Transcranial Electrical Stimulation for Associative Memory Enhancement: State-of-the-Art from Basic to Clinical Research
Abstract
:1. Introduction
2. Methods
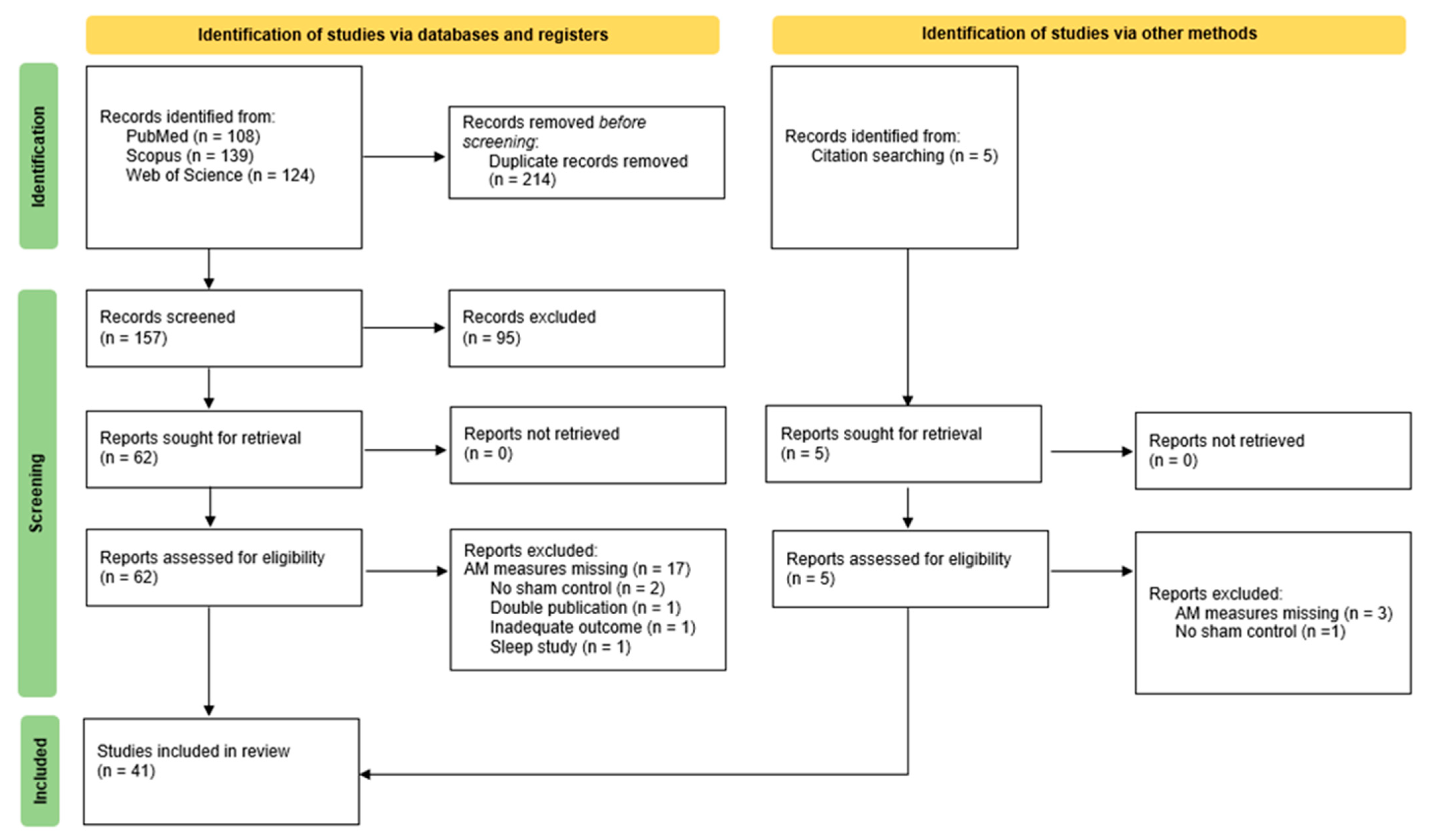
2.1. Search Strategy and Study Selection
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Analysis
3. Results
3.1. Studies on Healthy Adult Participants
3.2. Studies Conducted on Older Participants or Comparing Older vs. Younger Participants’ Effects
3.3. Studies on People with MCI and Alzheimer’s Disease
| Study | Sample n (Group), Age, Health Status | Design | tES 1 | Montage | AM Task: Measures | Result |
|---|---|---|---|---|---|---|
| Leach et al., 2016 [62] | 14 (7 + 7) 60–90 years, healthy | parallel 2-group online (encoding) | tDCS 2 mA 25 min | 1 × 1 left inferior PFC (F7-arm) | face-name task: cued recall and recognition | ↓ recognition (more false alarms in active group) = recall |
| Fiori, 2017 [67] | 30 (15 + 15) young: 29 ± 6, old: 72 ± 6, healthy | parallel 2-group online (retrieval) | tDCS 2 mA 20 min | 1 × 1 Wernicke’s area (CP5–CP4) | pseudowords-picture task: recognition | old: ↑ recognition young: = recognition |
| Prehn, 2017 [68] | 40 (10 + 10 + 10 + 10) young: 18–35 years, old: 50–80 years, healthy | parallel 4-group online (encoding; w/medical intervention) | tDCS 1 mA 20 min | 1 × 1 right temporoparietal cortex (T6—left frontopolar cortex) | object location task: cued recall | old: ↑ recall + medical intervention = recall young: ↑ recall + medical intervention = recall |
| Külzow et al., 2017 [61] | 32 (16 + 16) 53–79 years, healthy | parallel 2-group offline (before encoding; w/cognitive training) | tDCS 1 mA 20 min 3 sessions | 1 × 1 right temporal cortex (T6-eyebrow) | object location task: recognition (one month later) | = recognition |
| Antonenko et al., 2019 [63] | 34 63.1 ± 7.7 years, healthy | cross-over online (encoding) | tDCS 1 mA 20 min | 1 × 1 left temporoparietal cortex (CP5–AF4) | pseudowords-picture task: cued recall (immediate: 0 and 20 min) | ↑ recall (in both time points) |
| Huo et al., 2020 [64] | 49 (25 + 24) 66.6 ± 6.1 years, healthy | parallel 2-group offline | tDCS 2 mA 30 min 10 sessions | 1 × 1 left dlPFC (F3—deltoid muscle) | source memory task: cued recall (24 h after last simulation) | = recall |
| Klink et al., 2020 [65] | 28 71.1 ± 6.4 years, healthy | cross-over online (encoding) | tDCS 2 mA 20 min tACS (5 Hz) ± 1 mA 20 min | 1 × 1 left vl PFC (individualized position usually between T3–F3 and F7–C3) | face occupation task: cued recall and recognition | = recall = recognition |
| Luckey et al., 2020 [66] | 30 (15 + 15), 55–70 years, healthy | parallel 2 + group, online (encoding) | tDCS 1.5 mA 13 min | 1 × 1 occipital nerve (C1–C2) | word pairs task: cued recall (immediate, 7 and 24 days later) | ↑ cued recall |
| Leach, 2020 [69] | 96 (48 + 48), young: 22.4 ± 4.7 years, old: 65.6 ± 4.9 years, healthy | parallel 2 + group online (encoding) | tACS 1.5 mA | 1 × 1 left dl PFC (F3-arm) | face-name task: cued recall, recognition | old: = recall = recognition young: ↑ recall ↑ recognition |
| de Sousa et al., 2020 [70] | 48 50–90 years, 16 MCI (8 + 8) 32 healthy (16 + 16) | parallel 4-group offline (before encoding; w/cognitive training,) | tDCS 1 mA 20 min | 1 × 1 right temporal cortex (T6—supraorbital area) | object location task: cued recall (immediately and 24 h later) | MCI: ↑ cued recall (only immediate effects) healthy: = recall |
| Gu et al., 2022 [71] | 40 (20 + 20) 64 ± 6.6 years MCI | parallel 2-group offline (before encoding) | tDCS 2 mA 20 min 5 sessions | 1 × 1 left temporal area (T3-shoulder) | AM task form Wechsler Memory Scale, (immediate and 4 weeks later) | × no effects |
| Benussi et al., 2022 [72] | 60, 72.3 ± 7.0 years Alzheimer | cross-over offline (before encoding) | tACS (40 Hz) 1.5 mA 60 min | 1 × 1 PPC, precuneus (Pz—deltoid muscle) | face-name task: recognition | ↑ recognition |
4. Discussion
4.1. Defining the Optimal Stimulation Site/Were Do We Stimulate
4.2. Stimulation Protocol/How Do We Stimulate
4.3. Timing of Stimulation and the Duration of the Effects/When Do We Stimulate and How Often
4.4. Outcome Measures
4.5. Methodological Concerns: Sample Size, Power Issues, and Blinding
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. The Exact Syntaxes Used for Each Database
Appendix B. The PICO Strategy Table
| PICO Category | Included | Excluded |
|---|---|---|
