Classical Borrelia Serology Does Not Aid in the Diagnosis of Persistent Symptoms Attributed to Lyme Borreliosis: A Retrospective Cohort Study
Abstract
:1. Introduction
1.1. Two-Tier Approach in Serological Diagnostics
1.2. Serology in Early Lyme Borreliosis
1.3. Serology in Late Clinical Lyme Borreliosis (ACA, Arthritis, Myocarditis, and Neuroborreliosis)
1.4. Serology in Patients with Persistent Symptoms Attributed to Lyme Borreliosis (PSL)
2. Materials and Methods
2.1. Population
2.2. Serological Testing
2.3. Antibiotic Treatment
2.4. Statistical Analysis
3. Results
3.1. Subjects
3.2. Borrelia spp. Serological Testing—Manufacturer Performance in PSL Patient Group Compared to HC
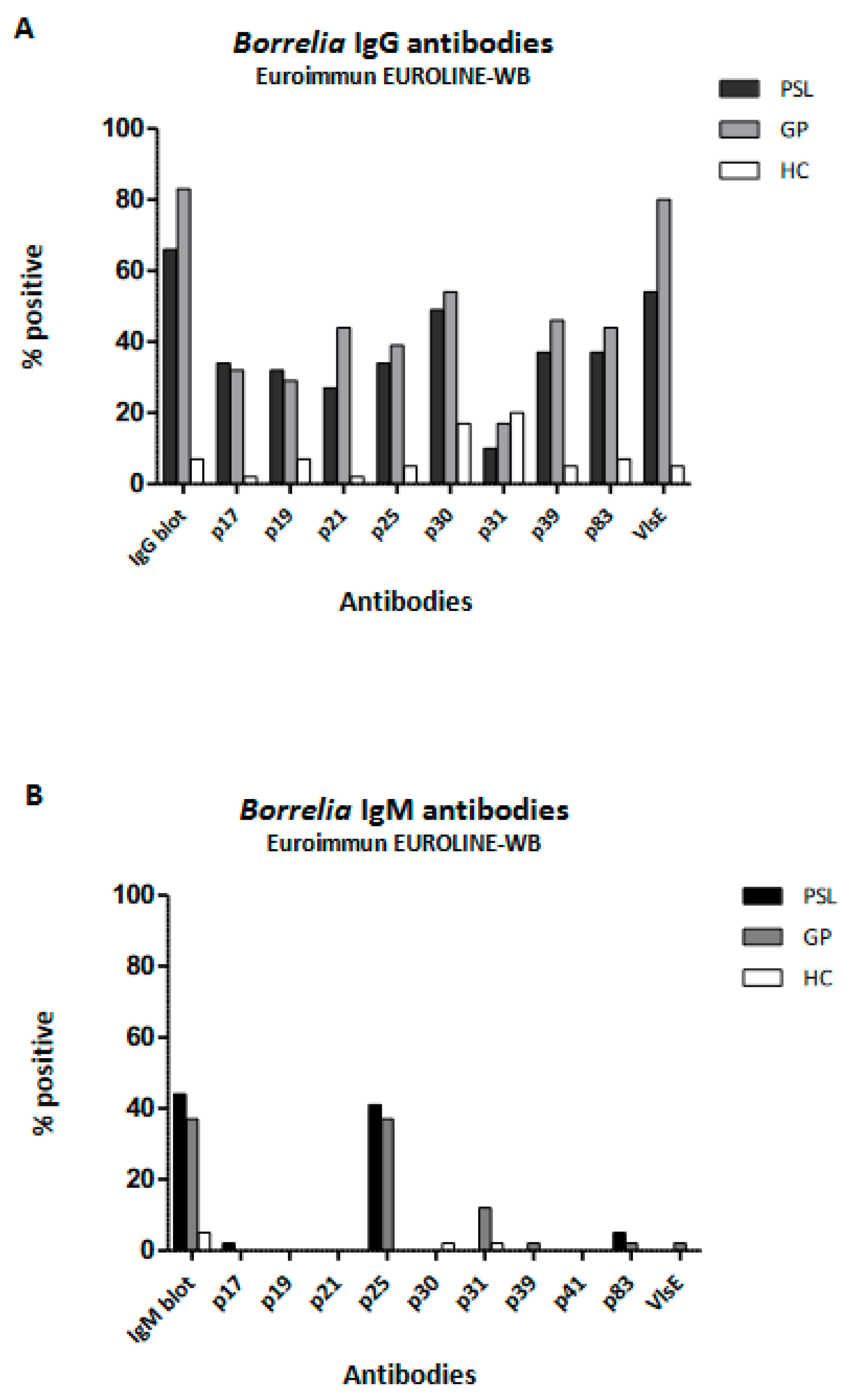
3.3. Comparison of Borrelia Serology among Patients with Persistent Symptoms Attributed to Lyme Borreliosis (PSL), Healthy Controls (HC), and Patients in Primary Care (GP)
3.4. Comparison of Borrelia Serology between Patients with Persistent Symptoms Attributed to Lyme Borreliosis (PSL) and Acute Borreliosis (EM)
3.5. Pre-Treatment Borrelia Serology in Patients with Persistent Symptoms Attributed to Lyme Borreliosis (PSL); Comparison between Patients with and without Response to Antibiotic Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- RIVM. Lyme Disease Cases Have Quadruppled. Available online: https://www.rivm.nl/en/news/lyme-disease-cases-have-quadrupled (accessed on 16 April 2018).
- Leeflang, M.M.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Van Bortel, W.; Brandenburg, A.H.; Van Burgel, N.D.; Van Dam, A.P.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- van den Wijngaard, C.C.; Hofhuis, A.; Harms, M.G.; Haagsma, J.A.; Wong, A.; de Wit, G.A.; Havelaar, A.H.; Lugner, A.K.; Suijkerbuijk, A.W.; van Pelt, W. The burden of Lyme borreliosis expressed in disability-adjusted life years. Eur. J. Public Health 2015, 25, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Cerar, T.; Strle, F.; Stupica, D.; Ruzic-Sabljic, E.; McHugh, G.; Steere, A.C.; Strle, K. Differences in Genotype, Clinical Features, and Inflammatory Potential of Borrelia burgdorferi sensu stricto Strains from Europe and the United States. Emerg. Infect Dis. 2016, 22, 818–827. [Google Scholar] [CrossRef]
- Saint Girons, I.; Gern, L.; Gray, J.S.; Guy, E.C.; Korenberg, E.; Nuttall, P.A.; Rijpkema, S.G.; Schonberg, A.; Stanek, G.; Postic, D. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentralbl. Bakteriol. 1998, 287, 190–195. [Google Scholar] [CrossRef]
- Feder, H.M., Jr.; Johnson, B.J.; O’Connell, S.; Shapiro, E.D.; Steere, A.C.; Wormser, G.P.; Ad Hoc International Lyme Disease, G.; Agger, W.A.; Artsob, H.; Auwaerter, P.; et al. A critical appraisal of “chronic Lyme disease”. N. Engl. J. Med. 2007, 357, 1422–1430. [Google Scholar] [CrossRef]
