Non-Compaction Ventricle and Associated Cardiovascular and Non-Cardiovascular Diseases; More Attention Is Needed!
Abstract
1. Introduction
2. Case Presentation
2.1. Case #1. NCLV and Coarctation of the Aorta
2.2. Case #2. NCLV, Patent Foramen Ovale (PFO), and Fast-Growing Aortic Aneurysm
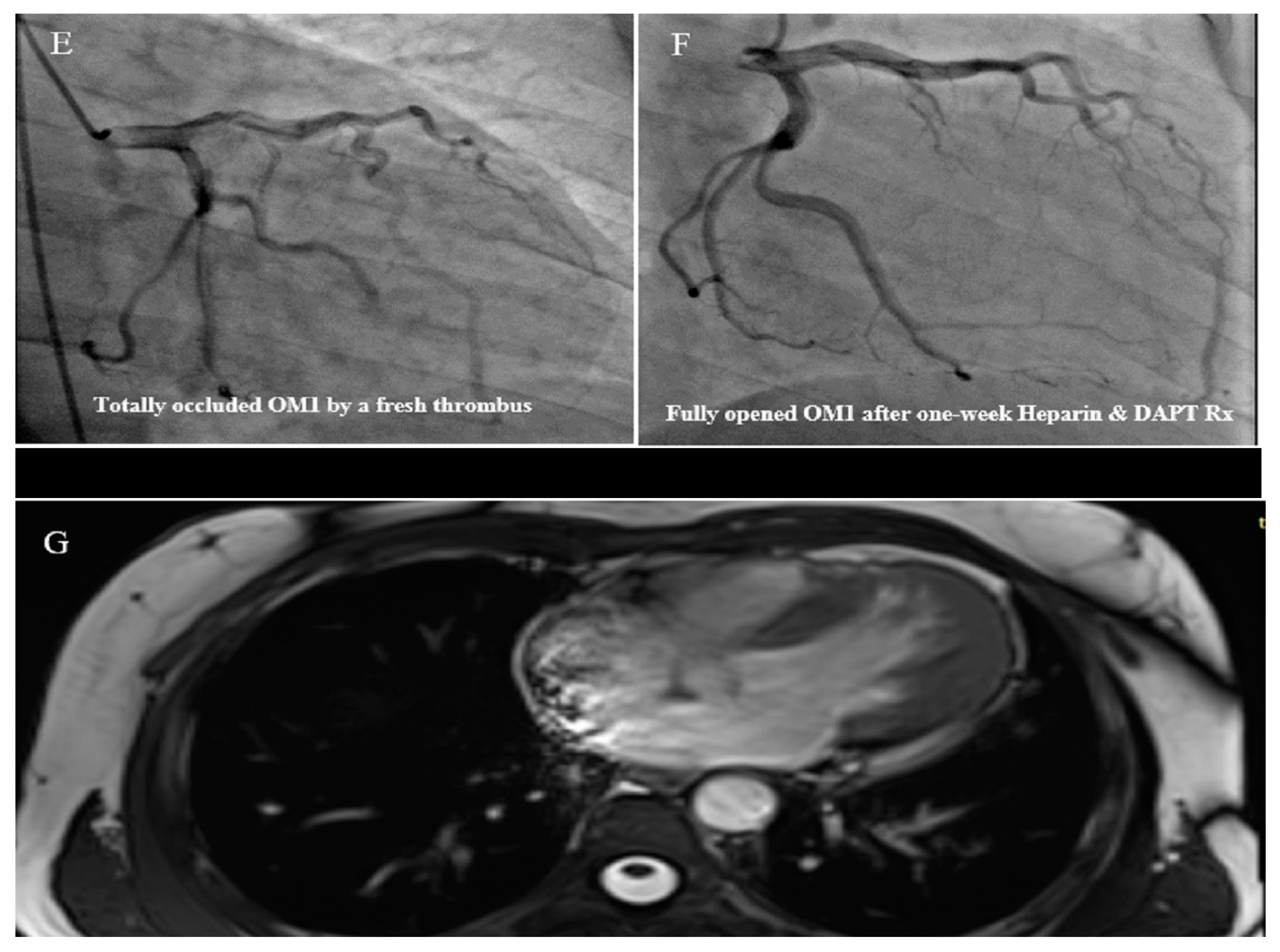
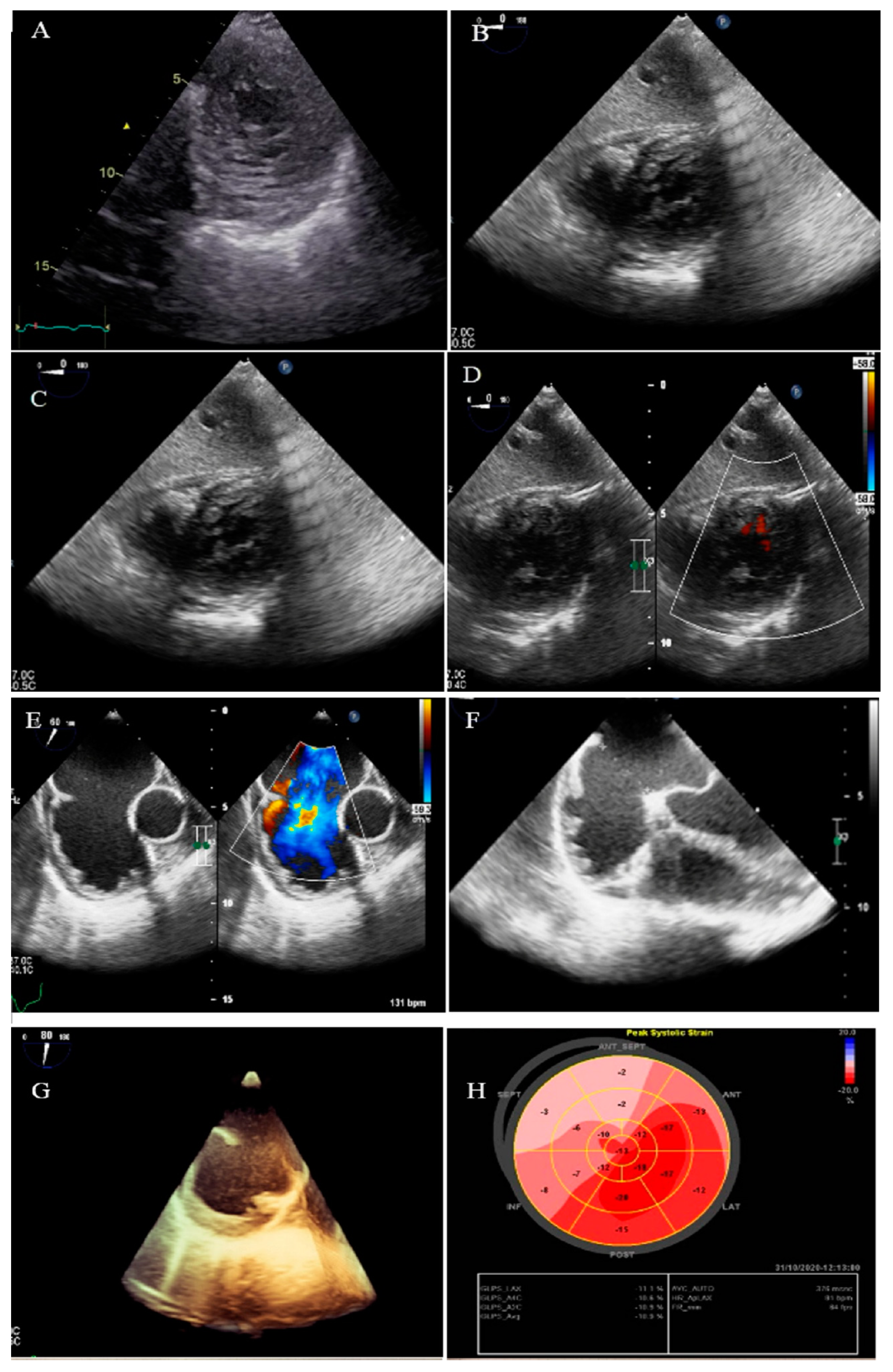
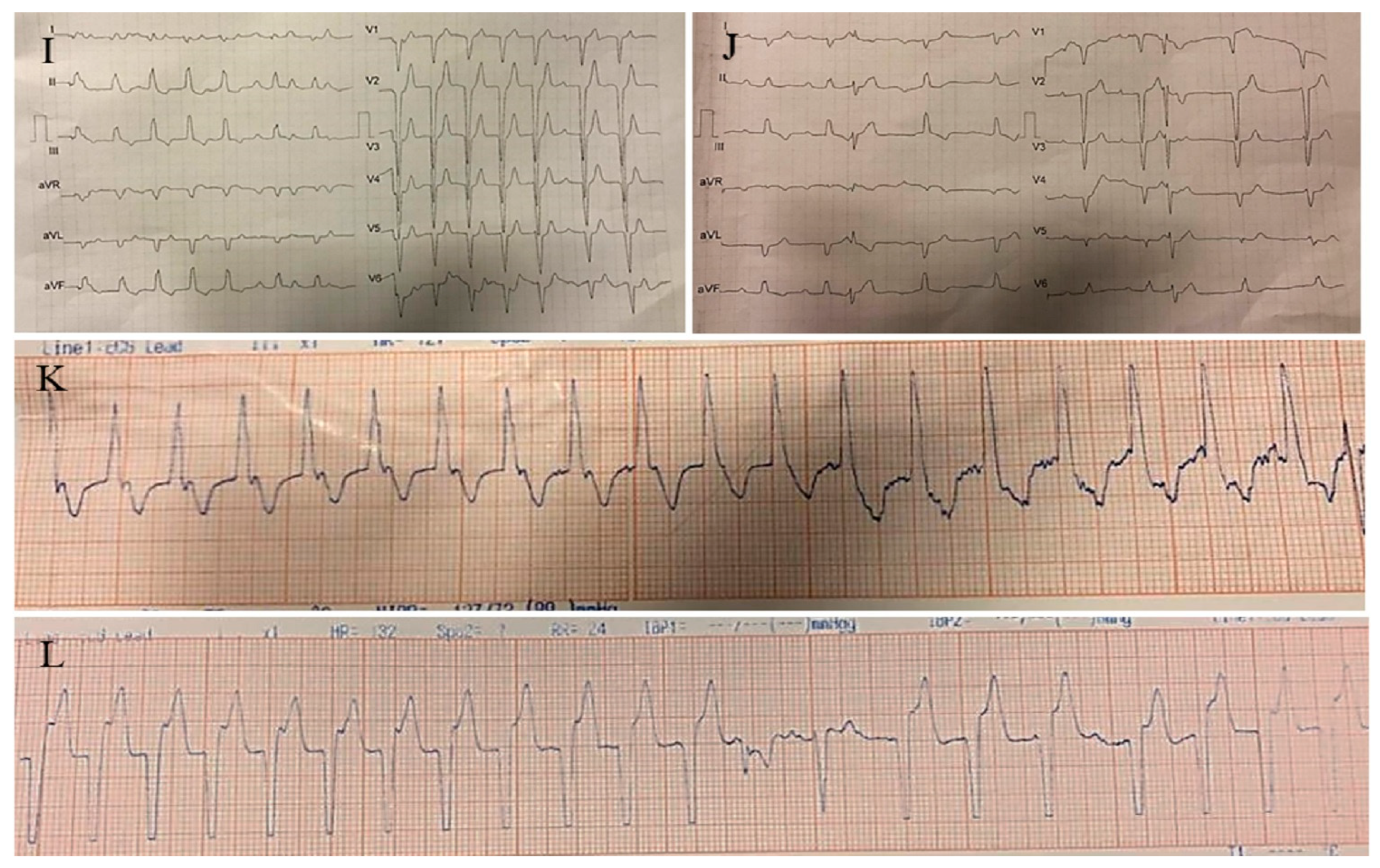
2.3. Case #3. NCLV with Aortic Dilation Complicated by Coronary Embolism
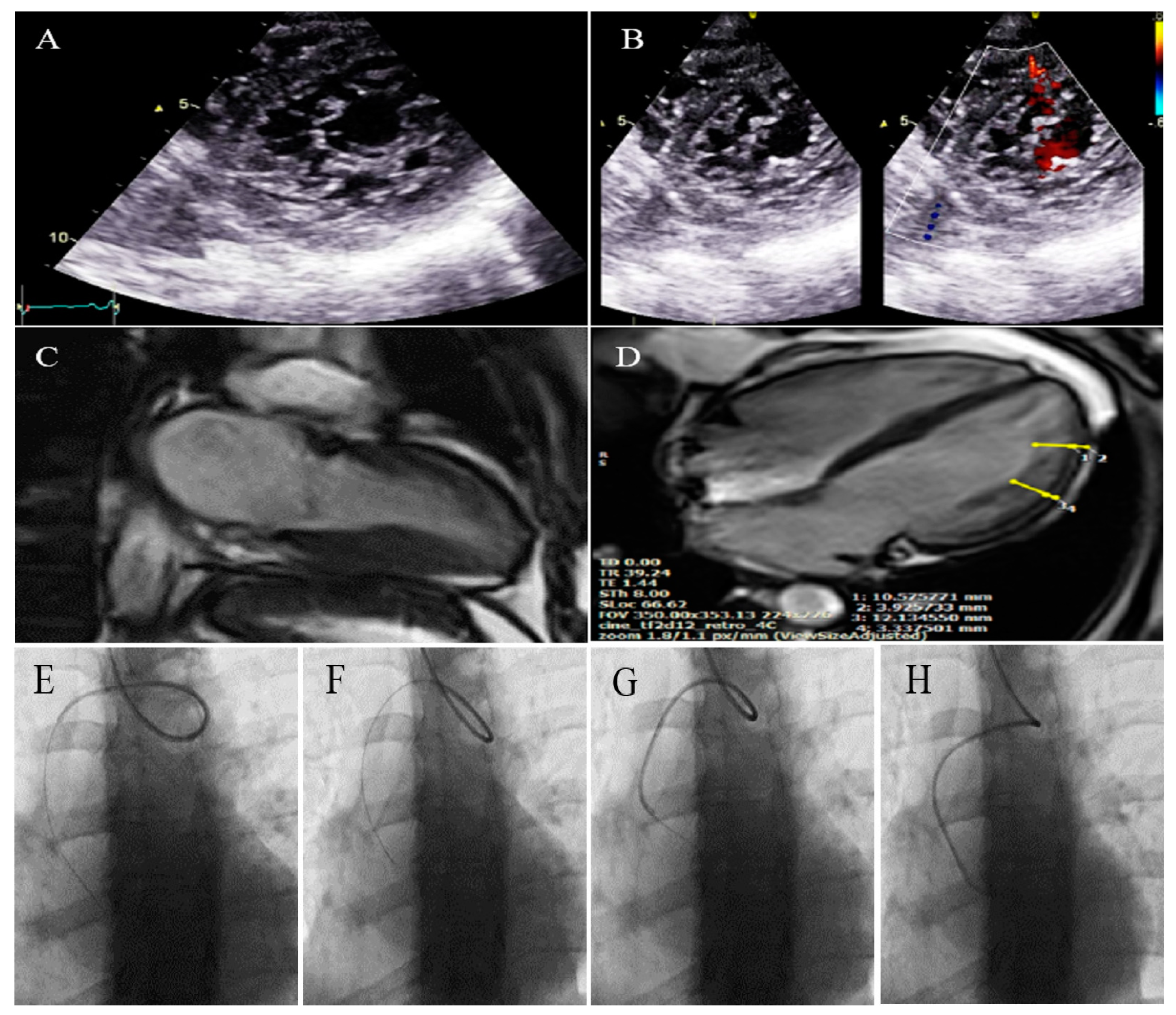
2.4. Case #4. Biventricular Non-Compaction (BVNC) with Ostium Primum Atrial Septal Defect (ASD) Plus Complete Heart Block
2.5. Case #5. NCLV and Arteria Lusoria
2.6. Case #6. BVNC, Bicuspid Aortic Valve (BAV), and Proximal Muscle Weakness in Lower Extremities
2.7. Case #7. BVNC, BAV, AS, and Dilated Aorta Ascending Aorta
2.8. Case #8. NCLV with BAV, Dilated Ascending Aorta, and Top Normal Size Main Pulmonary Artery
2.9. Case #9. NCLV and BAV
2.10. Case #10. NCLV with BAV, Highly Redundant and Oscillating Chiari Network
2.11. Case #11. NCLV, BAV, and Old Myocardial Infarction
2.12. Case #12. BVNC with a Dilated Aorta
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, B.; van der Wal, A.C.; Moorman, A.F.; Christoffels, V.M. Excessive trabeculations in noncompaction do not have the embryonic identity. Int. J. Cardiol. 