| Participants | Adults (over 18 years old): younger and older Healthy or with diagnosed memory deficits (e.g., Alzheimer’s, MCI) or with subjective memory complaints | Animal studies Memory-unrelated clinical conditions in human participants |
| Intervention | TES (tDCS, hd tDCS, TACS, hd TACS, os-tDCS) TES combined with other memory interventions (e.g., cognitive training) | Other types of NIBS intervention (e.g., TMS) |
| Comparator | Sham condition | No sham condition |
| Outcome | Associative memory measures (e.g., cued recall, recognition, reproduction) Immediate or immediate and delayed AM measures | Other memory type assessment tasks (e.g., working memory, episodic memory) Only delayed AM measures Sleep studies |
References
- Baddeley, A. Fractionating the Central Executive. In Principles of Frontal Lobe Function; Stuss, D.T., Knight, R.T., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 246–260. ISBN 978-0-19-513497-1. [Google Scholar]
- Baddeley, A. Working Memory, Thought, and Action; Oxford University Press: Oxford, UK, 2007; ISBN 978-0-19-852801-2. [Google Scholar]
- Atkinson, R.C.; Shiffrin, R.M. Human Memory: A Proposed System and Its Control Processes. In Psychology of Learning and Motivation; Elsevier: Amsterdam, The Netherlands, 1968; Volume 2, pp. 89–195. ISBN 978-0-12-543302-0. [Google Scholar]
- Tulving, E. How Many Memory Systems Are There? Am. Psychol. 1985, 40, 385–398. [Google Scholar] [CrossRef]
- Melton, A.W. Implications of Short-Term Memory for a General Theory of Memory. J. Verbal Learn. Verbal Behav. 1963, 2, 1–21. [Google Scholar] [CrossRef]
- Davachi, L.; Dobbins, I.G. Declarative Memory. Curr. Dir. Psychol. Sci. 2008, 17, 112–118. [Google Scholar] [CrossRef]
- Konkel, A. Relational Memory and the Hippocampus: Representations and Methods. Front. Neurosci. 2009, 3, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Birba, A.; Hesse, E.; Sedeño, L.; Mikulan, E.P.; García, M.D.C.; Ávalos, J.; Adolfi, F.; Legaz, A.; Bekinschtein, T.A.; Zimerman, M.; et al. Enhanced Working Memory Binding by Direct Electrical Stimulation of the Parietal Cortex. Front. Aging Neurosci. 2017, 9, 178. [Google Scholar] [CrossRef]
- Arnold, N.R.; Heck, D.W.; Bröder, A.; Meiser, T.; Boywitt, C.D. Testing Hypotheses About Binding in Context Memory With a Hierarchical Multinomial Modeling Approach: A Preregistered Study. Exp. Psychol. 2019, 66, 239–251. [Google Scholar] [CrossRef]
- Gold, J.J.; Hopkins, R.O.; Squire, L.R. Single-Item Memory, Associative Memory, and the Human Hippocampus. Learn. Mem. 2006, 13, 644–649. [Google Scholar] [CrossRef]
- Suzuki, W. Associative Learning and the Hippocampus; American Psychological Association: Washington, DC, USA, 2005. [Google Scholar]
- Old, S.R.; Naveh-Benjamin, M. Differential Effects of Age on Item and Associative Measures of Memory: A Meta-Analysis. Psychol. Aging 2008, 23, 104–118. [Google Scholar] [CrossRef]
- Kormas, C.; Zalonis, I.; Evdokimidis, I.; Kapaki, E.; Potagas, C. Face–Name Associative Memory Performance among Cognitively Healthy Individuals, Individuals with Subjective Memory Complaints, and Patients with a Diagnosis of AMCI. Front. Psychol. 2020, 11, 2173. [Google Scholar] [CrossRef]
- Hanseeuw, B.; Dricot, L.; Kavec, M.; Grandin, C.; Seron, X.; Ivanoiu, A. Associative Encoding Deficits in Amnestic Mild Cognitive Impairment: A Volumetric and Functional MRI Study. NeuroImage 2011, 56, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Rubiño, J.; Andrés, P. The Face-Name Associative Memory Test as a Tool for Early Diagnosis of Alzheimer’s Disease. Front. Psychol. 2018, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. JAD 2019, 67, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.J.; Sachdev, P.S.; Fiatarone Singh, M.A.; Valenzuela, M. Cognitive and Memory Training in Adults at Risk of Dementia: A Systematic Review. BMC Geriatr. 2011, 11, 55. [Google Scholar] [CrossRef]
- Khadka, N.; Bikson, M. Noninvasive Electrical Brain Stimulation of the Central Nervous System. In Handbook of Neuroengineering; Thakor, N.V., Ed.; Springer: Singapore, 2022; pp. 1–33. ISBN 9789811528484. [Google Scholar]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Miniussi, C.; Harris, J.A.; Ruzzoli, M. Modelling Non-Invasive Brain Stimulation in Cognitive Neuroscience. Neurosci. Biobehav. Rev. 2013, 37, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Antal, A.; Boros, K.; Poreisz, C.; Chaieb, L.; Terney, D.; Paulus, W. Comparatively Weak After-Effects of Transcranial Alternating Current Stimulation (TACS) on Cortical Excitability in Humans. Brain Stimul. 2008, 1, 97–105. [Google Scholar] [CrossRef]
