.M.; Chalchat, J.C. Chemical composition of carrot seeds (Daucus carota L.) cultivated in Turkey: Characterization of the seed oil and essential oil. Grasas Aceites 2007, 58, 359–365. [Google Scholar]
- Ali, M.A.; Sayeed, M.A.; Reza, M.S.; Yeasmin, M.S.; Khan, A.M. Characteristics of seed oils and nutritional compositions of seeds from different varieties of Momordica charantia Linn. cultivated in Bangladesh. Czech J. Food Sci. 2008, 26, 275–283. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Muddathir, A.M.; Hassa, A.B. The physical and chemical characteristics of seeds oil of local Sudanese pumpkin (Cucurbita moschata Duchesne). J. Oleo Sci. 2022, 71, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Shola, E. Extraction and physico-chemical properties of some edible seed oils sampled in Kano metropolis, Kano state. Bayero J. Pure Appl. Sci. 2016, 8, 239. [Google Scholar] [CrossRef]
- Jonas, M.; Ketlogetswe, C.; Gandure, J. Effect of fruit maturity stage on some physicochemical properties of jatropha seed oil and derived biodiesel. ACS Omega 2020, 5, 13473–13481. [Google Scholar] [CrossRef]
- Kabutey, A.; Herák, D.; Mizera, Č. Determination of maximum oil yield, quality indicators and absorbance spectra of hulled sunflower seeds oil extraction under axial loading. Foods 2022, 11, 2866. [Google Scholar] [CrossRef]
- Pulassery, S.; Abraham, B.; Ajikumar, N.; Munnilath, A.; Yoosaf, K. Rapid iodine value estimation using a handheld Raman spectrometer for on-site, reagent-free authentication of edible oils. ACS Omega 2022, 7, 9164–9171. [Google Scholar] [CrossRef]
- Fontanel, D. Unsaponifiable Matter in Plant Seed Oils; Springer: Berlin, Germany, 2013; pp. 1–366. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical analysis of minor bioactive components and cannabidiolic acid in commercial hemp seed oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Minguez-Mosquera, M.I. Chlorophyll and carotenoid composition in virgin olive oils from various Spanish olive varieties. J. Sci. Food Agric. 1996, 72, 31–39. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive compounds and antioxidant activities of different grape (Vitis vinifera L.) seed oils extracted by supercritical CO2 and organic solvent. LWT 2016, 74, 557–562. [Google Scholar] [CrossRef]
- Ušjak, L.; Sofrenić, I.; Tešević, V.; Drobac, M.; Niketić, M.; Petrović, S. Fatty acids, sterols, and triterpenes of the fruits of 8 Heracleum taxa. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Tsang, S. RIFM fragrance ingredient safety assessment, Octyl acetate, CAS Registry Number 112-14-1. Food Chem. Toxicol. 2018, 122, S34–S40. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D. Composition and herbicidal effect of Heracleum sosnowskyi essential oil. Open Life Sci. 2015, 10, 425–432. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Mohammad, N. Analysis of the oil of Heracleum persicum L. (seeds and stems). J. Essent. Oil Res. 2004, 16, 296–298. [Google Scholar] [CrossRef]