2017, 227, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Gati, S.; Rajani, R.; Carr-White, G.S.; Chambers, J.B. Adult left ventricular noncompaction: Reappraisal of current diagnostic imaging modalities. JACC Cardiovasc. Imaging 2014, 7, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Gi, W.-T.; Tugrul, O.F.; Amr, A.; Haas, J.; Zhu, F.; Ehlermann, P.; Uhlmann, L.; Katus, H.A. Clinical and genetic insights into non-compaction: A meta-analysis and systematic review on 7598 individuals. Clin. Res. Cardiol. 2019, 108, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, B.J.; Engberding, R. Noncompaction Cardiomyopathy—History and Current Knowledge for Clinical Practice. J. Clin. Med. 2021, 10, 2457. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Heart J. 2020, 41, 1428–1436. [Google Scholar]
- Wengrofsky, P.; Armenia, C.; Oleszak, F.; Kupferstein, E.; Rednam, C.; Mitre, C.A.; McFarlane, S.I. Left ventricular trabeculation and noncompaction cardiomyopathy: A review. EC Clin. Exp. Anat. 2019, 2, 267–283. [Google Scholar]
- Bennett, C.E.; Freudenberger, R. The current approach to diagnosis and management of left ventricular noncompaction cardiomyopathy: Review of the literature. Cardiol. Res. Pract. 2016, 2016, 5172308. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Morales, A.; Cowan, J. Is left ventricular noncompaction a trait, phenotype, or disease? The evidence points to phenotype. Circ. Cardiovasc. Genet. 2017, 10, e001968. [Google Scholar] [CrossRef]
