Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review
Abstract
1. Role of Echocardiography in TAVI Patients
2. Impact of TAVI on Cardiac Function and Structure
2.1. TAVI and Reverse Remodeling
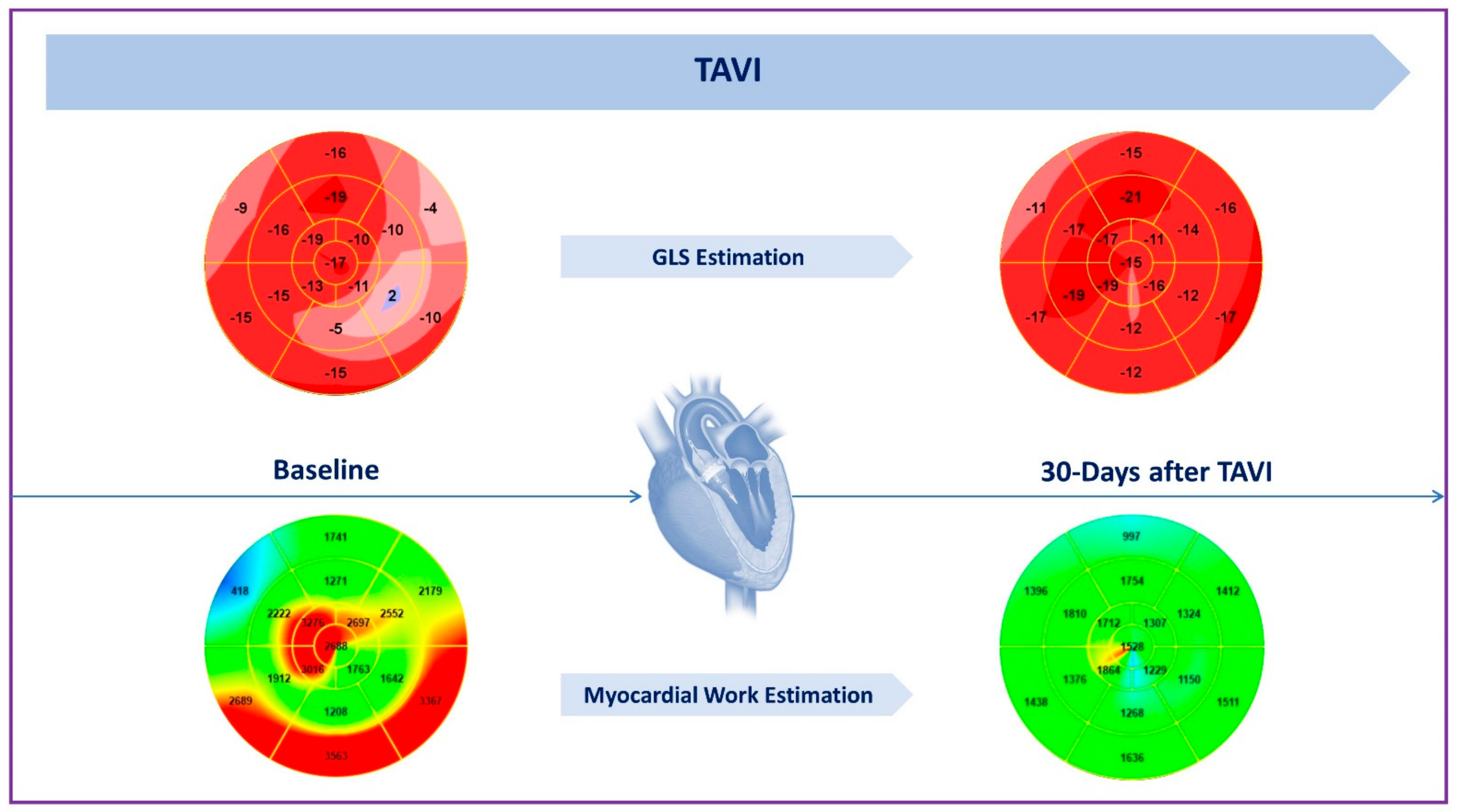
2.2. Impact on LV Systolic Function
2.3. Impact on LV Diastolic Function
2.4. Impact on Mitral Regurgitation
3. TAVI and Right Heart
3.1. Impact on Right Ventricle Function
3.2. Impact on Tricuspid Regurgitation and Pulmonary Hypertension
4. Endocarditis
5. Thrombosis
6. Strengths and Pitfalls of Echocardiography after TAVI
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Feghaly, J.; Das, D.; Oman, Z.; Smart, S. Cardiac Structural Remodeling and Hemodynamic Patterns Following Transcatheter Aortic Valve Replacement. Cureus 2021, 13, e19224. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Panoulas, V.; Jabbour, R.J.; Ruparelia, N.; Malik, I.S.; Hadjiloizou, N.; Frame, A.; Sen, S.; Sutaria, N.; Mikhail, G.W.; et al. Left Ventricular Speckle Tracking Echocardiographic Evaluation before and after TAVI. Echo Res. Pract. 2020, 7, 29–38. [Google Scholar] [CrossRef]
- Chau, K.H.; Douglas, P.S.; Pibarot, P.; Hahn, R.T.; Khalique, O.K.; Jaber, W.A.; Cremer, P.; Weissman, N.J.; Asch, F.M.; Zhang, Y.; et al. Regression of Left Ventricular Mass After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 75, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Puls, M.; Beuthner, B.E.; Topci, R.; Vogelgesang, A.; Bleckmann, A.; Sitte, M.; Lange, T.; Backhaus, S.J.; Schuster, A.; Seidler, T.; et al. Impact of Myocardial Fibrosis on Left Ventricular Remodelling, Recovery, and Outcome after Transcatheter Aortic Valve Implantation in Different Haemodynamic Subtypes of Severe Aortic Stenosis. Eur. Heart J. 2020, 41, 1903–1914. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and Clinical Impact of Prosthesis-Patient Mismatch in the Aortic Valve Position and Its Prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef]
- Rao, V.; Jamieson, W.R.; Ivanov, J.; Armstrong, S.; David, T.E. Prosthesis-Patient Mismatch Affects Survival after Aortic Valve Replacement. Circulation 2000, 102, III-5–III-9. [Google Scholar] [CrossRef]
- Tomoeda, H.; Ueda, T.; Teshima, H.; Arinaga, K.; Tayama, K.; Fukunaga, S.; Aoyagi, S. Postoperative Left Ventricular Mass Regression after Aortic Valve Replacement for Aortic Stenosis. Ann. Thorac. Surg. 2010, 89, 745–750. [Google Scholar] [CrossRef]
- Lindman, B.R.; Stewart, W.J.; Pibarot, P.; Hahn, R.T.; Otto, C.M.; Xu, K.; Devereux, R.B.; Weissman, N.J.; Enriquez-Sarano, M.; Wilson Szeto, Y.; et al. Early Regression of Severe Left Ventricular Hypertrophy After Transcatheter Aortic Valve Replacement Is Associated With Decreased Hospitalizations. JACC Cardiovasc. Interv. 2014, 7, 662–673. [Google Scholar] [CrossRef]
- Kampaktsis, P.N.; Kokkinidis, D.G.; Wong, S.-C.; Vavuranakis, M.; Skubas, N.J.; Devereux, R.B. The Role and Clinical Implications of Diastolic Dysfunction in Aortic Stenosis. Heart 2017, 103, 1481–1487. [Google Scholar] [CrossRef]
- Lisi, M.; Pastore, M.C.; Fiorio, A.; Cameli, M.; Mandoli, G.E.; Righini, F.M.; Cavigli, L.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; et al. Left Atrial Remodeling in Response to Aortic Valve Replacement: Pathophysiology and Myocardial Strain Analysis. Life 2022, 12, 2074. [Google Scholar] [CrossRef]