- Mustafavi, S.H.; Abbasi, A.; Morshedloo, M.R.; Pateiro, M.; Lorenzo, J.M. Essential oil variability in Iranian populations of Heracleum persicum Desf. ex Fischer: A rich source of hexyl butyrate and octyl acetate. Molecules 2022, 27, 6296. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Najafpour Navaei, M.; Behrad, Z. A comparative study of the essential oils of Heracleum anisactis Boiss. & Hohen. at different altitiudes. Iran. J. Med. Aromat. Plants Res. 2014, 30, 650–655. [Google Scholar] [CrossRef]
- Işcan, G.; Demirci, F.; Kürkçüoğlu, M.; Kivanç, M.; Başer, K.H.C. The bioactive essential oil of Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Z. Nat. C 2003, 58, 195–200. [Google Scholar] [CrossRef]
- Lee, K.-H.; Soine, T.O. Coumarins X: Spectral studies on some linear furanocoumarins. J. Pharm. Sci. 1969, 58, 681–683. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J.; Paradkar, M. Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem. 2005, 93, 25–32. [Google Scholar] [CrossRef]
- Ye, Q.; Meng, X. Highly efficient authentication of edible oils by FTIR spectroscopy coupled with chemometrics. Food Chem. 2022, 385, 132661. [Google Scholar] [CrossRef]
- Sinclair, R.G.; McKay, A.F.; Jones, R.N. The infrared absorption spectra of saturated fatty acids and esters. J. Am. Chem. Soc. 1952, 74, 2570–2575. [Google Scholar] [CrossRef]
- Kuznetsova, G.A. Natural Coumarins and Furanocoumarins; Nauka: Leningrad, Russia, 1967; pp. 35–38. [Google Scholar]
- Malikov, V.M.; Saidkhodzhaev, A.I.; Aripov, K.N. Coumarins: Plants, structure, properties. Part 1. Chem. Nat. Compd. 1998, 34, 202–264. [Google Scholar] [CrossRef]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins. Plants, structure, properties. Part 2. Chem. Nat. Compd. 1998, 34, 345–409. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Partilkhaev, V.V.; Rokhin, A.V. Chemical constituents of Caragana bungei shoots. Rev. Bras. Farmacogn. 2012, 22, 490–496. [Google Scholar] [CrossRef]
- Olennikov, D.N. Coumarins of lovage roots (Levisticum officinale W.D.J.Koch): LC-MS profile, quantification, and stability during postharvest storage. Metabolites 2023, 13, 3. [Google Scholar] [CrossRef]
- Kasaian, J.; Mohammadi, A. Biological activities of farnesiferol C: A review. J. Asian Nat. Prod. Res. 2017, 20, 27–35. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Sharifi-Rad, M. Auraptene and umbelliprenin: A review on their latest literature acquisitions. Phytochem. Rev. 2022, 21, 317–326. [Google Scholar] [CrossRef]
- Olennikov, D.N. New coumarins from roots and fruit of Peucedanum morisonii. Chem. Nat. Compd. 2022, 58, 816–821. [Google Scholar] [CrossRef]
- Benincasa, M.; Buiarelli, F.; Cartoni, G.P. Analysis of lemon and bergamot essential oils by HPLC with microbore columns. Chromatographia 1990, 30, 271–276. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Waksmundzka-Hajnos, M.; Skalicka-Woźniak, K.; Głowniak, K. Comparison of matrix-solid phase dispersion and liquid–solid extraction connected with solid-phase extraction in the quantification of selected furanocoumarins from fruits of Heracleum leskowii by high performance liquid chromatography. Ind. Crops Prod. 2013, 50, 131–136. [Google Scholar] [CrossRef]