- Koedel, U.; Pfister, H.W. Lyme neuroborreliosis. Curr. Opin. Infect Dis. 2017, 30, 101–107. [Google Scholar] [CrossRef]
- Shapiro, E.D. Lyme disease. N. Engl. J. Med. 2014, 371, 684. [Google Scholar] [CrossRef]
- Stanek, G.; Fingerle, V.; Hunfeld, K.P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. Off. Public Eur. Soc. Clin. Microbiol. Infect. Dis. 2011, 17, 69–79. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Cardenas-de la Garza, J.A.; De la Cruz-Valadez, E.; Ocampo-Candiani, J.; Welsh, O. Clinical spectrum of Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. Off. Public Eur. Soc. Clin. Microbiol. 2019, 38, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ursinus, J.; Vrijmoeth, H.D.; Harms, M.G.; Tulen, A.D.; Knoop, H.; Gauw, S.A.; Zomer, T.P.; Wong, A.; Friesema, I.H.M.; Vermeeren, Y.M.; et al. Prevalence of persistent symptoms after treatment for lyme borreliosis: A prospective observational cohort study. Lancet Reg. Health Eur. 2021, 6, 100142. [Google Scholar] [CrossRef] [PubMed]
- Cairns, V.; Godwin, J. Post-Lyme borreliosis syndrome: A meta-analysis of reported symptoms. Int. J. Epidemiol. 2005, 34, 1340–1345. [Google Scholar] [CrossRef]
- Ljostad, U.; Mygland, A. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. Eur. J. Neurol. 2010, 17, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C. Posttreatment Lyme disease syndromes: Distinct pathogenesis caused by maladaptive host responses. J. Clin. Investig. 2020, 130, 2148–2151. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Public Infect. Dis. Soc. Am. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Leth, T.A.; Dessau, R.B.; Moller, J.K. Discriminating between Lyme neuroborreliosis and other central nervous system infections by use of biomarkers CXCL13 and IL-6. Ticks Tick-Borne Dis. 2022, 13, 101984. [Google Scholar] [CrossRef]
- Waddell, L.A.; Greig, J.; Mascarenhas, M.; Harding, S.; Lindsay, R.; Ogden, N. The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS ONE 2016, 11, e0168613. [Google Scholar] [CrossRef]
- Pegalajar-Jurado, A.; Schriefer, M.E.; Welch, R.J.; Couturier, M.R.; MacKenzie, T.; Clark, R.J.; Ashton, L.V.; Delorey, M.J.; Molins, C.R. Evaluation of Modified Two-Tiered Testing Algorithms for Lyme Disease Laboratory Diagnosis Using Well-Characterized Serum Samples. J. Clin. Microbiol. 2018, 56, e01943-17. [Google Scholar] [CrossRef]
- Group, L.D.D.W. Modified two-tiered testing algorithm for Lyme disease serology: The Canadian context. Can. Commun. Dis. Rep. 2020, 46, 125–131. [Google Scholar] [CrossRef]