- Galli, E.; Fournet, M.; Chabanne, C.; Lelong, B.; Leguerrier, A.; Flecher, E.; Mabo, P.; Donal, E. Prognostic Value of Left Atrial Reservoir Function in Patients with Severe Aortic Stenosis: A 2D Speckle-Tracking Echocardiographic Study. Eur. Heart J. Cardiovasc. Imaging. 2016, 17, 533–541. [Google Scholar] [CrossRef]
- Angelillis, M.; Giannini, C.; de Carlo, M.; Adamo, M.; Nardi, M.; Colombo, A.; Chieffo, A.; Bedogni, F.; Brambilla, N.; Tamburino, C.; et al. Prognostic Significance of Change in the Left Ventricular Ejection Fraction After Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Stenosis and Left Ventricular Dysfunction. Am. J. Cardiol. 2017, 120, 1639–1647. [Google Scholar] [CrossRef]
- Elmariah, S.; Palacios, I.F.; McAndrew, T.; Hueter, I.; Inglessis, I.; Baker, J.N.; Kodali, S.; Leon, M.B.; Svensson, L.; Pibarot, P.; et al. Outcomes of Transcatheter and Surgical Aortic Valve Replacement in High-Risk Patients with Aortic Stenosis and Left Ventricular Dysfunction: Results from the Placement of Aortic Transcatheter Valves (PARTNER) Trial (Cohort A). Circ. Cardiovasc. Interv. 2013, 6, 604–614. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Ahn, J.-M.; Kang, D.-Y.; Park, H.; Ko, E.; Kim, H.J.; Kim, J.B.; Choo, S.J.; Lee, S.-A.; Park, S.-J.; et al. Incidence, Predictors, and Prognostic Impact of Immediate Improvement in Left Ventricular Systolic Function After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 152, 99–105. [Google Scholar] [CrossRef]
- Kuneman, J.H.; Butcher, S.C.; Singh, G.K.; Wang, X.; Hirasawa, K.; van der Kley, F.; Leon, M.B.; Knuuti, J.; Pibarot, P.; Ajmone Marsan, N.; et al. Prognostic Implications of Change in Left Ventricular Ejection Fraction After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2022, 177, 90–99. [Google Scholar] [CrossRef]
- Dimitriadis, Z.; Scholtz, S.; Ensminger, S.; Wiemer, M.; Fischbach, T.; Scholtz, W.; Piper, C.; Börgermann, J.; Bitter, T.; Horstkotte, D.; et al. Left Ventricular Adaptation after TAVI Evaluated by Conventional and Speckle-Tracking Echocardiography. Int. J. Cardiol. 2017, 228, 633–637. [Google Scholar] [CrossRef]
- Spethmann, S.; Baldenhofer, G.; Dreger, H.; Stüer, K.; Sanad, W.; Saghabalyan, D.; Müller, E.; Stangl, V.; Baumann, G.; Stangl, K.; et al. Recovery of Left Ventricular and Left Atrial Mechanics in Various Entities of Aortic Stenosis 12 Months after TAVI. Eur. Heart. J. Cardiovasc. Imaging 2014, 15, 389–398. [Google Scholar] [CrossRef]
- Schueler, R.; Sinning, J.-M.; Momcilovic, D.; Weber, M.; Ghanem, A.; Werner, N.; Nickenig, G.; Grube, E.; Hammerstingl, C. Three-Dimensional Speckle-Tracking Analysis of Left Ventricular Function after Transcatheter Aortic Valve Implantation. J. Am. Soc. Echocardiogr. 2012, 25, 827–834.e1. [Google Scholar] [CrossRef]
- Poulin, F.; Carasso, S.; Horlick, E.M.; Rakowski, H.; Lim, K.-D.; Finn, H.; Feindel, C.M.; Greutmann, M.; Osten, M.D.; Cusimano, R.J.; et al. Recovery of Left Ventricular Mechanics after Transcatheter Aortic Valve Implantation: Effects of Baseline Ventricular Function and Postprocedural Aortic Regurgitation. J. Am. Soc. Echocardiogr. 2014, 27, 1133–1142. [Google Scholar] [CrossRef]