- Wang, X.-B.; Li, G.-H.; Li, L.; Zheng, L.-J.; Huang, R.; Zhang, K.-Q. Nematicidal coumarins from Heracleum candicans Wall. Nat. Prod. Res. 2008, 22, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Razdan, T.K.; Kachroo, V.; Harkar, S.; Koul, G.L. Furanocoumarins from Heracleum canescens. Phytochemistry 1982, 21, 923–927. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A. The analysis of furanocoumarins in fruits of Heracleum sibiricum L. Acta Pol. Pharm. 1999, 56, 399–402. [Google Scholar]
- Komissarenko, N.F.; Derkach, A.I.; Kovalev, I.P. Coumarins of the roots of Heracleum leskovii. Chem. Nat. Compd. 1978, 14, 149–151. [Google Scholar] [CrossRef]
- Ibadullaeva, S.D.; Serkerov, S.V. Coumarins of Heracleum pastinacifolium. Chem. Nat. Compd. 2000, 36, 534. [Google Scholar] [CrossRef]
- Walasek, M.; Grzegorczyk, A.; Malm, A.; Skalicka-Woźniak, K. Bioactivity-guided isolation of antimicrobial coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) fruits by high-performance counter-current chromatography. Food Chem. 2015, 186, 133–138. [Google Scholar] [CrossRef]
- O׳Neill, T.; Johnson, J.A.; Webster, D.; Gray, C.A. The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins. J. Ethnopharmacol. 2013, 147, 232–237. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Buzjak Služek, V.; Cindrić, I.; Molnar, M. Coumarins in food and methods of their determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Głowniak, K. Pressurized liquid extraction of coumarins from fruits of Heracleum leskowii with application of solvents with different polarity under increasing temperature. Molecules 2012, 17, 4133–4141. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ozek, G.; Yur, S.; Goger, F.; Ozek, T.; Andjelkovic, B.; Todorova, M. Furanocoumarin content, antioxidant activity and inhibitory potential of Heracleum verticillatum, H. sibiricum, H. angustisectum and H. ternatum extracts against enzymes involved in Alzheimer’s disease and type II diabetes. Chem. Biodivers. 2019, 16, e1800672. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhao, J.; Ding, Y.; Li, L. A cost-effective method to prepare curcumin nanosuspensions with enhanced oral bioavailability. J. Colloid Interface Sci. 2017, 485, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Honda, M.; Fukaya, T.; Wahyudiono; Kanda, H.; Goto, M. One-step preparation of Z-isomer-rich β-carotene nanosuspensions utilizing a natural catalyst, allyl isothiocyanate, via supercritical CO2. Symmetry 2020, 12, 777. [Google Scholar] [CrossRef]
- Landucci, E.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced neuroprotective effects of Panax ginseng G115® and Ginkgo biloba GK501® combinations in vitro models of excitotoxicity. Int. J. Mol. Sci. 2019, 20, 5872. [Google Scholar] [CrossRef]
- Olennikov, D.N. The ethnopharmacological uses, metabolite diversity, and bioactivity of Rhaponticum uniflorum (Leuzea uniflora): A comprehensive review. Biomolecules 2022, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N. Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, separation methods, and bioactivity. Separations 2022, 9, 448. [Google Scholar] [CrossRef]
