Effectiveness of Epley–Canalith Repositioning Procedure versus Vestibular Rehabilitation Therapy in Diabetic Patients with Posterior Benign Paroxysmal Positional Vertigo: A Randomized Trial
Abstract
:1. Introduction
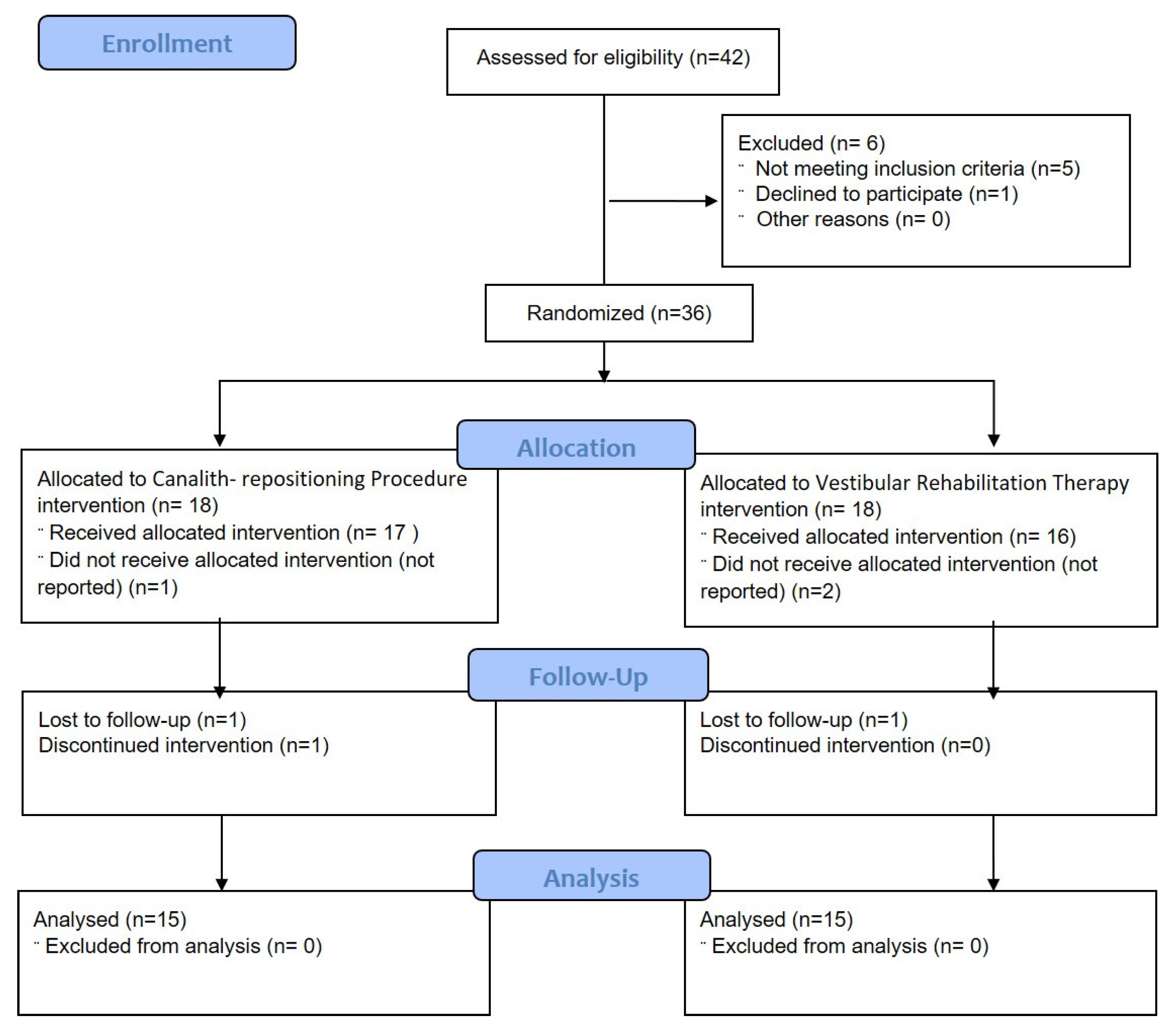
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Subject
2.3. Procedure
2.4. Interventions
2.4.1. Epley–Canalith Repositioning Procedure (Group A)
- Step 1: The patient was brought down with the head tilted 45 degrees towards the affected canal, as in Hallpike test. The neck was extended;
- Step 2: The head was rotated 90 degrees towards the unaffected side. The neck was extended;
- Step 3: The head and body were rotated further by 90 degrees from the previous position (now facedown). The neck was in a neutral position;
- Step 4: The patient was brought into a sitting position while having their head turned constantly in the direction of the unaffected side’
- Step 5: The head was turned forward and the chin was kept down by 20° for a minute.