- Marques, L.C.; Liguori, G.R.; Amarante Souza, A.C.; Aiello, V.D. Left Ventricular Noncompaction Is More Prevalent in Ventricular Septal Defect Than Other Congenital Heart Defects: A Morphological Study. J. Cardiovasc. Dev. Dis. 2020, 7, 39. [Google Scholar] [CrossRef]
- Stähli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Buechel, E.V.; Jost, C.H.A.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; Kaufmann, P. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Zuccarino, F.; Vollmer, I.; Sanchez, G.; Navallas, M.; Pugliese, F.; Gayete, A. Left ventricular noncompaction: Imaging findings and diagnostic criteria. AJR Am. J. Roentgenol. 2015, 204, W519–W530. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.V.d.; Salemi, V.M.C.; Alexandre, L.M.; Mady, C. Noncompaction cardiomyopathy: A current view. Arq. Bras. Cardiol. 2011, 97, e13–e19. [Google Scholar] [CrossRef]
- Udeoji, D.U.; Philip, K.J.; Morrissey, R.P.; Phan, A.; Schwarz, E.R. Left ventricular noncompaction cardiomyopathy: Updated review. Ther. Adv. Cardiovasc. Dis. 2013, 7, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Nguyen, C.; Xie, Y.; Deng, Z.; Zhou, Z.; Li, D.; Chang, H.J. Recent advances in cardiac magnetic resonance imaging. Korean Circ. J. 2019, 49, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stöllberger, C.; Blazek, G.; Sehnal, E. Familal left ventricular hypertrabeculation (noncompaction) is myopathic. Int. J. Cardiol. 2013, 164, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Ichida, F. Left ventricular noncompaction−Risk stratification and genetic consideration. J. Cardiol. 2020, 75, 1–9. [Google Scholar] [CrossRef]