- Razuvaeva, Y.G.; Toropova, A.A.; Olennikov, D.N.; Kharzheev, D.V. Antihypoxic activity of the dry extract from Nepeta multifida L. Nat. Prod. Rep. 2022, 36, 3105–3109. [Google Scholar] [CrossRef]
- Wei, H.; Wei-Wei, C.; Xian-Hua, H.; Yu-Mei, Z.; Fang, L. Protective effects of imperatorin against cerebral ischemia/reperfusion-induced oxidative stress through Nrf2 signaling pathway in rats. Chin. J. Pharmacol. Toxicol. 2017, 6, 988. [Google Scholar]
- Wang, N.; Wu, L.; Cao, Y.; Wang, Y.; Zhang, Y. The protective activity of imperatorin in cultured neural cells exposed to hypoxia re-oxygenation injury via anti-apoptosis. Fitoterapia 2013, 90, 38–43. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, X.; Wang, H.; Luo, J.; Zhong, S.; Zhang, Z.; Xie, F. Effects of imperatorin on apoptosis and synaptic plasticity in vascular dementia rats. Sci. Rep. 2021, 11, 8590. [Google Scholar] [CrossRef]
- Ge, J.; Deng, S.; Xue, Z. Imperatorin inhibits mitogen-activated protein kinase and nuclear factor kappa-B signaling pathways and alleviates neuroinflammation in ischemic stroke. CNS Neurosci. Ther. 2022, 28, 116–125. [Google Scholar] [CrossRef]
- Bertin, R.; Chen, Z.; Martínez-Vázquez, M.; García-Argaéz, A.; Froldi, G. Vasodilation and radical-scavenging activity of imperatorin and selected coumarinic and flavonoid compounds from genus Casimiroa. Phytomedicine 2014, 21, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Nakos-Bimpos, M.; Polissidis, A.; Dalla, C.; Kokras, N.; Skalicka-Woźniak, K.; Budzyńska, B. Imperatorin influences depressive-like behaviors: A preclinical study on behavioral and neurochemical sex differences. Molecules 2022, 27, 1179. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Thuy, T.T.T.; Thanh, L.T.; Okada, M. Antidepressive and anxiolytic effects of ostruthin, a TREK-1 channel activator. PLoS ONE 2018, 13, e0201092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Zhang, F. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef]



| Compound a | Organ (Origin b) [Ref.] |
|---|---|
| Alkyl glycosides | |
| n-Butyl-O-Frcp | Roots (CHI) [14] |
| Polyynes | |
| Falcarindiol | Roots (CHI) [15] |
| 4,6-Decadiyne 1-O-(2′-O-(6″-O-Glcp)-Glcp)-Glcp | Roots (CHI) [15] |
| (8Z)-Decaene-4,6-diyn 1-O-(2′-O-(6″-O-Glcp)-Glcp)-Glcp | Roots (CHI) [15] |
| (8E)-Decaene-4,6-diyn 1-O-(2′-O-(6″-O-Glcp)-Glcp)-Glcp | Roots (CHI) [15] |
| Semiterpene glycosides | |
| Butane-2,3-diol 2-O-Glcp | Roots (CHI) [14] |
| 2-Methyl-1-butanol 1-O-Glcp | Roots (CHI) [14] |
| 3-Methyl-1-butanol 1-O-Glcp | Roots (CHI) [14] |
| 3-Methylbutan-1,3-diol 1-O-Glcp | Roots (CHI) [14] |
| Monoterpene glycosides | |
| 3,7-Dimethyl-8-(Glcp)-1,6-octadiene-3-ol (betulalbuside A) | Roots (CHI) [14] |
| Vervenone 10-O-Glcp | Roots (CHI) [16] |
| Norsesquiterpene glycosides | |
| (9S)-Drummondol-9-O-Glcp | Roots (CHI) [14] |
| Phenols | |
| Catechol | Roots (CHI) [17] |
| Benzoic acids | |