- Baarsma, M.E.; Schellekens, J.; Meijer, B.C.; Brandenburg, A.H.; Souilljee, T.; Hofhuis, A.; Hovius, J.W.; van Dam, A.P. Diagnostic parameters of modified two-tier testing in European patients with early Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. Off. Public Eur. Soc. Clin. Microbiol. 2020, 39, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Dessau, R.B.; Bangsborg, J.M.; Ejlertsen, T.; Skarphedinsson, S.; Schonheyder, H.C. Utilization of serology for the diagnosis of suspected Lyme borreliosis in Denmark: Survey of patients seen in general practice. BMC Infect. Dis. 2010, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Tugwell, P.; Dennis, D.T.; Weinstein, A.; Wells, G.; Shea, B.; Nichol, G.; Hayward, R.; Lightfoot, R.; Baker, P.; Steere, A.C. Laboratory evaluation in the diagnosis of Lyme disease. Ann. Intern. Med. 1997, 127, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.W.; Brandenburg, A.H.; van Burgel, N.D.; Bijlmer, H.A.; Herremans, T.; Stelma, F.; Lunel, F.V.; van Dam, A.P.; Dutch Working Group on Diagnosis of Lyme, B. A Dutch nationwide evaluation of serological assays for detection of Borrelia antibodies in clinically well-defined patients. Diagn. Microbiol. Infect Dis. 2015, 83, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.M.; Miller, R.R.; Gardy, J.L.; Parker, S.M.; Morshed, M.G.; Steiner, T.S.; Singer, J.; Shojania, K.; Tang, P.; Complex Chronic Disease Study, G. Lyme Disease Diagnosed by Alternative Methods: A Phenotype Similar to That of Chronic Fatigue Syndrome. Clin. Infect. Dis. Off. Public Infect. Dis. Soc. Am. 2015, 61, 1084–1091. [Google Scholar] [CrossRef]
- van de Schoor, F.R.; Baarsma, M.E.; Gauw, S.A.; Joosten, L.A.B.; Kullberg, B.J.; van den Wijngaard, C.C.; Hovius, J.W. Validation of cellular tests for Lyme borreliosis (VICTORY) study. BMC Infect. Dis. 2019, 19, 732. [Google Scholar] [CrossRef]
- Branda, J.A.; Steere, A.C. Laboratory Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 2021, 34, e00018-19. [Google Scholar] [CrossRef]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective study of serologic tests for lyme disease. Clin. Infect. Dis. Off. Public Infect. Dis. Soc. Am. 2008, 47, 188–195. [Google Scholar] [CrossRef]
- Branda, J.A.; Aguero-Rosenfeld, M.E.; Ferraro, M.J.; Johnson, B.J.; Wormser, G.P.; Steere, A.C. 2-tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin. Infect. Dis. Off. Public Infect. Dis. Soc. Am. 2010, 50, 20–26. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Nowakowski, J.; Bittker, S.; Cooper, D.; Nadelman, R.B.; Wormser, G.P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 1996, 34, 1–9. [Google Scholar] [CrossRef]