- Shieh, J.T. Implications of genetic testing in noncompaction/hypertrabeculation. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163C, 206–211. [Google Scholar] [CrossRef]
- Wang, C.; Hata, Y.; Hirono, K.; Takasaki, A.; Ozawa, S.W.; Nakaoka, H.; Saito, K.; Miyao, N.; Okabe, M.; Ibuki, K. A Wide and Specific Spectrum of Genetic Variants and Genotype–Phenotype Correlations Revealed by Next-Generation Sequencing in Patients with Left Ventricular Noncompaction. J. Am. Heart Assoc. 2017, 6, e006210. [Google Scholar] [CrossRef]
- Cohen, P.J.; Prahlow, J.A. Sudden death due to biventricular non-compaction cardiomyopathy in a 14-year-old. Forensic Sci. Med. Pathol. 2015, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Handlos, P.; Gruszka, T.; Staňková, M.; Marecová, K.; Joukal, M. Biventricular noncompaction cardiomyopathy with malignant arrhythmia as a cause of sudden death. Forensic Sci. Med. Pathol. 2017, 13, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Saglam, M.; Saygin, H.; Kozan, H.; Ozturk, E.; Mutlu, H. Noncompaction of ventricular myocardium involving the right ventricle. Korean Circ. J. 2015, 45, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Açar, G.; Alizade, E.; Yazıcıoğlu, M.V.; Bayram, Z. A rare unclassified cardiomyopathy: Isolated right ventricle noncompaction. Turk Kardiyol. Dern. Ars. 2013, 41, 267. [Google Scholar] [PubMed]