2.4.2. Vestibular Rehabilitation Therapy (Group B)
2.5. Outcome Measurements
2.5.1. Vertigo Symptom Scale–Short Form
2.5.2. Berg Balance Scale
3. Results
3.1. Statistical Analysis
3.2. Demographic Characteristics
3.3. Outcome Measurements
3.4. Net Improvement
4. Discussion
5. Limitations of Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Kim, S.H.; Kim, H.J. Benign paroxysmal positional vertigo. J. Clin. Neurol. 2010, 6, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population-based study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Yoo, D.M.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Lee, J.S.; Choi, H.G. Association between benign paroxysmal positional vertigo and diabetes mellitus: A 1-year case-control study. J. Neurol. Sci. 2017, 378, 97–100. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- D’Silva, L.J.; Kluding, P.M.; Whitney, S.L.; Dai, H.; Santos, M. Postural sway in individuals with type 2 diabetes and concurrent benign paroxysmal positional vertigo. Int. J. Neurosci. 2017, 127, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical practice guideline: Benign paroxysmal positional vertigo. Otolaryngol. Head Neck Surg. 2008, 139 (Suppl. S4), S47–S81. [Google Scholar] [CrossRef] [PubMed]
- Han, B.I.; Song, H.S.; Kim, J.S. Vestibular rehabilitation therapy: Review of indications, mechanisms, and key exercises. J. Clin. Neurol. 2011, 7, 184–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, Y.; Carey, J.P.; Della Santina, C.C.; Schubert, M.C.; Minor, L.B. Diabetes, vestibular dysfunction, and falls: Analyses from the National Health and Nutrition Examination Survey. Otol. Neurotol. 2010, 31, 1445–1450. [Google Scholar] [CrossRef]
- Epley, J.M. The Canalith Repositioning Procedure: For treatment of Benign Paroxysmal Positional Vertigo. Otolaryngol. Head Neck Surg. 1992, 107, 399–404. [Google Scholar] [CrossRef]
- Verma, A. Paticle dislodgemengt procedure: A prospective study of 100 cases consecutive cases of posterior canal Benign Paroxysmal Positional Vertigo. Ann. Neurosci. 2010, 17, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Norre, M.E.; Beckers, A. Vestibular Habituation Training: Exercises treatment for Vertigo Based upon the habituation effect. J. Otolaryngol. Head Neck 1989, 101, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Banfield, G.K.; Wood, C.; Knight, J. Does vestibular habituation still have a place in the treatment of benign paroxysmal positional vertigo? J. Laryngol. Otol. 2000, 114, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Horak, F.B. Rehabilitation Strategies for Patients with Vestibular Deficits. Neurol. Clin. 1990, 8, 441–457. [Google Scholar] [CrossRef]
- Wilhelmsen, K.; Strand, L.I.; Nordahl, S.H.G.; Eide, G.E.; Ljunggren, A.E. Psychometric properties of the Vertigo symptom scale—Short form. BMC Ear Nose Throat Disord. 2008, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Downs, S.; Marquez, J.; Chiarelli, P. The Berg Balance Scale has high intra- and inter-rater reliability but absolute reliability varies across the scale: A systematic review. J. Physiother. 2013, 59, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundakci, B.; Sahan, B.; Ercan, I. Effectiveness of vestibular rehabilitation in patients with benign paroxysmal positional vertigo: A meta-analysis. Phys. Ther. Sport 2016, 17, 57–63. [Google Scholar]