- Wischnewski, M.; Alekseichuk, I.; Opitz, A. Neurocognitive, Physiological, and Biophysical Effects of Transcranial Alternating Current Stimulation. Trends Cogn. Sci. 2023, 27, 189–205. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Strüber, D. Transcranial Alternating Current Stimulation: A Review of the Underlying Mechanisms and Modulation of Cognitive Processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef]
- Chase, H.W.; Boudewyn, M.A.; Carter, C.S.; Phillips, M.L. Transcranial Direct Current Stimulation: A Roadmap for Research, from Mechanism of Action to Clinical Implementation. Mol. Psychiatry 2020, 25, 397–407. [Google Scholar] [CrossRef]
- Alekseichuk, I.; Turi, Z.; Amador de Lara, G.; Antal, A.; Paulus, W. Spatial Working Memory in Humans Depends on Theta and High Gamma Synchronization in the Prefrontal Cortex. Curr. Biol. 2016, 26, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- De Lara, G.A.; Alekseichuk, I.; Turi, Z.; Lehr, A.; Antal, A.; Paulus, W. Perturbation of Theta-Gamma Coupling at the Temporal Lobe Hinders Verbal Declarative Memory. Brain Stimul. 2018, 11, 509–517. [Google Scholar] [CrossRef]
- Bikson, M.; Esmaeilpour, Z.; Adair, D.; Kronberg, G.; Tyler, W.J.; Antal, A.; Datta, A.; Sabel, B.A.; Nitsche, M.A.; Loo, C.; et al. Transcranial Electrical Stimulation Nomenclature. Brain Stimul. 2019, 12, 1349–1366. [Google Scholar] [CrossRef] [PubMed]
- Borckardt, J.J.; Bikson, M.; Frohman, H.; Reeves, S.T.; Datta, A.; Bansal, V.; Madan, A.; Barth, K.; George, M.S. A Pilot Study of the Tolerability and Effects of High-Definition Transcranial Direct Current Stimulation (HD-TDCS) on Pain Perception. J. Pain 2012, 13, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Meinzer, M.; Jähnigen, S.; Copland, D.A.; Darkow, R.; Grittner, U.; Avirame, K.; Rodriguez, A.D.; Lindenberg, R.; Flöel, A. Transcranial Direct Current Stimulation over Multiple Days Improves Learning and Maintenance of a Novel Vocabulary. Cortex 2014, 50, 137–147. [Google Scholar] [CrossRef]
- Matzen, L.E.; Trumbo, M.C.; Leach, R.C.; Leshikar, E.D. Effects of Non-Invasive Brain Stimulation on Associative Memory. Brain Res. 2015, 1624, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, D.; Chua, E.F. Transcranial Direct Current Stimulation over the Parietal Cortex Alters Bias in Item and Source Memory Tasks. Brain Cogn. 2016, 108, 56–65. [Google Scholar] [CrossRef]
- Chen, N.-F.; Lo, C.-M.; Liu, T.-L.; Cheng, S.-K. Source Memory Performance Is Modulated by Transcranial Direct Current Stimulation over the Left Posterior Parietal Cortex. NeuroImage 2016, 139, 462–469. [Google Scholar] [CrossRef]
- Gaynor, A.M.; Chua, E.F. TDCS over the Prefrontal Cortex Alters Objective but Not Subjective Encoding. Cogn. Neurosci. 2017, 8, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Leshikar, E.D.; Leach, R.C.; McCurdy, M.P.; Trumbo, M.C.; Sklenar, A.M.; Frankenstein, A.N.; Matzen, L.E. Transcranial Direct Current Stimulation of Dorsolateral Prefrontal Cortex during Encoding Improves Recall but Not Recognition Memory. Neuropsychologia 2017, 106, 390–397. [Google Scholar] [CrossRef]
- de Lara, G.A.; Knechtges, P.N.; Paulus, W.; Antal, A. Anodal TDCS over the Left DLPFC Did Not Affect the Encoding and Retrieval of Verbal Declarative Information. Front. Neurosci. 2017, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Perceval, G.; Martin, A.K.; Copland, D.A.; Laine, M.; Meinzer, M. High-Definition TDCS of the Temporo-Parietal Cortex Enhances Access to Newly Learned Words. Sci. Rep. 2017, 7, 17023. [Google Scholar] [CrossRef] [PubMed]
- Brunyé, T.T.; Smith, A.M.; Horner, C.B.; Thomas, A.K. Verbal Long-Term Memory Is Enhanced by Retrieval Practice but Impaired by Prefrontal Direct Current Stimulation. Brain Cogn 2018, 128, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.; Mitrenga, K.J.; Ellison, A.; Fernyhough, C. Investigating the Roles of Medial Prefrontal and Superior Temporal Cortex in Source Monitoring. Neuropsychologia 2018, 120, 113–123. [Google Scholar] [CrossRef]
- Marián, M.; Szőllősi, Á.; Racsmány, M. Anodal Transcranial Direct Current Stimulation of the Right Dorsolateral Prefrontal Cortex Impairs Long-Term Retention of Reencountered Memories. J. Devoted Study Nerv. Syst. Behav. 2018, 108, 80–91. [Google Scholar] [CrossRef]