- Seecheran, R.V.; Ramdin, R.; Singh, S.; Seecheran, V.K.; Persad, S.A.; Peram, L.; Raza, S.; Seecheran, N.A. Isolated Right Ventricular Noncompaction Cardiomyopathy Causing Pulmonary Embolism. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211024027. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, E.K.; Wozniak, O.; Konka, M.; Piotrowska-Kownacka, D.; Walczak, E.; Hoffman, P. Isolated ventricular noncompaction mimicking arrhythmogenic right ventricular cardiomyopathy—A study of nine patients. Int. J. Cardiol. 2010, 145, 107–111. [Google Scholar] [CrossRef]
- Fazio, G.; Visconti, C.; D’angelo, L.; Grassedonio, E.; Re, G.L.; D’Amico, T.; Sutera, L.; Novo, G.; Ferrara, F.; Midiri, M. Diagnosis and definition of biventricular non-compaction associated to Ebstein’s anomaly. Int. J. Cardiol. 2011, 150, e20–e24. [Google Scholar] [CrossRef]
- Patra, S.; Singla, V.; Kharge, J.; Ravindranath, K.S.; Manjunath, C.N. A patient of Ebstein’s anomaly associated with biventricular noncompaction presented with Wolf Parkinson White syndrome-A rare presentation. J. Cardiovasc. Dis. Res. 2012, 3, 323–325. [Google Scholar] [CrossRef]
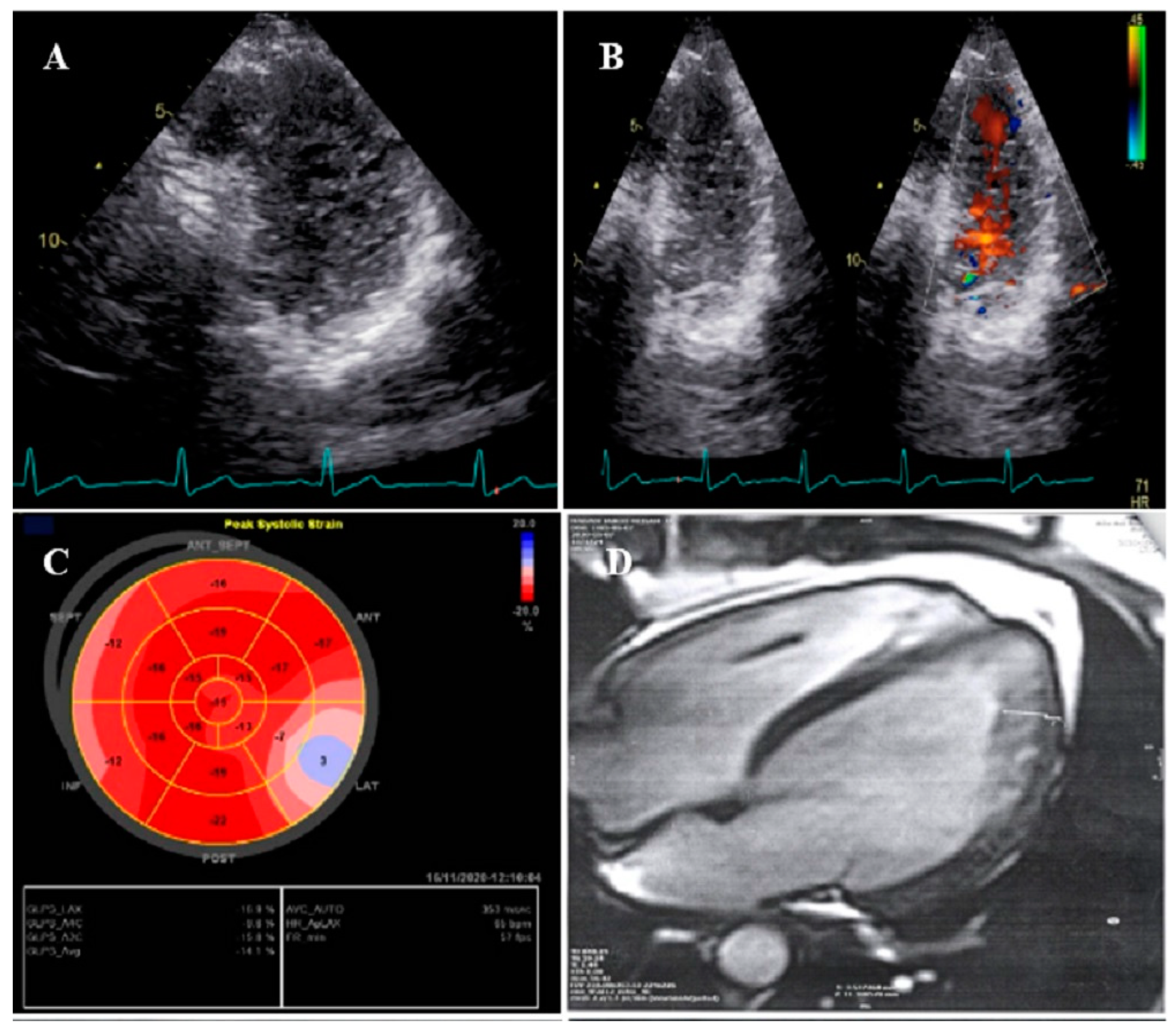
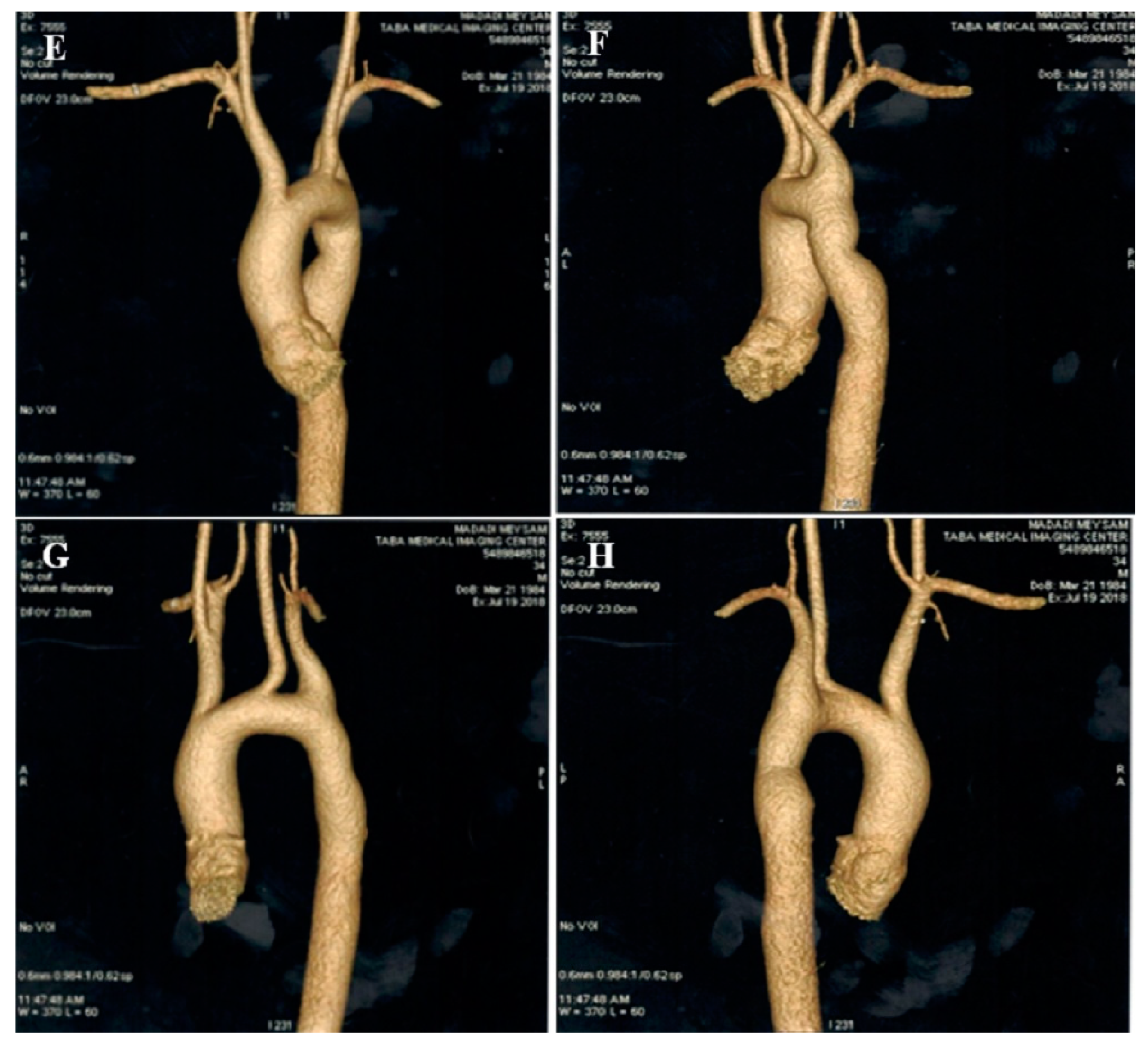
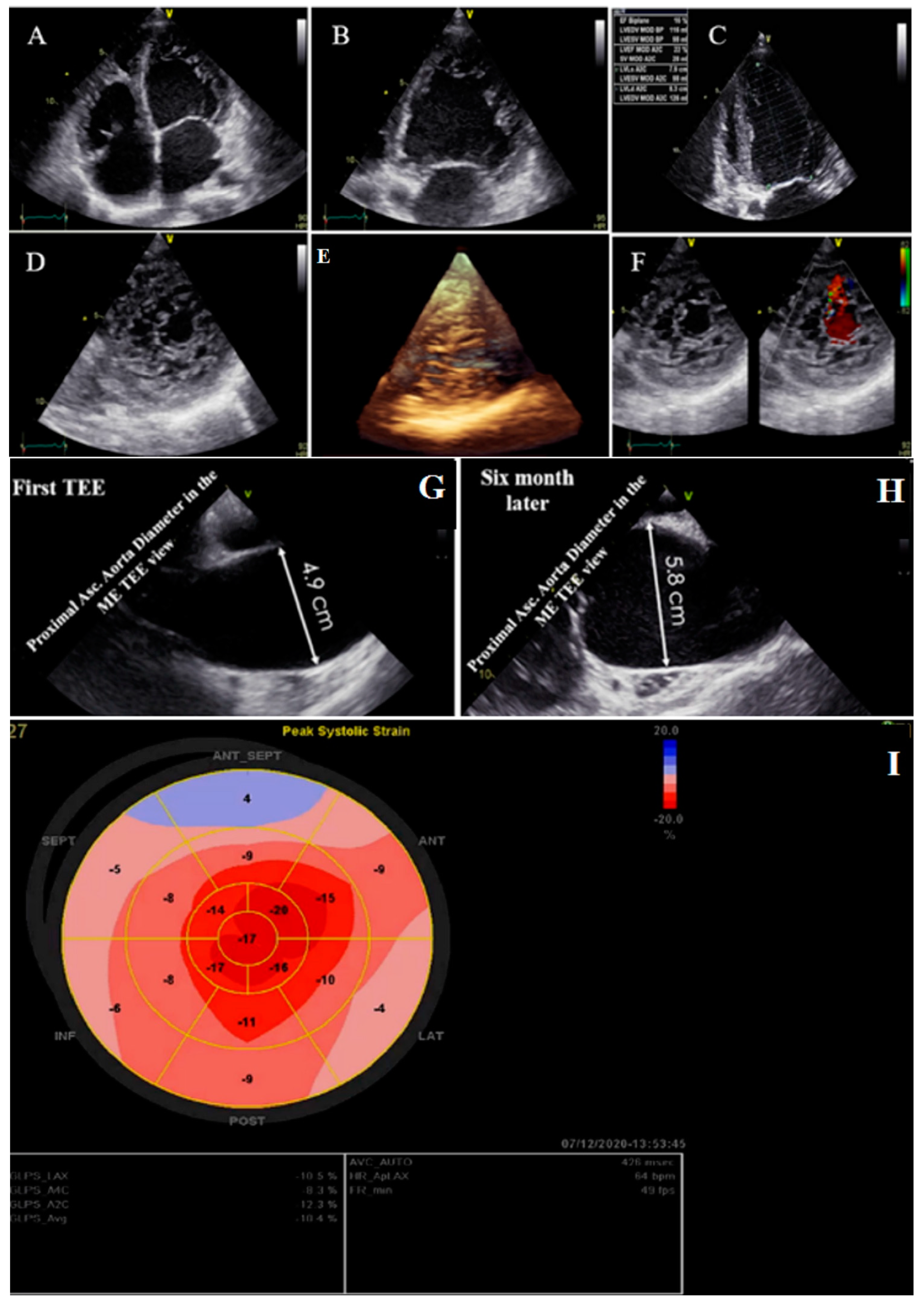
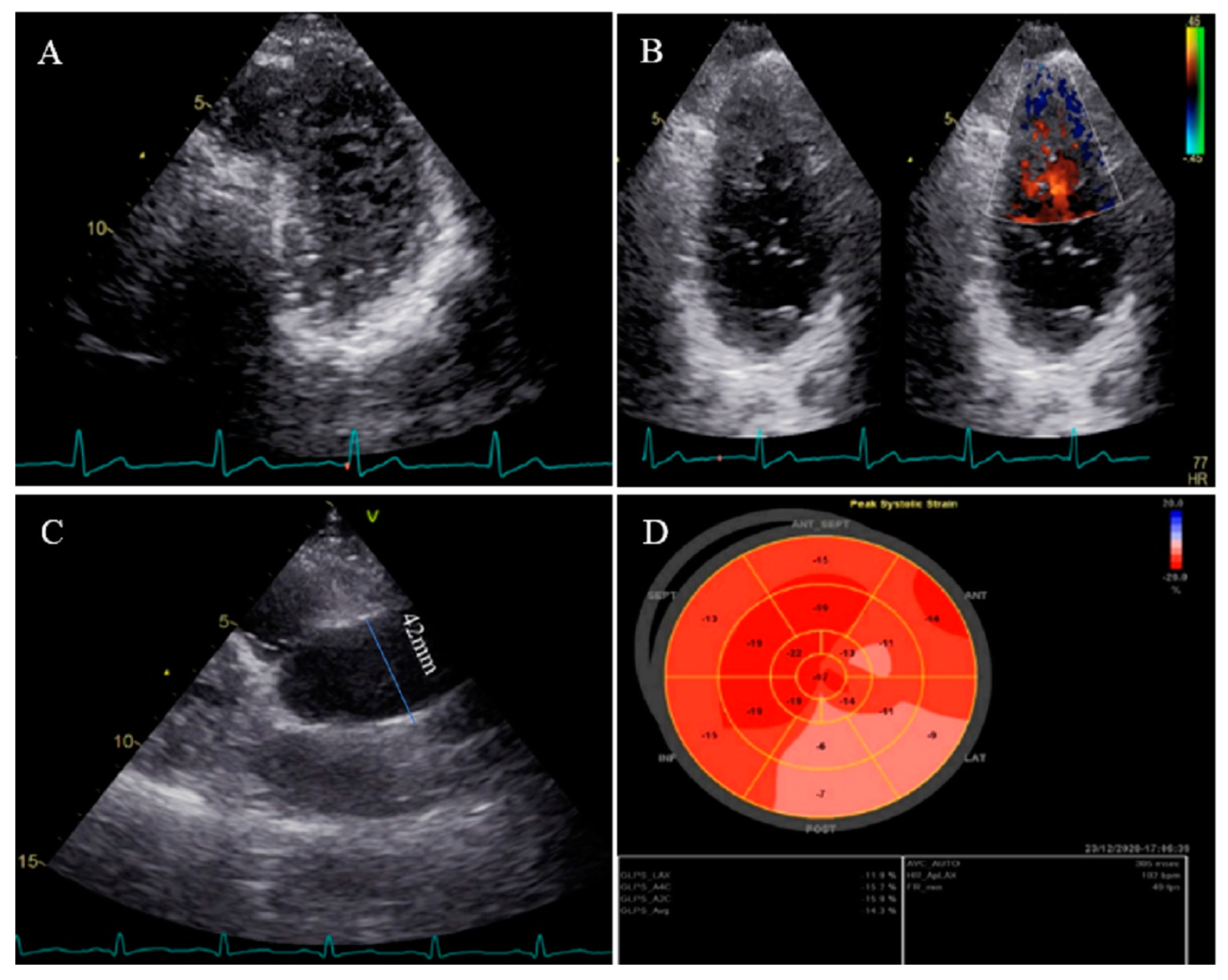
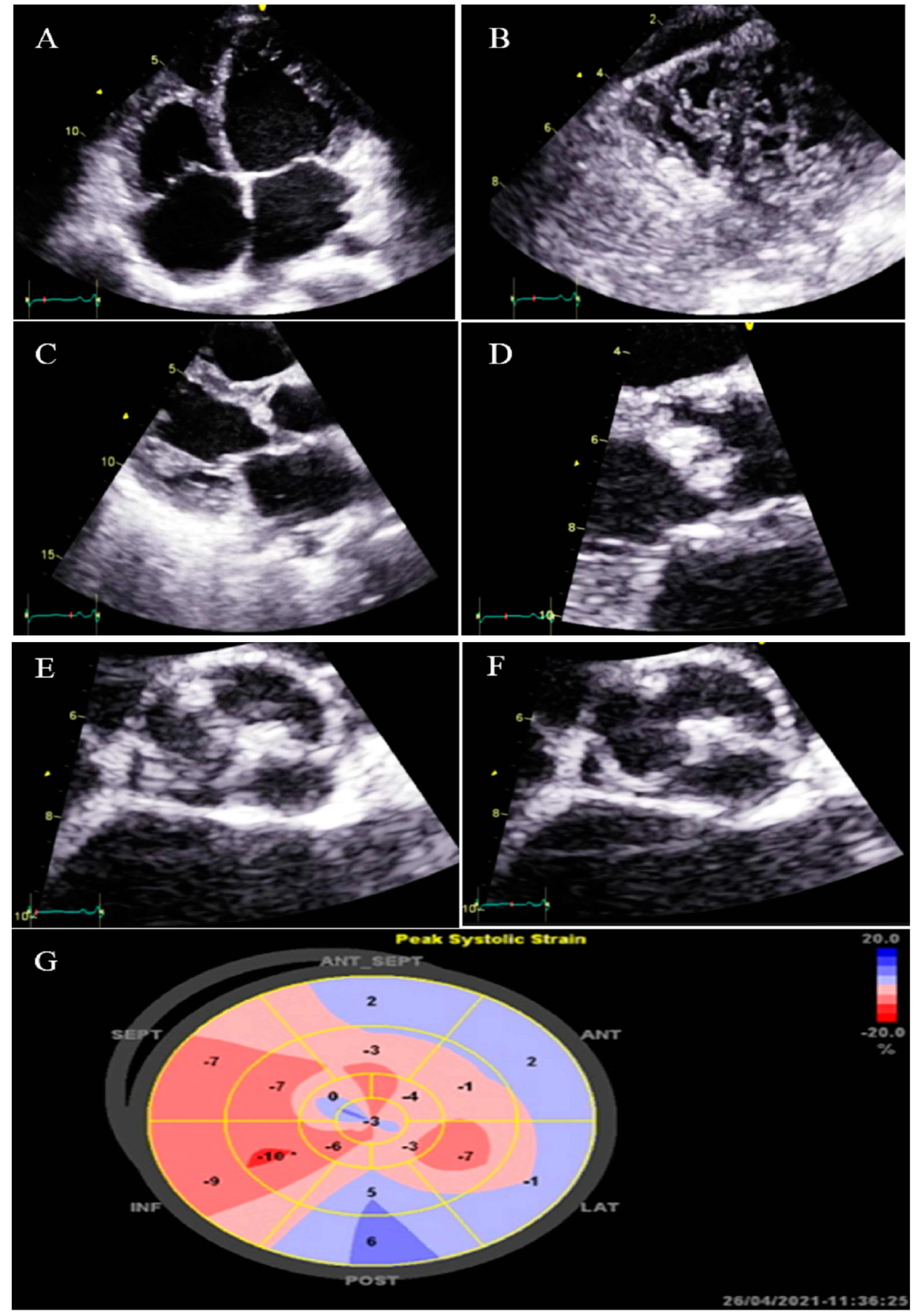

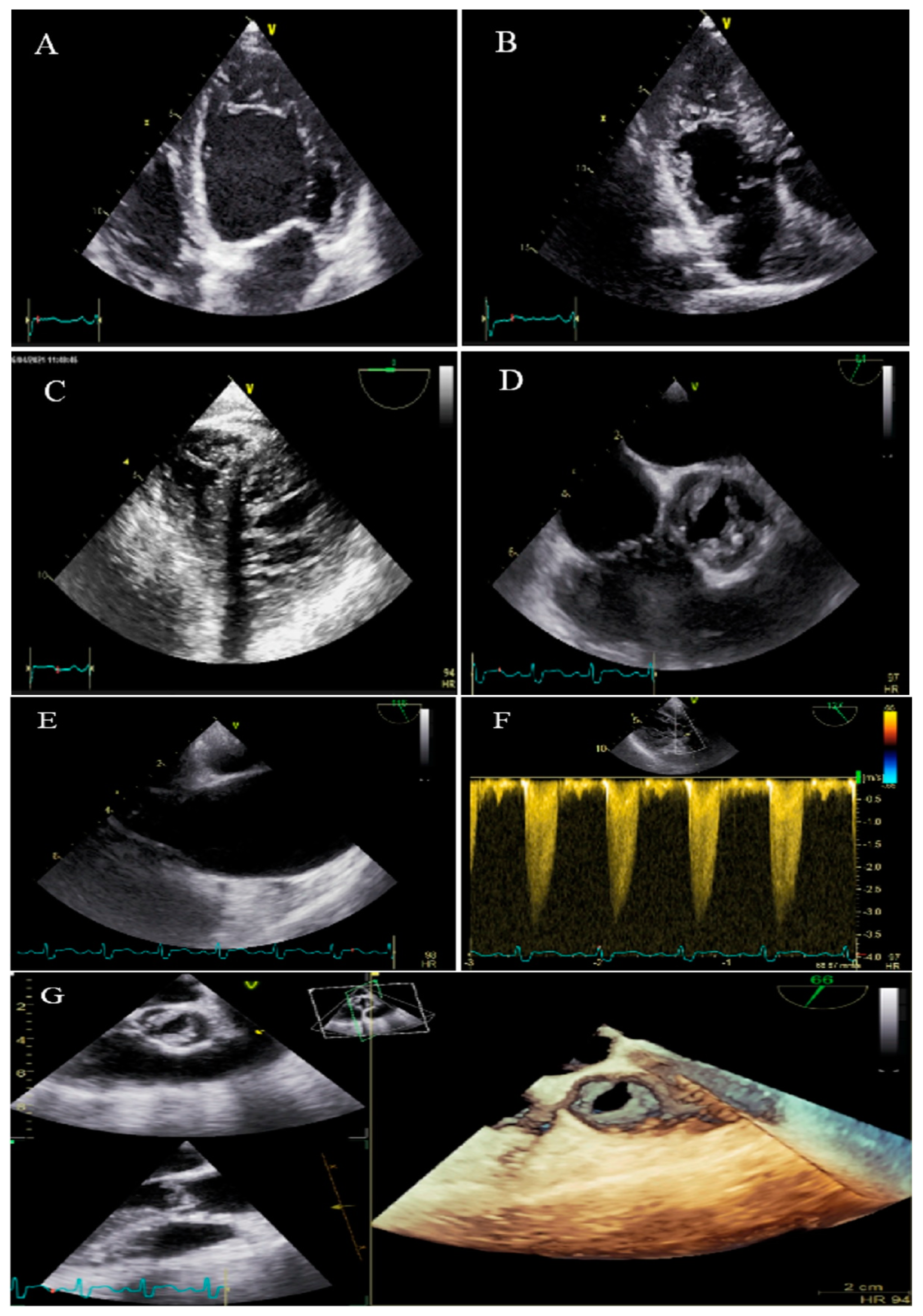