- Rebman, A.W.; Crowder, L.A.; Kirkpatrick, A.; Aucott, J.N. Characteristics of seroconversion and implications for diagnosis of post-treatment Lyme disease syndrome: Acute and convalescent serology among a prospective cohort of early Lyme disease patients. Clin. Rheumatol. 2015, 34, 585–589. [Google Scholar] [CrossRef] [PubMed]
- CBO. Richtlijn Lymeziekte. Dutch CBO Guidelines. 2013. Available online: https://www.rivm.nl/documenten/cbo-richtlijn-lymeziekte-juli-2013 (accessed on 1 July 2013).
- Hammers-Berggren, S.; Lebech, A.M.; Karlsson, M.; Svenungsson, B.; Hansen, K.; Stiernstedt, G. Serological follow-up after treatment of patients with erythema migrans and neuroborreliosis. J. Clin. Microbiol. 1994, 32, 1519–1525. [Google Scholar] [CrossRef]
- Halperin, J.J. Lyme neuroborreliosis. Curr. Opin. Infect Dis. 2019, 32, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Seriburi, V.; Ndukwe, N.; Chang, Z.; Cox, M.E.; Wormser, G.P. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin. Microbiol. Infect. 2012, 18, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Berende, A.; ter Hofstede, H.J.; Donders, A.R.; van Middendorp, H.; Kessels, R.P.; Adang, E.M.; Vos, F.J.; Evers, A.W.; Kullberg, B.J. Persistent Lyme Empiric Antibiotic Study Europe (PLEASE)—Design of a randomized controlled trial of prolonged antibiotic treatment in patients with persistent symptoms attributed to Lyme borreliosis. BMC Infect. Dis. 2014, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Berende, A.; ter Hofstede, H.J.; Vos, F.J.; van Middendorp, H.; Vogelaar, M.L.; Tromp, M.; van den Hoogen, F.H.; Donders, A.R.; Evers, A.W.; Kullberg, B.J. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N. Engl. J. Med. 2016, 374, 1209–1220. [Google Scholar] [CrossRef]
- Oosting, M.; Kerstholt, M.; Ter Horst, R.; Li, Y.; Deelen, P.; Smeekens, S.; Jaeger, M.; Lachmandas, E.; Vrijmoeth, H.; Lupse, M.; et al. Functional and Genomic Architecture of Borrelia burgdorferi-Induced Cytokine Responses in Humans. Cell Host Microbe 2016, 20, 822–833. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Ang, C.W.; Notermans, D.W.; Hommes, M.; Simoons-Smit, A.M.; Herremans, T. Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots. Eur. J. Clin. Microbiol. Infect. Dis. Off. Public Eur. Soc. Clin. Microbiol. 2011, 30, 1027–1032. [Google Scholar] [CrossRef]
- Botman, E.; Ang, C.W.; Joosten, J.H.K.; Slottje, P.; van der Wouden, J.C.; Maarsingh, O.R. Diagnostic behaviour of general practitioners when suspecting Lyme disease: A database study from 2010–2015. BMC Fam. Pract. 2018, 19, 43. [Google Scholar] [CrossRef]