- Mizrak, E.; Kim, K.; Roberts, B.; Ragl, D.J.; Carter, C.; Ranganath, C. Impact of Oscillatory TDCS Targeting Left Prefrontal Cortex on Source Memory Retrieval. Cogn. Neurosci. 2018, 9, 194–207. [Google Scholar] [CrossRef]
- Bjekić, J.; Vulić, K.; Živanović, M.; Vujičić, J.; Ljubisavljević, M.; Filipović, S.R. The Immediate and Delayed Effects of Single TDCS Session over Posterior Parietal Cortex on Face-Word Associative Memory. Behav. Brain Res. 2019, 366, 88–95. [Google Scholar] [CrossRef]
- Westphal, A.J.; Chow, T.E.; Ngoy, C.; Zuo, X.; Liao, V.; Storozuk, L.A.; Peters, M.A.K.; Wu, A.D.; Rissman, J. Anodal Transcranial Direct Current Stimulation to the Left Rostrolateral Prefrontal Cortex Selectively Improves Source Memory Retrieval. J. Cogn. Neurosci. 2019, 31, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.P.; Kellermann, T.; Freiherr, J.; Clemens, B.; Habel, U.; Regenbogen, C. Externalization Errors of Olfactory Source Monitoring in Healthy Controls-An FMRI Study. Chem. Senses 2019, 44, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Bjekić, J.; Čolić, M.V.; Živanović, M.; Milanović, S.D.; Filipović, S.R. Transcranial Direct Current Stimulation (TDCS) over Parietal Cortex Improves Associative Memory. Neurobiol. Learn. Mem. 2019, 157, 114–120. [Google Scholar] [CrossRef]
- Lang, S.; Gan, L.S.; Alrazi, T.; Monchi, O. Theta Band High Definition Transcranial Alternating Current Stimulation, but Not Transcranial Direct Current Stimulation, Improves Associative Memory Performance. Sci. Rep. 2019, 9, 8562. [Google Scholar] [CrossRef] [PubMed]
- Owusu, B.-A.K.; Burianová, H. Transcranial Direct Current Stimulation Improves Novel Word Recall in Healthy Adults. J. Neurolinguistics 2020, 53, 100862. [Google Scholar] [CrossRef]
- Ergo, K.; De Loof, E.; Debra, G.; Pastötter, B.; Verguts, T. Failure to Modulate Reward Prediction Errors in Declarative Learning with Theta (6 Hz) Frequency Transcranial Alternating Current Stimulation. PLoS ONE 2020, 15, e0237829. [Google Scholar] [CrossRef]
- Gilson, M.; Nitsche, M.A.; Peigneux, P. Prefrontal Transcranial Direct Current Stimulation Globally Improves Learning but Does Not Selectively Potentiate the Benefits of Targeted Memory Reactivation on Awake Memory Consolidation. Brain Sci. 2021, 11, 1104. [Google Scholar] [CrossRef]
- Vulić, K.; Bjekić, J.; Paunović, D.; Jovanović, M.; Milanović, S.; Filipović, S.R. Theta-Modulated Oscillatory Transcranial Direct Current Stimulation over Posterior Parietal Cortex Improves Associative Memory. Sci. Rep. 2021, 11, 3013. [Google Scholar] [CrossRef] [PubMed]
- Bolling, A.J.; King, V.L.; Enam, T.; McDonough, I.M. Using Transcranial Direct Current Stimulation (TDCS) on the Dorsolateral Prefrontal Cortex to Promote Long-Term Foreign Language Vocabulary Learning. Brain Cogn. 2021, 154, 105789. [Google Scholar] [CrossRef]
- Huang, Y.; Mohan, A.; McLeod, S.L.; Luckey, A.M.; Hart, J.J.; Vanneste, S. Polarity-Specific High-Definition Transcranial Direct Current Stimulation of the Anterior and Posterior Default Mode Network Improves Remote Memory Retrieval. Brain Stimul. 2021, 14, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Kaiser, M.; de Graaf, T.A.; Dücker, F.; Sack, A.T.; De Weerd, P.; van de Ven, V. Transcranial Alternating Current Stimulation at Theta Frequency to Left Parietal Cortex Impairs Associative, but Not Perceptual, Memory Encoding. Neurobiol. Learn. Mem. 2021, 182, 107444. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Cid-Fernández, S.; Díaz, F. Transcranial Direct Current Stimulation (Tdcs). an Effective Tool for Improving Episodic Memory in Young People? [Estimulación Transcraneal Por Corriente Eléctrica Directa (Tdcs). ¿una Herramienta Efectiva Para Mejorar La Memoria Episódica En Jóvenes?]. An. Psicol. 2021, 37, 468–477. [Google Scholar] [CrossRef]
- Pyke, W.; Vostanis, A.; Javadi, A.-H. Electrical Brain Stimulation During a Retrieval-Based Learning Task Can Impair Long-Term Memory. J. Cogn. Enhanc. 2021, 5, 218–232. [Google Scholar] [CrossRef]
- Luckey, A.M.; McLeod, S.L.; Mohan, A.; Vanneste, S. Potential Role for Peripheral Nerve Stimulation on Learning and Long-Term Memory: A Comparison of Alternating and Direct Current Stimulations. Brain Stimul. 2022, 15, 536–545. [Google Scholar] [CrossRef]
- Živanović, M.; Bjekić, J.; Konstantinović, U.; Filipović, S.R. Effects of Online Parietal Transcranial Electric Stimulation on Associative Memory: A Direct Comparison between TDCS, Theta TACS, and Theta-Oscillatory TDCS. Sci. Rep. 2022, 12, 14091. [Google Scholar] [CrossRef]