| Isovanillic acid | Roots (CHI) [17] |
| Benzyl glycosides | |
| Benzyl O-Glcp | Roots (CHI) [16] |
| 3-Methoxy-4-hydroxy-propiophenone 4-O-Glcp (praeroside) | Roots (CHI) [18] |
| Tachioside | Roots (CHI) [18] |
| Isotachioside | Roots (CHI) [17] |
| Allyl benzenes | |
| 2-Phenylethyl O-Glcp | Roots (CHI) [14] |
| 4-Hydroxy-1-allylbenzene 3-O-(6″-O-Xylp)-Glcp | Roots (CHI) [13] |
| Phenylpropanoids | |
| Tyrosol | Roots (CHI) [17] |
| Coniferin | Roots (CHI) [18] |
| Drupanin 4-O-(6″-O-Glcp)-Glcp (dissectumoside) | Roots (CHI) [17] |
| Ferulic acid | Roots (CHI) [16] |
| (E)-4-(3-Methoxy-prop-1-en-1-yl)-phenol | Roots (CHI) [16] |
| (E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid 2-(4-hydroxyphenyl) ethyl ester | Roots (CHI) [16] |
| Benzofurans | |
| 6-Carboxylethyl-benzofuran 5-O-(2″-O-Xylp)-Glcp | Roots (CHI) [14] |
| 6-Methoxycarbonylethyl-benzofuran 5-O-(2″-O-Xylp)-Glcp | Roots (CHI) [19] |
| Neolignans | |
| (7S,8R)-Dehydrodiconiferyl alcohol 4-O-Glcp | Roots (CHI) [13] |
| (7S,8R)-Dehydrodiconiferyl alcohol 4-O-Glcp-9′-n-butanol ether | Roots (CHI) [13] |
| (2S,3S,1′S,2′R)-2,3-Dihydro-5-(1′,2′-dihydroxypropyl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-3-methylbenzofuran | Roots (CHI) [13] |
| Simple coumarins | |
| Umbelliferone | Roots (TJK) [20] |
| Herb (CHI) [21] | |
| 7-Isopentyloxycoumarin | Roots (MON) [22] |
| Scopoletin | Roots (CHI) [18] |
| Herb (CHI) [21] | |
| Isoscopoletin 6-O-Glcp | Roots (CHI) [23] |
| Isofraxidin 6-O-Glcp (eleutheroside B1) | Roots (CHI) [23] |
| Furanocoumarins linear | |
| Bergaptene | Roots (CHI) [13], (TJK) [20] |
| Isopimpinellin | Roots (CHI) [13], (TJK) [20], (MON) [22] |
| Herb (CHI) [21] | |
| Phellopterin | Herb (CHI) [21] |
| Byakangelicin | Herb (CHI) [21] |
| Xanthotoxin | Roots (CHI) [13] |
| Xanthotoxol | Roots (CHI) [18] |
| Imperatorin | Roots (CHI) [13] |
| Heraclenol | Roots (CHI) [15] |
| Heraclenol 3″-O-methyl ester | Roots (CHI) [16] |
| Heraclenol 3″-O-Glcp | Roots (CHI) [23] |
| Heraclenol 2″-O-Fer | Roots (CHI) [23] |
| Pabularinone | Roots (CHI) [15] |
| Isogosferol | Roots (CHI) [15] |
| Furanocoumarins linear dimeric | |
| Candinol C | Roots (CHI) [16] |
| Rivulobirin C | Roots (CHI) [16] |
| Rivulobirin D | Roots (CHI) [16] |
| Furanocoumarins angular | |
| Angelicin | Roots (CHI) [13], (TJK) [20] |
| Isobergaptene | Roots (CHI) [18], (MON) [22] |
| Herb (CHI) [21] | |
| Heramotol 6-O-Glcp | Roots (CHI) [18] |
| Sphondin | Roots (TJK) [20] |
| Herb (CHI) [21] | |
| Pimpinellin | Roots (TJK) [20], (MON) [22] |
| Herb (CHI) [21] | |
| Dihydrofuranocoumarins linear | |
| (9R,10R)-9,10-Dihydro-10-hydroxy-9-methoxy-bergapten (dissectumol) | Roots (CHI) [16] |
| Dihydrofuranocoumarins angular | |
| Apterin | Roots (CHI) [13] |
| Apterin 6″-O-Glcp | Roots (CHI) [19] |
| Hermandiol 5′-O-Glcp (yunngnoside B) | Roots (CHI) [15] |
| Dihydropyranocoumarins angular | |
| 5,6-Dihydropyranocoumarin | Roots (CHI) [16] |
| Sterols | |
| β-Sitosterol | Herb (CHI) [21] |
| Daucosterol | Herb (CHI) [21] |