- Bruckbauer, H.R.; Preac-Mursic, V.; Fuchs, R.; Wilske, B. Cross-reactive proteins of Borrelia burgdorferi. Eur. J. Clin. Microbiol. Infect. Dis. Off. Public Eur. Soc. Clin. Microbiol. 1992, 11, 224–232. [Google Scholar] [CrossRef]
- Ulvestad, E.; Kanestrom, A.; Sonsteby, L.J.; Jureen, R.; Omland, T.; Edvardsen, B.; Lundervik, J.; Kristoffersen, E.; van Dam, A.P. Diagnostic and biological significance of anti-p41 IgM antibodies against Borrelia burgdorferi. Scand. J. Immunol. 2001, 53, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Crother, T.R.; Champion, C.I.; Whitelegge, J.P.; Aguilera, R.; Wu, X.Y.; Blanco, D.R.; Miller, J.N.; Lovett, M.A. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 2004, 72, 5063–5072. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Wormser, G.P.; Marques, A.R.; Latov, N.; Alaedini, A. Anti-Borrelia burgdorferi antibody profile in post-Lyme disease syndrome. Clin. Vaccine Immunol. CVI 2011, 18, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Lawrenz, M.B.; Hardham, J.M.; Owens, R.T.; Nowakowski, J.; Steere, A.C.; Wormser, G.P.; Norris, S.J. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 1999, 37, 3997–4004. [Google Scholar] [CrossRef]
- Glatz, M.; Golestani, M.; Kerl, H.; Mullegger, R.R. Clinical relevance of different IgG and IgM serum antibody responses to Borrelia burgdorferi after antibiotic therapy for erythema migrans: Long-term follow-up study of 113 patients. Arch. Dermatol. 2006, 142, 862–868. [Google Scholar] [CrossRef]
- Lovrich, S.D.; Jobe, D.A.; Schell, R.F.; Callister, S.M. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human lyme disease and do not occur in mice or hamsters. Clin. Diagn. Lab Immunol. 2005, 12, 746–751. [Google Scholar] [CrossRef]
- Markowicz, M.; Reiter, M.; Gamper, J.; Stanek, G.; Stockinger, H. Persistent Anti-Borrelia IgM Antibodies without Lyme Borreliosis in the Clinical and Immunological Context. Microbiol. Spectr. 2021, 9, e0102021. [Google Scholar] [CrossRef]
- Joyner, G.; Mavin, S.; Milner, R.; Lim, C. Introduction of IgM testing for the diagnosis of acute Lyme borreliosis: A study of the benefits, limitations and costs. Eur. J. Clin. Microbiol. Infect. Dis. Off. Public Eur. Soc. Clin. Microbiol. 2022, 41, 671–675. [Google Scholar] [CrossRef]
- Markowicz, M.; Kivaranovic, D.; Stanek, G. Testing patients with non-specific symptoms for antibodies against Borrelia burgdorferi sensu lato does not provide useful clinical information about their aetiology. Clin. Microbiol. Infect. Off. Public Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21, 1098–1103. [Google Scholar] [CrossRef]
