What Do We Know about Surface Proteins of Chicken Parasites Eimeria?
Abstract
1. Introduction
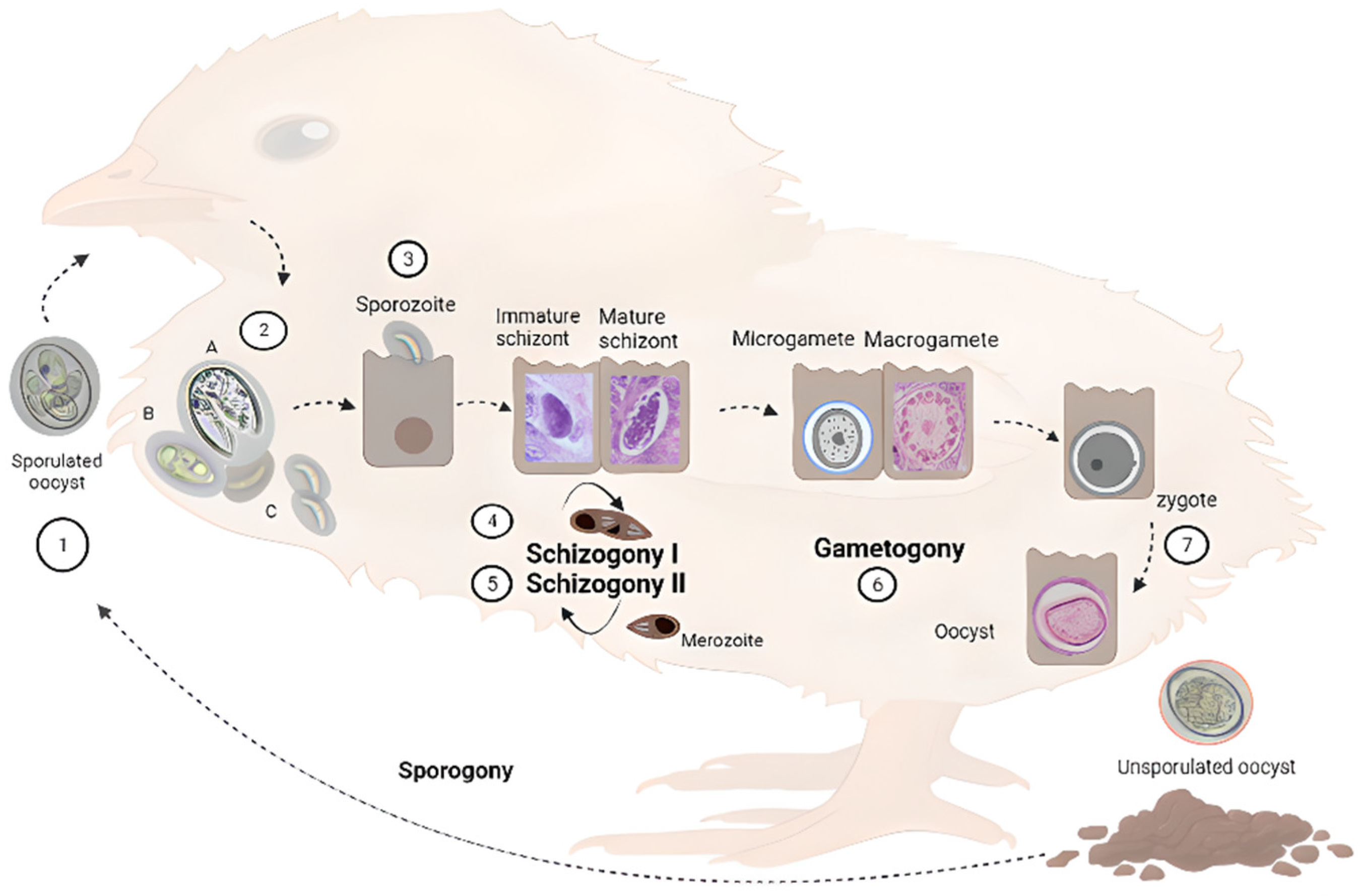
2. Life Cycle of Eimeria spp.
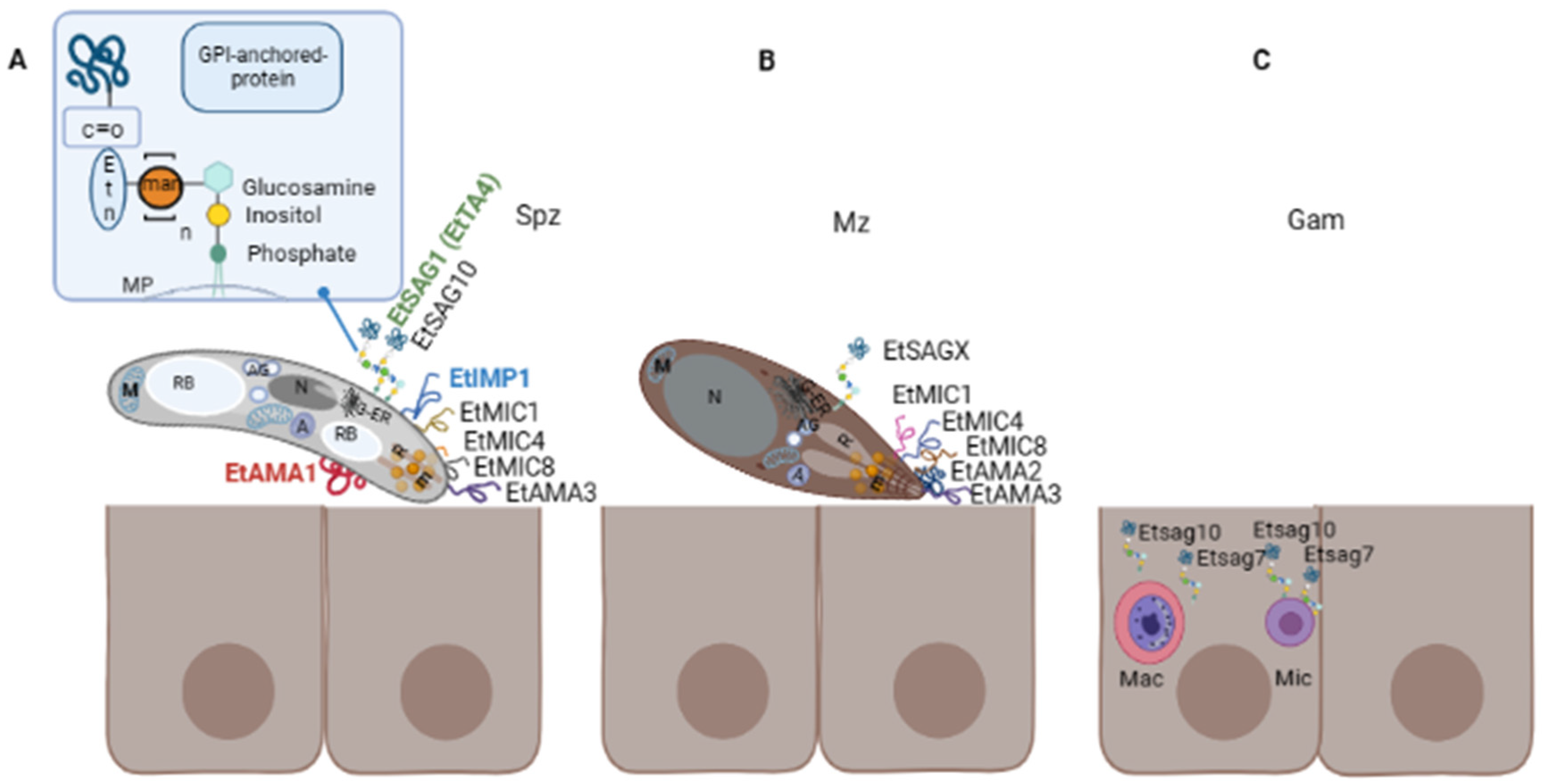
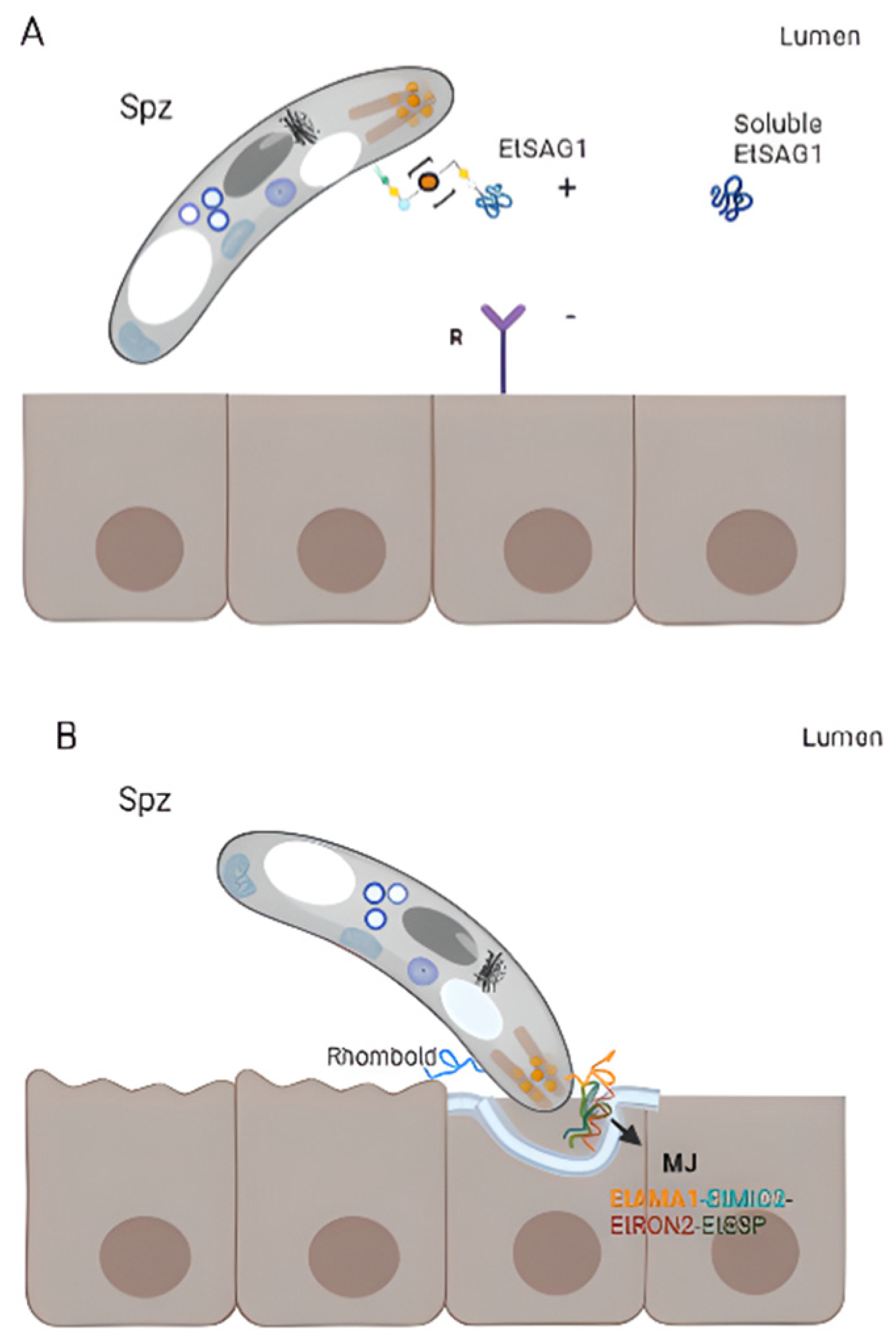
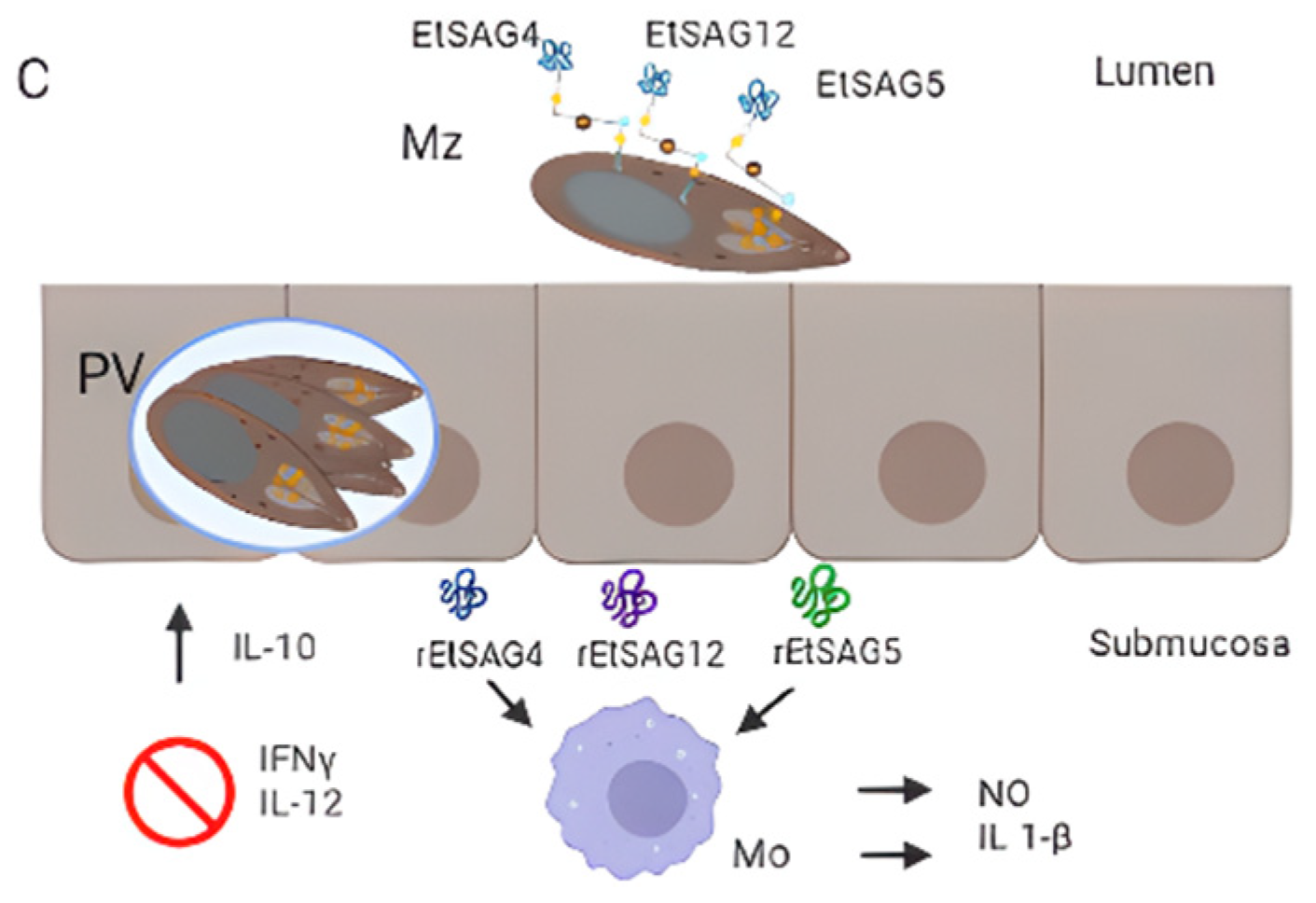
3. Surface Proteins of Eimeria spp.
3.1. Eimeria acervulina