- Bjekić, J.; Živanović, M.; Filipović, S.R. Transcranial direct current stimulation (tDCS) for memory enhancement. JoVE (J. Vis. Exp.) 2021, 175, e62681. [Google Scholar] [CrossRef]
- Külzow, N.; Cavalcanti de Sousa, A.V.; Cesarz, M.; Hanke, J.-M.; Günsberg, A.; Harder, S.; Koblitz, S.; Grittner, U.; Flöel, A. No Effects of Non-Invasive Brain Stimulation on Multiple Sessions of Object-Location-Memory Training in Healthy Older Adults. Front. Neurosci. 2017, 11, 746. [Google Scholar] [CrossRef]
- Leach, R.C.; McCurdy, M.P.; Trumbo, M.C.; Matzen, L.E.; Leshikar, E.D. Transcranial Stimulation over the Left Inferior Frontal Gyrus Increases False Alarms in an Associative Memory Task in Older Adults. Healthy Aging Res. 2016, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Hayek, D.; Netzb, J.; Grittner, U.; Flöel, A. TDCS-Induced Episodic Memory Enhancement and Its Association with Functional Network Coupling in Older Adults. Sci. Rep. 2019, 9, 2273. [Google Scholar] [CrossRef]
- Huo, L.; Zhu, X.; Zheng, Z.; Ma, J.; Ma, Z.; Gui, W.; Li, J. Effects of Transcranial Direct Current Stimulation on Episodic Memory in Older Adults: A Meta-Analysis. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2021, 76, 692–702, Corrigendum in J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2020, 75, 1931. https://doi.org/10.1093/geronb/gbz159. [Google Scholar] [CrossRef] [PubMed]
- Klink, K.; Peter, J.; Wyss, P.; Klöppel, S. Transcranial Electric Current Stimulation During Associative Memory Encoding: Comparing TACS and TDCS Effects in Healthy Aging. Front. Aging Neurosci. 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Luckey, A.M.; McLeod, S.L.; Robertson, I.H.; To, W.T.; Vanneste, S. Greater Occipital Nerve Stimulation Boosts Associative Memory in Older Individuals: A Randomized Trial. Neurorehabilit. Neural Repair 2020, 34, 1020–1029. [Google Scholar] [CrossRef]
- Fiori, V.; Nitsche, M.; Iasevoli, L.; Cucuzza, G.; Caltagirone, C.; Marangolo, P. Differential Effects of Bihemispheric and Unihemispheric Transcranial Direct Current Stimulation in Young and Elderly Adults in Verbal Learning. Behav. Brain Res. 2017, 321, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; Stengl, H.; Grittner, U.; Kosiolek, R.; Ölschläger, A.; Weidemann, A.; Flöel, A. Effects of Anodal Transcranial Direct Current Stimulation and Serotonergic Enhancement on Memory Performance in Young and Older Adults. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 551–561. [Google Scholar] [CrossRef]
- Leach, R.C.; McCurdy, M.P.; Trumbo, M.C.; Matzen, L.E.; Leshikar, E.D. Differential Age Effects of Transcranial Direct Current Stimulation on Associative Memory. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2019, 74, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.V.C.; Grittner, U.; Rujescu, D.; Külzow, N.; Flöel, A. Impact of 3-Day Combined Anodal Transcranial Direct Current Stimulation-Visuospatial Training on Object-Location Memory in Healthy Older Adults and Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2020, 75, 223–244. [Google Scholar] [CrossRef]
- Gu, J.; Li, D.; Li, Z.; Guo, Y.; Qian, F.; Wang, Y.; Tang, L. The Effect and Mechanism of Transcranial Direct Current Stimulation on Episodic Memory in Patients With Mild Cognitive Impairment. Front. Neurosci. 2022, 16, 811403. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Brattini, C.; Datta, A.; Thomas, C.; Santarnecchi, E.; Pascual-Leone, A.; Borroni, B. Exposure to Gamma TACS in Alzheimer’s Disease: A Randomized, Double-Blind, Sham-Controlled, Crossover, Pilot Study. Brain Stimul. 2021, 14, 531–540. [Google Scholar] [CrossRef]
- Mayes, A.; Montaldi, D.; Migo, E. Associative Memory and the Medial Temporal Lobes. Trends Cogn. Sci. 2007, 11, 126–135. [Google Scholar] [CrossRef]
- Eichenbaum, H. The Hippocampus and Declarative Memory: Cognitive Mechanisms and Neural Codes. Behav. Brain Res. 2001, 127, 199–207. [Google Scholar] [CrossRef]
- Eichenbaum, H. A Cortical–Hippocampal System for Declarative Memory. Nat. Rev. Neurosci. 2000, 1, 41–50. [Google Scholar] [CrossRef]
- Kim, K.; Ekstrom, A.D.; Tandon, N. A Network Approach for Modulating Memory Processes via Direct and Indirect Brain Stimulation: Toward a Causal Approach for the Neural Basis of Memory. Neurobiol. Learn. Mem. 2016, 134, 162–177. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Gilmore, A.W.; Nelson, S.M.; McDermott, K.B. A Parietal Memory Network Revealed by Multiple MRI Methods. Trends Cogn. Sci. 2015, 19, 534–543. [Google Scholar] [CrossRef]