- Ilardi, F.; Marchetta, S.; Martinez, C.; Sprynger, M.; Ancion, A.; Manganaro, R.; Sugimoto, T.; Tsugu, T.; Postolache, A.; Piette, C.; et al. Impact of Aortic Stenosis on Layer-Specific Longitudinal Strain: Relationship with Symptoms and Outcome. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Shiino, K.; Yamada, A.; Scalia, G.M.; Putrino, A.; Chamberlain, R.; Poon, K.; Walters, D.L.; Chan, J. Early Changes of Myocardial Function after Transcatheter Aortic Valve Implantation Using Multilayer Strain Speckle Tracking Echocardiography. Am. J. Cardiol. 2019, 123, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Monosilio, S.; Luongo, F.; Neccia, M.; Birtolo, L.I.; Salvi, N.; Filomena, D.; Mancone, M.; Fedele, F.; Agati, L.; et al. Myocardial Contractility Recovery Following Acute Pressure Unloading after Transcatheter Aortic Valve Intervention (TAVI) in Patients with Severe Aortic Stenosis and Different Left Ventricular Geometry: A Multilayer Longitudinal Strain Echocardiographicanalysis. Int. J. Cardiovasc. Imaging 2021, 37, 965–970. [Google Scholar] [PubMed]
- Ilardi, F.; Postolache, A.; Dulgheru, R.; Trung, M.-L.N.; de Marneffe, N.; Sugimoto, T.; Go, Y.Y.; Oury, C.; Esposito, G.; Lancellotti, P. Prognostic Value of Non-Invasive Global Myocardial Work in Asymptomatic Aortic Stenosis. J. Clin. Med. 2022, 11, 1555. [Google Scholar] [CrossRef]
- Jain, R.; Bajwa, T.; Roemer, S.; Huisheree, H.; Allaqaband, S.Q.; Kroboth, S.; Perez Moreno, A.C.; Tajik, A.J.; Khandheria, B.K. Myocardial Work Assessment in Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 715–721. [Google Scholar] [CrossRef]
- Sato, K.; Kumar, A.; Jones, B.M.; Mick, S.L.; Krishnaswamy, A.; Grimm, R.A.; Desai, M.Y.; Griffin, B.P.; Rodriguez, L.L.; Kapadia, S.R.; et al. Reversibility of Cardiac Function Predicts Outcome after Transcatheter Aortic Valve Replacement in Patients with Severe Aortic Stenosis. J. Am. Heart Assoc. 2017, 6, e005798. [Google Scholar] [CrossRef]
- Asami, M.; Lanz, J.; Stortecky, S.; Räber, L.; Franzone, A.; Heg, D.; Hunziker, L.; Roost, E.; Siontis, G.C.; Valgimigli, M.; et al. The Impact of Left Ventricular Diastolic Dysfunction on Clinical Outcomes after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 593–601. [Google Scholar] [CrossRef]
- Blair, J.E.A.; Atri, P.; Friedman, J.L.; Thomas, J.D.; Brummel, K.; Sweis, R.N.; Mikati, I.; Malaisrie, S.C.; Davidson, C.J.; Flaherty, J.D. Diastolic Function and Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2017, 30, 541–551. [Google Scholar] [CrossRef]
- Muratori, M.; Fusini, L.; Tamborini, G.; Gripari, P.; Delgado, V.; Marsan, N.A.; Ghulam Ali, S.; Barbier, P.; Bartorelli, A.L.; Alamanni, F.; et al. Sustained Favourable Haemodynamics 1 Year after TAVI: Improvement in NYHA Functional Class Related to Improvement of Left Ventricular Diastolic Function. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1269–1278. [Google Scholar] [CrossRef]