| Compound | a a | b a | Correlation Coefficient (r2) | SYX | LOD/LOQ (µg/mL) | Linear Range (µg/mL) | RSD% (Intra-Day) | RSD% (Inter-Day) | Recovery of Spiked Sample REC% |
|---|---|---|---|---|---|---|---|---|---|
| Heraclenin | 3.4511 | −0.9526 | 0.9896 | 0.36∙10−2 | 0.003/0.010 | 0–250 | 0.96 | 1.43 | 99.63 |
| Oxypeucedanin | 2.5481 | −0.5231 | 0.9963 | 0.22∙10−2 | 0.002/0.009 | 0–250 | 0.99 | 1.28 | 100.70 |
| Imperatorin | 4.6210 | −0.8694 | 0.9906 | 0.42∙10−2 | 0.003/0.009 | 0–250 | 1.03 | 1.52 | 98.94 |
| Phellopterin | 3.8637 | −0.9005 | 0.9922 | 0.28∙10−2 | 0.002/0.007 | 0–250 | 0.97 | 1.11 | 99.52 |
| Isoimperatorin | 2.8631 | −0.2634 | 0.9850 | 0.14∙10−2 | 0.002/0.005 | 0–250 | 1.06 | 1.27 | 100.93 |
| Ostruthin | 2.5387 | −0.2622 | 0.9899 | 0.10∙10−2 | 0.001/0.004 | 0–250 | 0.99 | 1.14 | 100.52 |
| Parameter | Value |
|---|---|
| Yield (% dry seed weight) | 10.52 ± 0.15 |
| Viscosity (cP) | 62.1 ± 1.2 |
| Specific gravity (g/mL) | 0.929 ± 0.018 |
| Refractive index | 1.472 ± 0.044 |
| pH | 6.20 ± 0.05 |
| Peroxide value (mEq. peroxide/kg) | 6.28 ± 0.18 |
| Acid value (mg KOH/g) | 0.52 ± 0.01 |
| Saponification value (mg KOH/g) | 173.82 ± 3.47 |
| Iodine value (g of I2/100 g) | 105.37 ± 2.10 |
| Unsaponifiable matter (% w/w) | 0.92 ± 0.02 |
| Melting point (°C) | −25.3 ± −0.3 |
| Chlorophyll a content (mg/L) | 297.38 ± 5.94 |
| Chlorophyll b content (mg/L) | 66.70 ± 1.42 |
| Carotenoid content (mg/L) | 233.94 ± 4.67 |
| Essential oil content (% v/v) | 32.31 ± 0.62 |
| Coumarin content (% w/w) | 24.52 ± 0.51 |
| Fatty acids (% of total FA content) | |
| Lauric acid (C12:0) | 0.1 ± 0.0 |
| Myristic acid (C14:0) | 0.1 ± 0.0 |
| Pentadecanoic acid (C15:0) | 0.1 ± 0.0 |
| Palmitic acid (C16:0) | 5.2 ± 0.1 |
| Palmitoleic acid (C16:1n7c) | 0.1 ± 0.0 |
| Heptadecanoic acid (C17:0) | 0.1 ± 0.0 |
| Stearic acid (C18:0) | 1.2 ± 0.0 |
| Petroselinic acid (C18:1n12c) | 48.3 ± 0.9 |
| Oleic acid (C18:1n9c) | 10.2 ± 0.2 |
| cis-Vaccenic acid (C18:1n7c) | 0.8 ± 0.0 |
| Linoleic acid (C18:2n6c) | 28.3 ± 0.6 |
| α-Linolenic acid (C18:3n3) | 0.9 ± 0.0 |
| Arachidic acid (C20:0) | 0.4 ± 0.0 |
| Behenic acid (C22:0) | 0.1 ± 0.0 |
| Lignoceric acid (C24:0) | 0.1 ± 0.0 |
| Compound | RI a | Content, % | Identification b |
|---|---|---|---|
| Octanal | 1003 | 1.2 | i, ii, iii |
| Limonene | 1027 | 0.4 | i, ii, iii |
| Benzyl alcohol | 1033 | 0.7 | i, ii, iii |
| Octanol | 1071 | 1.4 | i, ii, iii |
| Hexyl butyrate | 1192 | 3.0 | i, ii |
| Decanal | 1205 | 0.6 | i, ii, iii |
| Octyl acetate | 1214 | 67.8 | i, ii, iii |
| Hexyl 2-methylbutyrate | 1237 | 8.5 | i, ii |
| Bornyl acetate | 1286 | 0.1 | i, ii, iii |
| Octyl isobutyrate | 1345 | 1.4 | i, ii |
| Octyl 2-methyl isobutyrate | 1354 | 9.6 | i, ii |
| Hexyl hexanoate | 1387 | 0.5 | i, ii, iii |
| Octyl butyrate | 1391 | 0.6 | i, ii |
| Decyl acetate | 1411 | 0.2 | i, ii, iii |
| Octyl 2-methylbutyrate | 1437 | 0.9 | i, ii |
| Germacrene D | 1485 | 0.2 | i, ii, iii |
| δ-Cadinene | 1525 | 0.1 | i, ii, iii |
| E-Nerolidol | 1564 | 0.1 | i, ii, iii |
| Octyl hexanoate | 1585 | 1.1 | i, ii, iii |
| Nonyl pentanoate | 1588 | 0.1 | i, ii |