- Wagner, A.D.; Shannon, B.J.; Kahn, I.; Buckner, R.L. Parietal Lobe Contributions to Episodic Memory Retrieval. Trends Cogn. Sci. 2005, 9, 445–453. [Google Scholar] [CrossRef]
- Tambini, A.; Nee, D.E.; D’Esposito, M. Hippocampal-Targeted Theta-Burst Stimulation Enhances Associative Memory Formation. J. Cogn. Neurosci. 2018, 30, 1452–1472. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted Enhancement of Cortical-Hippocampal Brain Networks and Associative Memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef]
- Hermiller, M.S.; VanHaerents, S.; Raij, T.; Voss, J.L. Frequency-specific Noninvasive Modulation of Memory Retrieval and Its Relationship with Hippocampal Network Connectivity. Hippocampus 2018, 29, 595–609. [Google Scholar] [CrossRef]
- Nilakantan, A.S.; Bridge, D.J.; Gagnon, E.P.; VanHaerents, S.A.; Voss, J.L. Stimulation of the Posterior Cortical-Hippocampal Network Enhances Precision of Memory Recollection. Curr. Biol. 2017, 27, 465–470. [Google Scholar] [CrossRef]
- Lee, J.S.A.; Bestmann, S.; Evans, C. A Future of Current Flow Modelling for Transcranial Electrical Stimulation? Curr. Behav. Neurosci. Rep. 2021, 8, 150–159. [Google Scholar] [CrossRef]
- Miniussi, C.; Brignani, D.; Pellicciari, M.C. Combining Transcranial Electrical Stimulation with Electroencephalography: A Multimodal Approach. Clin. EEG Neurosci. 2012, 43, 184–191. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Axmacher, N.; Inman, C.S. Modulating Human Memory via Entrainment of Brain Oscillations. Trends Neurosci. 2019, 42, 485–499. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.; Fietsam, A.; Ponto, L. Imaging Transcranial Direct Current Stimulation (TDCS) with Positron Emission Tomography (PET). Brain Sci. 2020, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low Intensity Transcranial Electric Stimulation: Safety, Ethical, Legal Regulatory and Application Guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic Evaluation of the Impact of Stimulation Intensity on Neuroplastic After-Effects Induced by Transcranial Direct Current Stimulation: Effects of DC Intensity on Cortical Excitability. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef]
- Bjekić, J.; Paunovic, D.; Živanović, M.; Stanković, M.; Griskova-Bulanova, I.; Filipović, S.R. Determining the Individual Theta Frequency for Associative Memory Targeted Personalized Transcranial Brain Stimulation. J. Pers. Med. 2022, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, K.A.; Badran, B.W.; DeVries, W.H.; Summers, P.M.; Kofmehl, E.; Li, X.; Borckardt, J.J.; Bikson, M.; George, M.S. Transcranial Electrical Stimulation Motor Threshold Can Estimate Individualized TDCS Dosage from Reverse-Calculation Electric-Field Modeling. Brain Stimul. 2020, 13, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Bachmann, C.; Lee, J.S.A.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-Controlled TDCS Reduces Electric Field Intensity Variability at a Cortical Target Site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, M.; Laakso, I.; Tanaka, S.; Hirata, A. Cost of Focality in TDCS: Interindividual Variability in Electric Fields. Brain Stimul. 2020, 13, 117–124. [Google Scholar] [CrossRef]
- Staresina, B.P.; Davachi, L. Differential Encoding Mechanisms for Subsequent Associative Recognition and Free Recall. J. Neurosci. 2006, 26, 9162–9172. [Google Scholar] [CrossRef] [PubMed]
- Herweg, N.A.; Solomon, E.A.; Kahana, M.J. Theta Oscillations in Human Memory. Trends Cogn. Sci. 2020, 24, 208–227. [Google Scholar] [CrossRef]
- Osth, A.F.; Dennis, S. Associative Recognition and the List Strength Paradigm. Mem. Cogn. 2014, 42, 583–594. [Google Scholar] [CrossRef]
- Stanković, M.; Živanović, M.; Bjekić, J.; Filipović, S.R. Blinding in TDCS Studies: Correct End-of-Study Guess Does Not Moderate the Effects on Associative and Working Memory. Brain Sci. 2021, 12, 58. [Google Scholar] [CrossRef]
| Study | Sample n (Group), Age | Design | tES | Montage | AM Task: Measures | Result |
|---|---|---|---|---|---|---|
| Meinzer, 2014 [32] | n = 40 (20 + 20) 23.9 ± 3.6 years | parallel 2-group online (encoding) | tDCS 1 mA 20 min 5 sessions | 1 × 1 left PFC (CP5-supraorbital) | object-nonword task: cued recall, recognition | ↑ cued recall = recognition |