- Kalish, R.A.; McHugh, G.; Granquist, J.; Shea, B.; Ruthazer, R.; Steere, A.C. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin. Infect. Dis. Off. Public Infect. Dis. Soc. Am. 2001, 33, 780–785. [Google Scholar] [CrossRef] [PubMed]


| Group | PSL (N = 40) | HC (N = 40) | GP (N = 41) | EM (N = 41) | |
|---|---|---|---|---|---|
| Group definition | Persistent symptoms attributed to Lyme disease | Controls | Primary care | Acute Lyme (erythema migrans) | |
| Borrelia assays | Serion ELISA ↓ Euroimmun EUROLINE–WB Euroimmun EUROLINE–RN–AT Mikrogen RecomLine * Mikrogen RecomBead * Serion Multianalyte system $ | Serion ELISA ↓ Euroimmun EUROLINE–WB Euroimmun EUROLINE–RN–AT Mikrogen RecomLine Mikrogen RecomBead Serion Multianalyte system | Serion ELISA ↓ Euroimmun EUROLINE–WB | Euroimmun ELISA/BioMérieuxVidas Lyme ELFA ↓ Euroimmun EUROLINE–RN–AT | |
| p-value | |||||
| Female gender | 20 (49%) | 26 (65%) | 23 (56%) | 25 (61%) | 0.62 # |
| Average age (years) | 48 | 48 | 61 | 48 | <0.001 ** |
| Country | Netherlands | Netherlands | Netherlands | Romania | |
| PSL N = 40 | HC N = 40 | p-Value | ||
|---|---|---|---|---|
| VlsE | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 22 | 4 | <0.001 |
| IgM | 0 | 0 | ||
| Positive Euroimmun EUROLINE–RN–AT | IgG | 22 | 3 | <0.001 |
| IgM | 0 | 0 | ||
| Positive Mikrogen RecomLine | IgG | 26 | 4 | <0.001 |
| IgM | 0 | 2 | 0.358 | |
| Positive Mikrogen RecomBead | IgG | 26 | 3 | <0.001 |
| IgM | 1 | 1 | 0.603 | |
| Positive Serion Multianalyte system | IgG | 24 | 4 | <0.001 |
| IgM | 2 | 1 | 0.556 | |
| p17/p18/DbpA | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 14 | 1 | 0.002 |
| IgM | 1 | 0 | 0.484 | |
| Positive Euroimmun EUROLINE–RN–AT | IgG | 4 | 0 | 0.148 |
| Positive Mikrogen RecomLine (B. afzelii) | IgG | 13 | 2 | 0.007 |
| IgM | 0 | 0 | ||
| Positive Mikrogen RecomBead (B. afzelii) | IgG | 12 | 2 | 0.007 |
| IgM | 0 | 0 | ||
| Positive Serion Multianalyte system | IgG | 21 | 3 | <0.001 |
| IgM | 0 | 0 | ||
| p19 | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 13 | 3 | 0.027 |
| IgM | 0 | 0 | ||
| Positive Euroimmun EUROLINE–RN–AT | IgG | 1 | 0 | 0.484 |
| p20 | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 11 | 1 | 0.01 |
| IgM | 0 | 0 | ||
| Positive Euroimmun EUROLINE–RN–AT | IgG | 1 | 0 | 0.390 |
| P25/OspC | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 14 | 2 | <0.001 |
| IgM | 17 | 0 | <0.001 | |
| IgM | 18 | 3 | <0.001 | |
| Positive Mikrogen RecomLine (IgG positive and equivocal) | IgG | 1 | 1 | |
| IgM | 14 | 0 | <0.001 | |
| Positive Mikrogen RecomBead | IgG | 1 | 1 | |
| IgM | 11 | 0 | <0.001 | |
| Positive Serion Multianalyte system | IgG | 5 | 3 | 0.156 |