| N | Sex | Age | Presenting Symptom | CVD History or Risk Factor | Type of Non-Compaction | Associated Cardiovascular Anomalies | Genetic Study | Cardiac Magnetic Resonance Imaging | Treatment | Final Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 47 | Easy fatigability and mild hypertension since two years ago | HTN | NCLV, reduced LVEF (45%) | Coarctation of aorta | + | + | Carvedilol 6.25 mg TID plus spironolactone 25 mg daily | Good conditions |
| 2 | M | 56 | Progressive dyspnea | − | NCLV, LVEF = 16%, Global hypokinesia | Hypoplasia of ascending and arch of aorta plus dilated main pulmonary artery | + | − | Scheduled for a valve-sparing aortic root replacement surgery + post-op carvedilol 6.25 mg TID, furosemide 40 mg daily plus spironolactone 25 mg daily | Doing well |
| 3 | M | 37 | Acute retrosternal pain with radiation to both shoulders since three hours prior to admission | − | NCLV, LVEF = 55% | Aortic dilation and coronary embolism | + | + | Anticoagulation plus dual antiplatelet therapy for two weeks switched to lifelong warfarin | Doing well |
| 4 | F | 34 | Dyspnea, two hours after admission, she experienced sudden cardiac death, resuscitated successfully with no sequela | − | BVNC, LVEF = 45% | BAV, ostium primum atrial septal defect plus complete heart block | − | − | Single-chamber implantable cardioverter-defibrillator | No high ventricular rate for 4 months |
| 5 | F | 41 | Echocardiography after angiography | Positive family history for CAD | NCLV, LVEF = 55% | BAV and Arteria Lusoria | + | + | Nil, suggested being under cardiologist follow-up at home country | Did not refer for follow-up |