| Matzen, 2015 [33] | n = 26 (13 + 13) 19-30 years | parallel 2-group online (encoding) | tDCS 2 mA 30 min | 1 × 1 left PFC (F9-arm) | face-word task: cued recall, recognition | ↑ cued recall = recognition |
| Pergolizzi, 2016 [34] | n = 54 (18 + 18 + 18) 19.6 ± 3.06 years | parallel 3-group online (retrieval) | tDCS 2 mA 20 min | 1 × 1 left PPC (CP3-CP4) left dlPFC (F3-F4) | source memory task: cued recall, source bias | ↓ source bias = cued recall |
| Chen, 2016 [35] | n = 36 (18 + 18), 21.2 years | mixed parallel 2-group: anodal/cathodal, repeated: stimulation site online (retrieval) | tDCS anodal/cathodal +1.5 mA/−1.5 mA 10 min | 1 × 1 left PPC (P3-cheek) right M1 (M1-cheek) | source memory task: recognition, memory confidence | anodal tDCS: = recognition (PPC, M1) = confidence (PPC, M1) cathodal tDCS: ↓ recognition (PPC) = recognition (M1) = confidence (PPC, M1) |
| Gaynor, 2017 [36] | n = 72 (24), 20.8 ± 3.3 years | parallel 3-group online (encoding) | tDCS 2 mA 20 min | 1 × 1 left PPC (CP3-CP4) left dlPFC (F3—supraorbital bridge) | unrelated word pairs: recognition (24 h later) | ↓ recognition (dlPFC) = recognition (PPC) |
| Leshikar 2017 [37] | n = 42 (21) 22.5 years | parallel 2-group online (encoding) | tDCS 1.5 mA 25 min | 1 × 1 left dlPFC (F3-arm) | face-name task: cued recall, recognition | ↑ cued recall = recognition |
| de Lara, 2017 [38] | n = 30 (15), 24.8 ± 3.5 years | mixed parallel 2-group: encoding/retrieval repeated: active/sham online (encoding/retrieval) | HD-tDCS 1 mA 20 min | 1 × 4 left dlPFC (AF3—6 cm distance from AF3 and 10 cm between each other) | semantically related word pairs: cued recall | = cued recall |
| Perceval, 2017 [39] | n = 50 (25), 23.16 ± 3.79 years | parallel 2-group online (encoding) | HD-tDCS 1 mA 20 min | ring electrode left temporoparietal cortex (CP5) | pseudoword-picture task: recognition | = recognition ↑ RT for correct pairs |
| de Lara, 2018 [28] | n = 24 23.5 ± 3.1 years | cross-over, online (encoding) | tACS (5 Hz + 80 Hz at peaks) 1 mA 10 min | 1 × 2 left temporal lobe (T7–T8 and FPz) | semantically related word pairs, cued recall | = cued recall |
| n = 24 24.3 ± 2.9 years | cross-over, online (encoding) | tACS (5 Hz + 80 Hz at troughs) 1 mA 10 min | 1 × 2 left temporal lobe (T7–T8 and FPz) | semantically related word pairs, cued recall | ↓ cued recall | |
| n = 24 23.2 ± 2.2 years | cross-over, online (encoding) | tACS (5 Hz + 80 Hz throughout) 1 mA 10 min | 1 × 2 left temporal lobe (T7–T8 and FPz) | semantically related word pairs, cued recall | = cued recall | |
| Brunye, 2018 [40] | n = 50 (25) 22.5 years | parallel 2-group online (encoding) | tDCS 1.5 mA 20 min | 2 × 3 PFC (FP1, FP2–AF3, F4, P8) | face-picture task, recognition | = recognition |
| Moseley, 2018 [41] | n = 36 20.14 ± 2.5 years | cross-over, online (encoding) | tDCS 1.5 mA 15 min | 1 × 1 right amPFC—left STG; right amPFC—left V5 | source memory task: recognition | = recognition |
| n = 36 22.69 ± 5.7 years | cross-over, online (retrieval) | tDCS 1.5 mA 15 min | 1 × 1 right amPFC—left STG; right amPFC—left V5 | source memory task: recognition | = recognition | |
| Marián, 2018 [42] | n = 66 (33) 23.2 ± 2.5 years | parallel 2-group offline (consolidation) | tDCS 2 mA 15 min | 1 × 1 right dlPFC (F4–Cz) | word pairs task: cued recall | ↓ cued recall |
| n = 52 (26) 23.2 ± 2.5 years | parallel 2-group offline (consolidation) | tDCS 2 mA 15 min | 1 × 1 right dlPFC (F4–Cz) | word pairs task: cued recall | = cued recall | |
| Mizrak, 2018 [43] | n = 21 / | cross-over offline (consolidation) | otDCS (5.5 Hz) 0.5–1 mA 20 min | 1 × 1 left dlPFC (F3—supraorbital area) | source memory task: cued recall | ↓ cued recall |
| Bjekić, 2019 [44] | n = 37 25.34 ± 3.59 years | cross-over offline (before encoding) | tDCS 1.5 mA 20 min | 1 × 1 left PPC (P3–cheek) | face word task: cued recall | ↑ cued recall |
| Westphal, 2019 [45] | n = 72 (24) 20 years | parallel -3 group online (retrieval) | tDCS anodal/cathodal 1.5 mA/−1.5 mA 30 min | 1 × 1 left rtPFC (midpoint between FP1 and F7–C4) | source memory task: recognition | anodal tDCS: ↑ recognition cathodal tDCS: = recognition |
| Leclerc, 2019 [46] | n = 48 (23/25) 24.77 ± 5.33 years | parallel 2-group offline (before encoding) | tDCS 2 mA 20 min | 1 × 1 premotor cortex (Fz–deltoid) | source memory task: cued recall | = cued recall |