| Octyl octanoate | 1778 | 1.2 | i, ii, iii |
| Total | 99.7 |
| Storage Duration | Heraclenin | Oxypeucedanin | Imperatorin | Phellopterin | Isoimperatorin | Ostruthin | Total |
|---|---|---|---|---|---|---|---|
| Before storage (control samples) | |||||||
| Sample 1 | 10.48 ± 0.21 | 3.23 ± 0.06 | 153.05 ± 3.06 | 37.12 ± 0.74 | 29.52 ± 0.59 | 5.02 ± 0.11 | 238.42 |
| Sample 2 | 9.53 ± 0.19 | 1.18 ± 0.02 | 126.11 ± 2.53 | 42.10 ± 0.84 | 25.86 ± 0.52 | 4.59 ± 0.09 | 209.37 |
| Sample 3 | 10.26 ± 0.21 | 5.76 ± 0.11 | 108.83 ± 2.19 | 30.83 ± 0.63 | 20.82 ± 0.40 | 4.64 ± 0.08 | 181.14 |
| Sample 4 | 5.14 ± 0.11 | 0.93 ± 0.02 | 128.41 ± 2.57 | 32.63 ± 0.66 | 11.67 ± 0.23 | 5.09 ± 0.10 | 183.87 |
| Room storage (20 °C; treated sample 1) | |||||||
| 1 year | 9.43 ± 0.19 * | 2.93 ± 0.04 * | 140.81 ± 2.85 * | 33.04 ± 0.67 * | 26.56 ± 0.54 * | 4.41 ± 0.08 * | 217.18 |
| 2 years | 8.91 ± 0.17 * | 2.77 ± 0.04 * | 128.52 ± 2.59 * | 31.12 ± 0.61 * | 24.21 ± 0.47 * | 4.16 ± 0.07 * | 199.69 |
| 3 years | 8.17 ± 0.16 * | 2.42 ± 0.03 * | 110.19 ± 2.26 * | 26.76 ± 0.52 * | 20.66 ± 0.40 * | 3.81 ± 0.05 * | 172.01 |
| Cold storage (1 °C; treated sample 1) | |||||||
| 1 year | 10.37 ± 0.20 | 3.18 ± 0.06 | 149.99 ± 3.00 | 36.75 ± 0.74 | 29.20 ± 0.58 | 4.97 ± 0.09 | 234.46 |
| 2 years | 10.15 ± 0.19 | 3.10 ± 0.06 | 148.45 ± 2.96 | 36.37 ± 0.71 | 28.38 ± 0.54 | 4.86 ± 0.09 | 231.31 |
| 3 years | 9.85 ± 0.18 * | 3.04 ± 0.06 * | 143.87 ± 2.85 * | 32.26 ± 0.63 * | 27.75 ± 0.52 * | 4.76 ± 0.08 * | 221.53 |
| Freeze storage (−20 °C; treated sample 1) | |||||||
| 1 year | 10.45 ± 0.21 | 3.21 ± 0.06 | 152.80 ± 3.05 | 37.04 ± 0.73 | 29.06 ± 0.60 | 4.92 ± 0.11 | 237.48 |
| 2 years | 10.37 ± 0.20 | 3.17 ± 0.06 | 151.48 ± 3.01 | 36.70 ± 0.72 | 29.14 ± 0.58 | 4.90 ± 0.11 | 235.79 |
| 3 years | 10.16 ± 0.19 | 3.10 ± 0.06 | 149.90 ± 2.99 | 36.10 ± 0.70 | 28.82 ± 0.56 | 4.84 ± 0.11 | 232.92 |
| Parameter | Experimental Group (n = 15 For All Groups) | |||||
|---|---|---|---|---|---|---|
| Sham-Operated Group | Negative Control | EGB761 | HSO, 0.1 mL/kg | HSO, 0.5 mL/kg | HSO, 1 mL/kg | |
| Cerebral Blood Flow, sm/sec | 4.10 ± 0.25 | 1.25 ± 0.10 a | 2.63 ± 0.21 ab | 1.45 ± 0.11 ac | 2.28 ± 0.22 ab | 3.11 ± 0.26 abc |
| Necrosis Zone Area, % | - | 41.52 ± 3.73 | 21.60 ± 1.95 b | 42.62 ± 4.69 c | 30.38 ± 2.78 bc | 18.56 ± 1.69 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Chirikova, N.K. Hogweed Seed Oil: Physico–Chemical Characterization, LC-MS Profile, and Neuroprotective Activity of Heracleum dissectum Nanosuspension. Life 2023, 13, 1112. https://doi.org/10.3390/life13051112
Olennikov DN, Chirikova NK. Hogweed Seed Oil: Physico–Chemical Characterization, LC-MS Profile, and Neuroprotective Activity of Heracleum dissectum Nanosuspension. Life. 2023; 13(5):1112. https://doi.org/10.3390/life13051112
Chicago/Turabian StyleOlennikov, Daniil N., and Nadezhda K. Chirikova. 2023. "Hogweed Seed Oil: Physico–Chemical Characterization, LC-MS Profile, and Neuroprotective Activity of Heracleum dissectum Nanosuspension" Life 13, no. 5: 1112. https://doi.org/10.3390/life13051112