- Kampaktsis, P.N.; Bang, C.N.; Chiu Wong, S.; Skubas, N.J.; Singh, H.; Voudris, K.; Baduashvili, A.; Pastella, K.; Swaminathan, R.V.; Kaple, R.K.; et al. Prognostic Importance of Diastolic Dysfunction in Relation to Post Procedural Aortic Insufficiency in Patients Undergoing Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2017, 89, 445–451. [Google Scholar] [CrossRef]
- Conte, L.; Fabiani, I.; Pugliese, N.R.; Giannini, C.; La Carruba, S.; Angelillis, M.; Spontoni, P.; De Carlo, M.; Petronio, A.S.; Di Bello, V. Left Ventricular Stiffness Predicts Outcome in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. Echocardiography 2017, 34, 6–13. [Google Scholar] [CrossRef]
- Khan, F.; Okuno, T.; Malebranche, D.; Lanz, J.; Praz, F.; Stortecky, S.; Windecker, S.; Pilgrim, T. Transcatheter Aortic Valve Replacement in Patients With Multivalvular Heart Disease. JACC Cardiovasc. Interv. 2020, 13, 1503–1514. [Google Scholar] [CrossRef]
- Sengupta, A.; Biswas, M.; Zaid, S.; Alexis, S.L.; Tang, G.H.L. Effect & Implications of Transcatheter Aortic Valve Replacement on Concomitant Functional Mitral Regurgitation. Struct. Heart 2020, 4, 192–194. [Google Scholar]
- Nombela-Franco, L.; Ribeiro, H.B.; Urena, M.; Allende, R.; Amat-Santos, I.; DeLarochellière, R.; Dumont, E.; Doyle, D.; DeLarochellière, H.; Laflamme, J.; et al. Significant Mitral Regurgitation Left Untreated at the Time of Aortic Valve Replacement. J. Am. Coll. Cardiol. 2014, 63, 2643–2658. [Google Scholar] [CrossRef]
- Chakravarty, T.; van Belle, E.; Jilaihawi, H.; Noheria, A.; Testa, L.; Bedogni, F.; Rück, A.; Barbanti, M.; Toggweiler, S.; Thomas, M.; et al. Meta-Analysis of the Impact of Mitral Regurgitation on Outcomes after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2015, 115, 942–949. [Google Scholar] [CrossRef]
- Cortés, C.; Amat-Santos, I.J.; Nombela-Franco, L.; Muñoz-Garcia, A.J.; Gutiérrez-Ibanes, E.; de La, J.M.; Hernandez, T.; Córdoba-Soriano, J.G.; Jimenez-Quevedo, P.; Hernández-García, J.M.; et al. Mitral Regurgitation After Transcatheter Aortic Valve Replacement Prognosis, Imaging Predictors, and Potential Management. JACC Cardiovasc. Interv. 2016, 9, 1603–1614. [Google Scholar] [CrossRef]
- Muratori, M.; Fusini, L.; Tamborini, G.; Ghulam Ali, S.; Gripari, P.; Fabbiocchi, F.; Salvi, L.; Trabattoni, P.; Roberto, M.; Agrifoglio, M.; et al. Mitral Valve Regurgitation in Patients Undergoing TAVI: Impact of Severity and Etiology on Clinical Outcome. Int. J. Cardiol. 2020, 299, 228–234. [Google Scholar] [CrossRef]
- Vollenbroich, R.; Stortecky, S.; Praz, F.; Lanz, J.; Franzone, A.; Zuk, K.; Heg, D.; Valgimigli, M.; O’Sullivan, C.J.; Heinisch, C.; et al. The Impact of Functional vs. Degenerative Mitral Regurgitation on Clinical Outcomes among Patients Undergoing Transcatheter Aortic Valve Implantation. Am. Heart J. 2017, 184, 71–80. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Eltchaninoff, H.; Zahn, R.; Testa, L.; Leon, M.B.; Trillo-Nouche, R.; Donofrio, A.; Smith, C.R.; Webb, J.; Bleiziffer, S.; et al. Clinical Impact and Evolution of Mitral Regurgitation Following Transcatheter Aortic Valve Replacement: A Meta-Analysis. Heart 2015, 101, 1395–1405. [Google Scholar] [CrossRef]
- De Chiara, B.; Moreo, A.; de Marco, F.; Musca, F.; Oreglia, J.; Lobiati, E.; Bruschi, G.; Belli, O.; Mauri, F.; Klugmann, S. Influence of CoreValve ReValving System Implantation on Mitral Valve Function: An Echocardiographic Study in Selected Patients. Catheter. Cardiovasc. Interv. 2011, 78, 638–644. [Google Scholar] [CrossRef]