| Bjekić, 2019 [47] | n = 20 26.4 ± 3.71 years | cross-over offline (before encoding) | tDCS 1.5 mA 20 min | 1 × 1 left PPC (P3 -cheek) | face word task: cued recall | ↑ cued recall |
| n = 21 24.15 ± 2.74 years | cross-over offline (before encoding) | tDCS 1.5 mA 20 min | 1 × 1 right PPC (P4-cheek) | object location task: cued recall | ↑ cued recall | |
| Lang, 2019 [48] | n = 59 (19/21/19) 18–45 years | parallel 3-group online (encoding) | HD-tACS (6 Hz)/HD-tDCS 2 mA 10 min | 1 × 4 dlPFC (P10–FP1, P2, P3, PO7) | face scene task: recognition | hd tACS: ↑ recognition hd tDCS: = recognition |
| Owusu, 2020 [49] | n = 20 21–34 years | cross-over online (encoding) | tDCS 1.5 mA 20 min | 1 × 1 left posterior temporoparietal junction (CP5–FP2) | polysemous words and meaning task: cued recall and recognition | ↑ recognition ↑ cued recall |
| Ergo, 2020 [50] | n = 76 (38) 20.8 ± 2.4 years | parallel 2-group online (encoding) | tACS (6 Hz) 2 mA 16.6 min | 1 × 1 dlPFC (FCz-neck) | word pairs task: recognition, memory confidence | = recognition ↑ confidence in correct recognition |
| Gilson, 2021 [51] | n = 69 (16/16/17/20) 22.4 years | parallel 4-group offline (consolidation) | tDCS anodal/cathodal with or without cognitive training 1 mA 20 min | 1 × 1 dlPFC (F3–F4) | affective word pairs task: cued recall | anodal + training: ↑ cued-recall cathodal + training: ↑ cued-recall anodal: = cued recall cathodal: = cued recall |
| Vulić, 2021 [52] | n = 36; 18 follow up 23.8 ± 1.8 years | cross-over offline (before task) | tDCS, otDCS (5 Hz) 1.5 mA 20 min | 1 × 1 left PPC (P3-cheek) | face word pairs task, cued recall | tDCS: ↑ cued recall otDCS: ↑ cued recall |
| Bolling, 2021 [53] | n = 56 (34/41/39) 18-25 years | parallel 3-group online (encoding) | tDCS 1.5 mA vs. 1 mA 20 min | 1 × 1 dlPFC (F3–F4) | word pairs task, cued recall | ↑ cued recall (1.5 mA) = cued recall (1 mA) |
| Huang, 2021 [54] | n = 84 (aDMN:13/14/13 pDMN:16/11/17) 19 ± 1.2 years | parallel 6-group online (retrieval) | HD-tDCS 1 mA 10 min | 1 × 3 aDMN (FPz–Fz, FP1, FP2) pDMN (Pz–Oz, PO7, PO8) | word pairs task: cued recall | ↑ recall in HD-tDCS anodal stimulation on pDMN ↑ recall in HD-tDCS cathodal stimulation on aDMN |
| Meng, 2021 [55] | n = 20 21.7 ± 8.2 years | cross-over online (encoding) | HD-tACS (6 Hz) 2 mA 15 min | ring electrode left PPC (P3) | face scene task: recognition | ↓ recognition |
| Fernández, 2021 [56] | n = 30 (15) 21.3 years | parallel 2-group online (encoding) | tDCS 2 mA 18 min | 1 × 1 dlPFC (F3–FP2) | word pairs task: recognition | = recognition ↑ RT for correct 24 h later |
| Pyke, 2021 [57] | n = 25 19.2 ± 0.8 years | cross-over online (encoding) | tDCS 1.5 mA 15 min | 1 × 1 dlPFC (F3-wrist) | word picture pairs task: cued recall | ↑ cued recall |
| Luckey, 2022 [58] | n = 84 (25 + 25 + 24 + 11) 21.6 ± 2.1 years | parallel 4-group online (encoding) | tDCS tACS (40 Hz) tACS (1 Hz) 1.5 mA 8 min | 1 × 1 occipital nerve (occipital lobe) | word pairs task: cued recall | tDCS: = cued recall (immediate) ↑ cued recall (delayed) tACS (40 Hz): ↑ cued recall (immediate) ↑ cued recall (delayed) tACS (1 Hz): = cued recall (immediate & delayed) |
| Živanović, 2022 [59] | n = 40 25.2 ± 3.7 years | cross-over online (encoding) | tDCS 1.5 mA 20 min tACS (ITF) ±1 mA 20 min otDCS (ITF) 1 mA–2 mA 20 min | 1 × 1 left PPC (P3-cheek) | short-term AM task number-color pairs, cued recall | tDCS: ↑ cued recall tACS: ↑ cued recall otDCS: ↑ cued recall |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjekić, J.; Manojlović, M.; Filipović, S.R. Transcranial Electrical Stimulation for Associative Memory Enhancement: State-of-the-Art from Basic to Clinical Research. Life 2023, 13, 1125. https://doi.org/10.3390/life13051125
Bjekić J, Manojlović M, Filipović SR. Transcranial Electrical Stimulation for Associative Memory Enhancement: State-of-the-Art from Basic to Clinical Research. Life. 2023; 13(5):1125. https://doi.org/10.3390/life13051125
Chicago/Turabian StyleBjekić, Jovana, Milica Manojlović, and Saša R. Filipović. 2023. "Transcranial Electrical Stimulation for Associative Memory Enhancement: State-of-the-Art from Basic to Clinical Research" Life 13, no. 5: 1125. https://doi.org/10.3390/life13051125
APA StyleBjekić, J., Manojlović, M., & Filipović, S. R. (2023). Transcranial Electrical Stimulation for Associative Memory Enhancement: State-of-the-Art from Basic to Clinical Research. Life, 13(5), 1125. https://doi.org/10.3390/life13051125