| IgM | 13 | 9 | <0.001 | |
| p30 | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 20 | 6 | 0.002 |
| IgM | 0 | 1 | 0.360 | |
| p31/OspA | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 4 | 7 | 0.541 |
| IgM | 0 | 1 | 0.06 | |
| Positive Mikrogen RecomLine | IgG | 0 | 1 | 0.314 |
| IgM | 0 | 0 | ||
| Positive Mikrogen RecomBead | IgG | 0 | 0 | |
| IgM | 0 | 0 | ||
| p39 | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 15 | 2 | <0.001 |
| IgM | 0 | 0 | ||
| Positive Euroimmun EUROLINE–RN–AT | IgG | 12 | 1 | 0.003 |
| IgM | 0 | 0 | ||
| Positive Mikrogen RecomLine | IgG | 11 | 2 | 0.006 |
| IgM | 2 | 1 | 0.717 | |
| Positive Mikrogen RecomBead | IgG | 1 | 1 | 0.603 |
| IgM | 0 | 0 | ||
| p41 | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 0 | 0 | |
| IgM | 0 | 0 | ||
| Positive Euroimmun EUROLINE–RN–AT | IgG | 39 | 37 | 0.256 |
| IgM | 19 | 10 | 0.041 | |
| Positive Mikrogen RecomLine | IgG | 33 | 27 | 0.273 |
| IgM | 29 | 22 | 0.121 | |
| Positive Serion Multianalyte system | IgG | 2 | 1 | 0.309 |
| IgM | 0 | 0 | ||
| p58 | ||||
| Positive Euroimmun EUROLINE–RN–AT | IgG | 4 | 1 | 0.123 |
| Positive Mikrogen RecomLine | IgG | 14 | 2 | <0.001 |
| IgM | 0 | 1 | 0.314 | |
| Positive Mikrogen RecomBead | IgG | 15 | 2 | <0.001 |
| IgM | 0 | 0 | ||
| Positive Serion Multianalyte system | IgG | 2 | 1 | 0.556 |
| IgM | 0 | 0 | ||
| p83/p100 | ||||
| Positive Euroimmun EUROLINE–WB | IgG | 15 | 3 | 0.001 |
| IgM | 2 | 0 | 0.384 | |
| Positive Euroimmun EUROLINE–RN–AT | IgG | 14 | 3 | 0.014 |
| Positive Mikrogen RecomLine | IgG | 16 | 2 | <0.001 |
| IgM | 2 | 3 | 0.697 | |
| Positive Mikrogen RecomBead | IgM | 2 | 0 | 0.152 |
| Positive Serion Multianalyte system | IgG | 5 | 4 | 0.801 |
| IgM | 0 | 0 | ||
| Others | ||||
| Positive Euroimmun EUROLINE–RN–AT Lipid | IgG | 1 | 1 | 0.708 |
| Positive Serion Multianalyte system DbpAPBr | IgG | 8 | 2 | 0.103 |
| IgM | 0 | 0 | ||
| Positive Serion Multianalyte system Lysate | IgG | 9 | 2 | 0.074 |
| IgM | 12 | 3 | 0.026 |
| PSL N = 40 (%) | HC N = 40 (%) | p-Value | ||
|---|---|---|---|---|
| Positive Serion ELISA (%) | IgG | 23 (58) | 2 (5) | <0.05 |
| IgM | 16 (40) | 2 (5) | <0.05 | |
| Positive Euroimmun EUROLINE–WB | IgG | 27 (68) | 3 (8) | <0.05 |
| IgM | 18 (45) | 2 (5) | <0.05 | |
| Positive Euroimmun EUROLINE–RN–AT | IgG | 25 (63) | 5 (13) | <0.05 |
| IgM | 18 (45) | 4 (10) | <0.05 | |
| Positive Mikrogen RecomLine | IgG | 20 (50) | 3 (8) | <0.05 |
| IgM | 14 (35) | 1 (3) | <0.05 | |
| Positive Mikrogen RecomBead | IgG | 19 (48) | 2 (5) | <0.05 |
| IgM | 11 (28) | 0 (0) | <0.05 | |
| Positive Serion Multianalyte system | IgG | 24 (60) | 4 (10) | <0.05 |