- Bedogni, F.; Latib, A.; de Marco, F.; Agnifili, M.; Oreglia, J.; Pizzocri, S.; Latini, R.A.; Lanotte, S.; Petronio, A.S.; de Carlo, M.; et al. Interplay between Mitral Regurgitation and Transcatheter Aortic Valve Replacement with the CoreValve Revalving System: A Multicenter Registry. Circulation 2013, 128, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Codner, P.; Landes, U.; Schwartzenberg, S.; Barbanti, M.; Valvo, R.; de Backer, O.; Ooms, J.F.; Islas, F.; Marroquin, L.; et al. Effect of Transcatheter Aortic Valve Replacement on Concomitant Mitral Regurgitation and Its Impact on Mortality. JACC Cardiovasc. Interv. 2021, 14, 1181–1192. [Google Scholar] [CrossRef]
- Mavromatis, K.; Thourani, V.H.; Stebbins, A.; Vemulapalli, S.; Devireddy, C.; Guyton, R.A.; Matsouaka, R.; Ghasemzadeh, N.; Block, P.C.; Leshnower, B.G.; et al. Transcatheter Aortic Valve Replacement in Patients With Aortic Stenosis and Mitral Regurgitation. Ann. Thorac. Surg. 2017, 104, 1977–1985. [Google Scholar] [CrossRef]
- Mauri, V.; Körber, M.I.; Kuhn, E.; Schmidt, T.; Frerker, C.; Wahlers, T.; Rudolph, T.K.; Baldus, S.; Adam, M.; ten Freyhaus, H. Prognosis of Persistent Mitral Regurgitation in Patients Undergoing Transcatheter Aortic Valve Replacement. Clin. Res. Cardiol. 2020, 109, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.; Plein, D.; van Camp, G.; Cosyns, B.; Pasquet, A.; Henrard, V.; de Cannière, D.; Melot, C.; Piérard, L.A.; Lancellotti, P. Effects of Valve Replacement for Aortic Stenosis on Mitral Regurgitation. Am. J. Cardiol. 2008, 102, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Mao, W.; McKay, R.; Sun, W. The Impact of Balloon-Expandable Transcatheter Aortic Valve Replacement on Concomitant Mitral Regurgitation: A Comprehensive Computational Analysis. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef]
- Caballero, A.; Mao, W.; McKay, R.; Sun, W. The Impact of Self-Expandable Transcatheter Aortic Valve Replacement on Concomitant Functional Mitral Regurgitation: A Comprehensive Engineering Analysis. Struct. Heart 2020, 4, 179–191. [Google Scholar] [CrossRef]
- Boerlage-van Dijk, K.; Wiegerinck, E.M.A.; Takama, T.; Koch, K.T.; Vis, M.M.; de Mol, B.A.J.M.; Piek, J.J.; Bouma, B.J.; Baan, J. Mitral Regurgitation Prior to Transcatheter Aortic Valve Implantation Influences Survival but Not Symptoms. Int. J. Cardiol. 2016, 204, 95–100. [Google Scholar] [CrossRef]
- Asami, M.; Stortecky, S.; Praz, F.; Lanz, J.; Räber, L.; Franzone, A.; Piccolo, R.; Siontis, G.C.M.; Heg, D.; Valgimigli, M.; et al. Prognostic Value of Right Ventricular Dysfunction on Clinical Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 577–587. [Google Scholar] [CrossRef]
- Eleid, M.F.; Padang, R.; Pislaru, S.V.; Greason, K.L.; Crestanello, J.; Nkomo, V.T.; Pellikka, P.A.; Jentzer, J.C.; Gulati, R.; Sandhu, G.S.; et al. Effect of Transcatheter Aortic Valve Replacement on Right Ventricular–Pulmonary Artery Coupling. JACC Cardiovasc. Interv. 2019, 12, 2145–2154. [Google Scholar] [CrossRef]