- Herdman, S.J.; Tusa, R.J.; Zee, D.S.; Proctor, L.R.; Mattox, D.E. Single treatment approaches to benign paroxysmal positional vertigo. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 450–454. [Google Scholar] [CrossRef]
- Boulton, A.J.; Malik, R.A.; Arezzo, J.C.; Sosenko, J.M. Diabetic somatic neuropathies. Diabetes Care 2004, 27, 1458–1486. [Google Scholar] [CrossRef] [Green Version]
- Whitney, S.L.; Wrisley, D.M.; Brown, K.E.; Furman, J.M. Physical therapy for migraine-related vestibulopathy and vestibular dysfunction with history of migraine. Laryngoscope 2000, 110, 1528–1534. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.; Kim, J.S. Update on benign paroxysmal positional vertigo [published correction appears in J Neurol. 23 February 2021]. J. Neurol. 2021, 268, 1995–2000. [Google Scholar] [CrossRef]
- Bahadır, C.; Dıraçoğlu, D.; Kurtuluş, D.; Garipoğlu, İ. Efficacy of canalith repositioning maneuvers for benign paroxysmal positional vertigo. Clin. Chiropr. 2009, 12, 95–100. [Google Scholar] [CrossRef]
- Cohen-Shwartz, Y.; Nechemya, Y.; Kalron, A. Canalith repositioning procedure improves gait and static balance in people with posterior semicircular canal benign paroxysmal positional vertigo. J. Vestib. Res. 2020, 30, 335–343. [Google Scholar] [CrossRef]
- Ribeiro, K.M.; Freitas, R.V.; Ferreira, L.M.; Deshpande, N.; Guerra, R.O. Effects of balance Vestibular Rehabilitation Therapy in elderly with Benign Paroxysmal Positional Vertigo: A randomized controlled trial. Disabil. Rehabil. 2017, 39, 1198–1206. [Google Scholar] [CrossRef]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.; Goebel, J.A.; Shepard, N.T.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: From the american physical therapy association neurology section. J. Neurol. Phys. Ther. 2016, 40, 124–155. [Google Scholar] [CrossRef] [Green Version]
- Bayat, A.; Hoseinabadi, R.; Saki, N.; Sanayi, R. Disability and Anxiety in Vestibular Diseases: A Cross-Sectional Study. Cureus 2020, 12, e11813. [Google Scholar] [CrossRef] [PubMed]
- Bressi, F.; Vella, P.; Casale, M.; Moffa, A.; Sabatino, L.; Lopez, M.A.; Carinci, F.; Papalia, R.; Salvinelli, F.; Sterzi, S.; et al. Vestibular rehabilitation in benign paroxysmal positional vertigo: Reality or fiction? Int. J. Immunopathol. Pharmacol. 2017, 30, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, J.I.; Lee, H.M.; Yoo, J.H.; Lee, D.K. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J. Clin. Neurol. 2008, 4, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martellucci, S.; Stolfa, A.; Castellucci, A.; Pagliuca, G.; Clemenzi, V.; Terenzi, V.; Malara, P.; Attanasio, G.; Gazia, F.; Gallo, A. Recovery of Regular Daily Physical Activities Prevents Residual Dizziness after Canalith Repositioning Procedures. Int. J. Environ. Res. Public Health 2022, 19, 490. [Google Scholar] [CrossRef]
| Sequence | Direction | Change of Position | ||
|---|---|---|---|---|
| S1 | S2 | From | To | |
| M1 | Mi | Sitting | Supine | |
| M2 | Le | Supine | Left side | |
| M3 | Ri | Supine | Right side | |