| 6 | M | 43 | Dyspnea on exertion and recently at rest | − | BVNC LVEF = 24% and fractional area change = 16% | BVNC, BAV, and proximal muscle weakness in lower extremities | − | − | Daily furosemide 40 mg, spironolactone 25 mg, losartan 25 mg and carvedilol 12.5 mg | He left the hospital and did not refer for a follow-up |
| 7 | M | 48 | Dyspnea on moderate exercise and easy fatigability for six months | − | BVNC, LVEF = 55% | BVNC, BAV, aortic stenosis, and dilated ascending aorta | + | Denied because of claustrophobia | Spironolactone 25 mg, carvedilol 12.5 mg daily | Doing well 2 months later. He did not return for a follow-up |
| 8 | F | 25 | History of palpitation referred for the echocardiographic assessment | − | NCLV, LVEF = 32% | BAV, dilated ascending aorta and top normal size main pulmonary artery | − | − | Referred for CMR | Did not refer again to our center |
| 9 | M | 37 | Atypical chest pain for a month | − | NCLV, LVEF = 55% | Medial-lateral directed BAV’s cusps | − | + | Nil | Not yet referred |
| 10 | M | 48 | History of worsening palpitation, and a suspected right atrial mass on echocardiography, reported by a cardiologist | − | NCLV, LVEF = 55% | BAV, highly redundant and oscillating Chiari network | − | − | Suggested referring to his cardiologist in charge for further management | Did not refer again to our center |