| IgM | 14 (35) | 2 (5) | <0.05 |
| IgG | Euroimmun EUROLINE–WB | Euroimmun EUROLINE–RN–AT | Mikrogen RecomLine | Mikrogen RecomBead | Serion MultianalyteTM System | Serion ELISA |
|---|---|---|---|---|---|---|
| Euroimmun, EUROLINE–WB | ||||||
| Euroimmun, EUROLINE–RN–AT | 0.680 | |||||
| Mikrogen, RecomLine | 0.692 | 0.636 | ||||
| Mikrogen, RecomBead | 0.688 | 0.631 | 0.875 | |||
| Serion MultianalyteTM System | 0.676 | 0.622 | 0.685 | 0.679 | ||
| Serion, ELISA | 0.807 | 0.641 | 0.822 | 0.817 | 0.803 | |
| IgM | Euroimmun EUROLINE–WB | Euroimmun EUROLINE–RN–AT | Mikrogen RecomLine | Mikrogen RecomBead | Serion MultianalyteTM System | Serion ELISA Borrelia |
| Euroimmun, EUROLINE–WB | ||||||
| Euroimmun, EUROLINE–RN–AT | 0.613 | |||||
| Mikrogen, RecomLine | 0.600 | 0.409 | ||||
| Mikrogen, RecomBead | 0.647 | 0.592 | 0.451 | |||
| Serion, MultianalyteTM System | 0.643 | 0.589 | 0.400 | 0.690 | ||
| Serion, ELISA | 0.586 | 0.535 | 0.429 | 0.543 | 0.552 |
| PSL n = 40 (%) | HC n = 40 (%) | GP n = 41 (%) | p-Value | ||
|---|---|---|---|---|---|
| Positive Serion screening ELISA | IgG | 23 (58) | 2 (5) | 32 (78) | <0.001 * 0.098 # |
| IgM | 16 (39) | 2 (5) | 16 (39) | <0.001 * 0.589 # | |
| Positive Euroimmun EUROLINE–WB | IgG | 27 (68) | 3 (8) | 34 (83) | <0.001 * 0.176 # |
| IgM | 18 (45) | 2 (5) | 15 (37) | <0.001 * 0.326 # | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelma, F.F.; Berende, A.; Ter Hofstede, H.; Vrijmoeth, H.D.; Vos, F.; Kullberg, B.-J. Classical Borrelia Serology Does Not Aid in the Diagnosis of Persistent Symptoms Attributed to Lyme Borreliosis: A Retrospective Cohort Study. Life 2023, 13, 1134. https://doi.org/10.3390/life13051134
Stelma FF, Berende A, Ter Hofstede H, Vrijmoeth HD, Vos F, Kullberg B-J. Classical Borrelia Serology Does Not Aid in the Diagnosis of Persistent Symptoms Attributed to Lyme Borreliosis: A Retrospective Cohort Study. Life. 2023; 13(5):1134. https://doi.org/10.3390/life13051134
Chicago/Turabian StyleStelma, Foekje F., Anneleen Berende, Hadewych Ter Hofstede, Hedwig D. Vrijmoeth, Fidel Vos, and Bart-Jan Kullberg. 2023. "Classical Borrelia Serology Does Not Aid in the Diagnosis of Persistent Symptoms Attributed to Lyme Borreliosis: A Retrospective Cohort Study" Life 13, no. 5: 1134. https://doi.org/10.3390/life13051134
APA StyleStelma, F. F., Berende, A., Ter Hofstede, H., Vrijmoeth, H. D., Vos, F., & Kullberg, B.-J. (2023). Classical Borrelia Serology Does Not Aid in the Diagnosis of Persistent Symptoms Attributed to Lyme Borreliosis: A Retrospective Cohort Study. Life, 13(5), 1134. https://doi.org/10.3390/life13051134