- Hutter, A.; Bleiziffer, S.; Richter, V.; Opitz, A.; Hettich, I.; Mazzitelli, D.; Ruge, H.; Lange, R. Transcatheter Aortic Valve Implantation in Patients with Concomitant Mitral and Tricuspid Regurgitation. Ann. Thorac. Surg. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Barbanti, M.; Binder, R.K.; Dvir, D.; Tan, J.; Freeman, M.; Thompson, C.R.; Cheung, A.; Wood, D.A.; Leipsic, J.; Webb, J.G. Prevalence and Impact of Preoperative Moderate/Severe Tricuspid Regurgitation on Patients Undergoing Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2015, 85, 677–683. [Google Scholar] [CrossRef]
- Galli, E.; Guirette, Y.; Feneon, D.; Daudin, M.; Fournet, M.; Leguerrier, A.; Flecher, E.; Mabo, P.; Donal, E. Prevalence and Prognostic Value of Right Ventricular Dysfunction in Severe Aortic Stenosis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 531–538. [Google Scholar] [CrossRef]
- Ren, B.; Spitzer, E.; Geleijnse, M.L.; Zijlstra, F.; de Jaegere, P.P.T.; van Mieghem, N.M.; Tijssen, J.G. Right Ventricular Systolic Function in Patients Undergoing Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2018, 257, 40–45. [Google Scholar] [CrossRef]
- Lindman, B.R.; Maniar, H.S.; Jaber, W.A.; Lerakis, S.; Mack, M.J.; Suri, R.M.; Thourani, V.H.; Babaliaros, V.; Kereiakes, D.J.; Whisenant, B.; et al. Effect of Tricuspid Regurgitation and the Right Heart on Survival after Transcatheter Aortic Valve Replacement: Insights from the Placement of Aortic Transcatheter Valves II Inoperable Cohort. Circ. Cardiovasc. Interv. 2015, 8, e002073. [Google Scholar] [CrossRef]
- Grevious, S.N.; Fernandes, M.F.; Annor, A.K.; Ibrahim, M.; Saint Croix, G.R.; de Marchena, E.; Cohen, M.G.; Alfonso, C.E. Prognostic Assessment of Right Ventricular Systolic Dysfunction on Post–Transcatheter Aortic Valve Replacement Short-Term Outcomes: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, 14463. [Google Scholar] [CrossRef]
- Poch, F.; Thalmann, R.; Olbrich, I.; Fellner, C.; Stundl, A.; Barthel, P.; Bradaric, C.; Laugwitz, K.L.; Kupatt, C.; Ledwoch, J. Changes of Right Ventricular Function After Transcatheter Aortic Valve Replacement and Association With Outcomes. J. Card. Fail. 2021, 27, 1337–1344. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Harjola, V.P.; Mebazaa, A.; Čelutkiene, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary Management of Acute Right Ventricular Failure: A Statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, H.; Durmaz, T.; Keleş, T.; Sari, C.; Aslan, A.N.; Kasapkara, H.A.; Bozkurt, E. Improvement of Right Ventricular Function with Transcatheter Aortic Valve Implantation. Scand. Cardiovasc. J. 2014, 48, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Avvedimento, M.; Franzone, A.; Leone, A.; Piccolo, R.; Castiello, D.S.; Ilardi, F.; Mariani, A.; Esposito, R.; Iapicca, C.; Angellotti, D.; et al. Extent of Cardiac Damage and Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2021, 10, 4563. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, F.; Lorca, L.; Agullo, A.; Bouchdoug, K.; Macia, J.C.; Delseny, D.; Roubille, F.; Gandet, T.; Lattuca, B.; Robert, P.; et al. Evolution of Right Ventricular Dysfunction and Tricuspid Regurgitation after TAVI: A Prospective Study. Int. J. Cardiol. 2022, 353, 29–34. [Google Scholar] [CrossRef]