| 11 | F | 62 | Echocardiography before diagnostic angiography | Old MI, DM, HLP | NCLV, LVEF = 34% | BAV | − | − | Indicated for CABG according to the result of coronary angiography, she refused and left the hospital | Did not refer again to our center |
| 12 | M | 58 | History of palpitation and shortness of breath on heavy exercise | − | BVNC, LVEF = 55% | Dilated aorta | − | + | carvedilol; 6.25 mg BID | Doing well |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifkazemi, M.; Mohseni-Badalabadi, R.; Kasaie, M.; Ahmadi, L. Non-Compaction Ventricle and Associated Cardiovascular and Non-Cardiovascular Diseases; More Attention Is Needed! Life 2023, 13, 1231. https://doi.org/10.3390/life13061231
Sharifkazemi M, Mohseni-Badalabadi R, Kasaie M, Ahmadi L. Non-Compaction Ventricle and Associated Cardiovascular and Non-Cardiovascular Diseases; More Attention Is Needed! Life. 2023; 13(6):1231. https://doi.org/10.3390/life13061231
Chicago/Turabian StyleSharifkazemi, Mohammadbagher, Reza Mohseni-Badalabadi, Mohammad Kasaie, and Leila Ahmadi. 2023. "Non-Compaction Ventricle and Associated Cardiovascular and Non-Cardiovascular Diseases; More Attention Is Needed!" Life 13, no. 6: 1231. https://doi.org/10.3390/life13061231
APA StyleSharifkazemi, M., Mohseni-Badalabadi, R., Kasaie, M., & Ahmadi, L. (2023). Non-Compaction Ventricle and Associated Cardiovascular and Non-Cardiovascular Diseases; More Attention Is Needed! Life, 13(6), 1231. https://doi.org/10.3390/life13061231