| M4 | Mi | Supine | Sitting | |
| M5 | Turning to the right | |||
| M6 | Turning to the left | |||
| M7 | Ri | Nose on left knee | Right ear on right shoulder | |
| M8 | Le | Nose on right knee | Left ear on left shoulder | |
| M9 | Turning head counter-clockwise | |||
| M10 | Turning head clockwise | |||
| M11 | Bending forward | |||
| M12 | From sitting to erected standing position | |||
| M13 | Moving head and backward | |||
| M14 | Le | Sitting | Head hanging and turning to the left | |
| M15 | Le | Sitting | Head hanging and turning to the left | |
| M16 | Ri | Sitting | Head hanging and turning to the right | |
| M17 | Ri | Sitting | Head hanging and turning to the right | |
| M18 | Mi | Sitting | Head hanging and turning to the right | |
| M19 | Mi | Sitting | Head hanging and turning to the left | |
| A. In bed |
|
| B. Sitting |
|
| C. Standing |
|
| Variable | Group A (n = 15) (%) | Group B (n = 15) (%) | t/χ2 Value | p Value |
|---|---|---|---|---|
| Age (years): Mean ± SE | 39.80 ± 3.89 | 39.00 ± 2.69 | 0.17 | 0.867 |
| Gender: | ||||
| Female | 6 (40.0) | 5 (33.3) | 0.14 | 0.705 |
| Male | 9 (60.0) | 10 (66.7) | ||
| Dix–Hallpik3 test: | ||||
| Positive nystagmus present | 15 (100.0) | 15 (100.0) | 0.00 | 1.000 |
| Outcome Measures | Group | Pre (n = 15) | Post (n = 15) | Mean Change (Pre–Post) | t Value | p Value |
|---|---|---|---|---|---|---|
| VSS–sf score | Group A | 38.93 ± 0.93 | 8.80 ± 0.83 | 30.13 ± 1.43 | 21.06 | <0.001 |
| Group B | 38.87 ± 1.32 | 4.00 ± 0.46 | 34.87 ± 1.52 | 22.97 | <0.001 | |
| BBS Score | Group A | 18.80 ± 0.64 | 43.40 ± 0.69 | 24.60 ± 1.07 | 22.93 | <0.001 |
| Group B | 23.13 ± 1.26 | 49.07 ± 1.22 | 25.93 ± 1.70 | 15.23 | <0.001 |
| Outcome Measures | Group | Mean Change (Pre–Post) | t Value | p Value |
|---|---|---|---|---|
| VSS–sf score | Group A | 30.13 ± 1.43 | 2.27 | 0.031 |
| Group B | 34.87 ± 1.52 | |||
| BBS Score | Group A | 24.60 ± 1.07 | 0.66 | 0.513 |
| Group B | 25.93 ± 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaphe, M.A.; Alshehri, M.M.; Alajam, R.A.; Beg, R.A.; Hamdi, N.I.A.; Nanjan, S.; Esht, V.; Aljahni, M.A.; Ahmed, H.; Ahmad, A.; et al. Effectiveness of Epley–Canalith Repositioning Procedure versus Vestibular Rehabilitation Therapy in Diabetic Patients with Posterior Benign Paroxysmal Positional Vertigo: A Randomized Trial. Life 2023, 13, 1169. https://doi.org/10.3390/life13051169
Shaphe MA, Alshehri MM, Alajam RA, Beg RA, Hamdi NIA, Nanjan S, Esht V, Aljahni MA, Ahmed H, Ahmad A, et al. Effectiveness of Epley–Canalith Repositioning Procedure versus Vestibular Rehabilitation Therapy in Diabetic Patients with Posterior Benign Paroxysmal Positional Vertigo: A Randomized Trial. Life. 2023; 13(5):1169. https://doi.org/10.3390/life13051169
Chicago/Turabian StyleShaphe, Mohammad Abu, Mohammed M. Alshehri, Ramzi Abdu Alajam, Rashid Ali Beg, Najat Ibrahim A. Hamdi, Saravanakumar Nanjan, Vandana Esht, Mohammed A. Aljahni, Hashim Ahmed, Ausaf Ahmad, and et al. 2023. "Effectiveness of Epley–Canalith Repositioning Procedure versus Vestibular Rehabilitation Therapy in Diabetic Patients with Posterior Benign Paroxysmal Positional Vertigo: A Randomized Trial" Life 13, no. 5: 1169. https://doi.org/10.3390/life13051169