- Testa, L.; Latib, A.; de Marco, F.; de Carlo, M.; Fiorina, C.; Barbanti, M.; Montone, R.A.; Agnifili, M.; Petronio, A.S.; Ettori, F.; et al. The Failing Right Heart: Implications and Evolution in High-Risk Patients Undergoing Transcatheter Aortic Valve Implantation. EuroIntervention 2016, 12, 1542–1549. [Google Scholar] [CrossRef]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of Pulmonary Artery Pressure by Echocardiography—A Comprehensive Review. Int. J. Cardiol. Heart Vasc. 2016, 12, 45. [Google Scholar] [CrossRef]
- Muraishi, M.; Tabata, M.; Shibayama, K.; Ito, J.; Shigetomi, K.; Obunai, K.; Watanabe, H.; Yamamoto, M.; Watanabe, Y.; Naganuma, T.; et al. Late Progression of Tricuspid Regurgitation After Transcatheter Aortic Valve Replacement. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Testa, L.; Latib, A.; de Marco, F.; de Carlo, M.; Fiorina, C.; Montone, R.; Agnifili, M.; Barbanti, M.; Petronio, A.S.; Zoccai, G.B.; et al. Persistence of Severe Pulmonary Hypertension after Transcatheter Aortic Valve Replacement: Incidence and Prognostic Impact. Circ. Cardiovasc. Interv. 2016, 9, e003563. [Google Scholar] [CrossRef]
- Alushi, B.; Beckhoff, F.; Leistner, D.; Franz, M.; Reinthaler, M.; Stähli, B.E.; Morguet, A.; Figulla, H.R.; Doenst, T.; Maisano, F.; et al. Pulmonary Hypertension in Patients With Severe Aortic Stenosis: Prognostic Impact After Transcatheter Aortic Valve Replacement: Pulmonary Hypertension in Patients Undergoing TAVR. JACC Cardiovasc. Imaging 2019, 12, 591–601. [Google Scholar] [CrossRef]
- Sinning, J.M.; Hammerstingl, C.; Chin, D.; Ghanem, A.; Schueler, R.; Sedaghat, A.; Bence, J.; Spyt, T.; Werner, N.; Kovac, J.; et al. Decrease of Pulmonary Hypertension Impacts on Prognosis after Transcatheter Aortic Valve Replacement. EuroIntervention 2014, 9, 1042–1049. [Google Scholar] [CrossRef]
- Eisen, A.; Shapira, Y.; Sagie, A.; Kornowski, R. Infective Endocarditis in the Transcatheter Aortic Valve Replacement Era: Comprehensive Review of a Rare Complication. Clin. Cardiol. 2012, 35, E1–E5. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation: The Valve Academic Research Consortium-2 Consensus Document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef]
- Durack, D.T.; Phil, D.; Lukes, A.S.; Bright, D.K.; Service, E. New Criteria for Diagnosis of Infective Endocarditis: Utilization of Specific Echocardiographic Findings. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; del Zotti, F.; Dulgheru, R.; el Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis. Eur. Heart J. 2015, 36, 3075–3123. [Google Scholar] [CrossRef]
- Akins, C.W.; Miller, D.C.; Turina, M.I.; Kouchoukos, N.T.; Blackstone, E.H.; Grunkemeier, G.L.; Takkenberg, J.J.M.; David, T.E.; Butchart, E.G.; Adams, D.H.; et al. Guidelines for Reporting Mortality and Morbidity After Cardiac Valve Interventions. Ann. Thorac. Surg. 2008, 85, 1490–1495. [Google Scholar] [CrossRef]
- Latib, A.; Naganuma, T.; Abdel-Wahab, M.; Danenberg, H.; Cota, L.; Barbanti, M.; Baumgartner, H.; Finkelstein, A.; LeGrand, V.; de Lezo, J.S.; et al. Treatment and Clinical Outcomes of Transcatheter Heart Valve Thrombosis. Circ. Cardiovasc. Interv. 2015, 8, e001779. [Google Scholar] [CrossRef]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Roudaut, R.; Serri, K.; Lafitte, S. Thrombosis of Prosthetic Heart Valves: Diagnosis and Therapeutic Considerations. Heart 2007, 93, 137–142. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A.; et al. Recommendations for Evaluation of Prosthetic Valves With Echocardiography and Doppler Ultrasound. A Report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, Developed in Conjunction With the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014. [Google Scholar]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the Imaging Assessment of Prosthetic Heart Valves: A Report from the European Association of Cardiovascular Imaging Endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [PubMed]
- Freitas-Ferraz, A.B.; Rodés-Cabau, J.; Junquera Vega, L.; Beaudoin, J.; O’Connor, K.; Turgeon, P.Y.; Paradis, J.-M.; Ferreira-Neto, A.; Asmarats, L.; Champagne, J.; et al. Transesophageal Echocardiography Complications Associated with Interventional Cardiology Procedures. Am. Heart J. 2020, 221, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hasnie, A.A.; Parcha, V.; Hawi, R.; Trump, M.; Shetty, N.S.; Ahmed, M.I.; Booker, O.J.; Arora, P.; Arora, G. Complications Associated With Transesophageal Echocardiography in Transcatheter Structural Cardiac Interventions. J. Am. Soc. Echocardiogr. 2023, 36, 381–390. [Google Scholar] [CrossRef] [PubMed]
| Factors Predicting MR Improvement | Factors Predicting MR Persistence or Worsening | |
|---|---|---|
| Functional etiology | Organic etiology | PPM |
| LV dilatation | Baseline severe MR | Use of SEV |
| Low ejection fraction | Permanent AF | Deep valve implantation |
| Coronary artery disease | Pulmonary hypertension | Calcified mitral valve disease |
| High transvalvular aortic gradient | Moderate or severe PVL | Mitral annular diameter > 35.5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angellotti, D.; Manzo, R.; Castiello, D.S.; Immobile Molaro, M.; Mariani, A.; Iapicca, C.; Nappa, D.; Simonetti, F.; Avvedimento, M.; Leone, A.; et al. Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review. Life 2023, 13, 1079. https://doi.org/10.3390/life13051079
Angellotti D, Manzo R, Castiello DS, Immobile Molaro M, Mariani A, Iapicca C, Nappa D, Simonetti F, Avvedimento M, Leone A, et al. Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review. Life. 2023; 13(5):1079. https://doi.org/10.3390/life13051079
Chicago/Turabian StyleAngellotti, Domenico, Rachele Manzo, Domenico Simone Castiello, Maddalena Immobile Molaro, Andrea Mariani, Cristina Iapicca, Dalila Nappa, Fiorenzo Simonetti, Marisa Avvedimento, Attilio Leone, and et al. 2023. "Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review" Life 13, no. 5: 1079. https://doi.org/10.3390/life13051079
APA StyleAngellotti, D., Manzo, R., Castiello, D. S., Immobile Molaro, M., Mariani, A., Iapicca, C., Nappa, D., Simonetti, F., Avvedimento, M., Leone, A., Canonico, M. E., Spaccarotella, C. A. M., Franzone, A., Ilardi, F., Esposito, G., & Piccolo, R. (2023). Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review. Life, 13(5), 1079. https://doi.org/10.3390/life13051079