| Species | Protein | Life Stage | Tested as Vaccine Candidate | Function | References |
|---|---|---|---|---|---|
| E. acervulina | 3-1E | Sz, Mz (A) | Yes | Host-parasite interaction | [70,72,79] |
| SAG (TA4) | Sz (A) | No | Binding to duodenal cells | [78] | |
| E. brunetti | AMA1 | NR | Yes | NR | [80] |
| E. maxima | AMA1 | Sz (A) | Yes | Host-parasite interaction | [62,81] |
| IMP-1 | Sz (A) | Yes | Invasion and immunoprotection | [62,82,83] | |
| SAG | Sz, Mz (A) | Yes | Host-parasite interaction | [84,85] | |
| E. mitis | MIC3 | Sz, Mz (A) | Yes | Host-parasite interaction, Immunoprotection | [86] |
| E. necatrix | NA4 | NR | Yes | Immunoprotection | [87] |
| SAGs (2) | Mz | No | Possibly attachment and host evasion | [88] | |
| 3-1E | Sz, Mz (A) | Yes | Invasion, immunoprotection | [89] | |
| E. tenella | SAG1 (TA4) | sOo, Sz (A) | Yes | Attachment, immunoprotection | [45,90,91] |
| SAG | Sz (A) | Yes | Host-parasite interaction | [92] | |
| SAG2 | Mz (A) | Yes | Elicitation humoral response | [92,93] | |
| SAG4 | Mz (A) | Yes | Elicitation humoral response; cell-mediated immunity impairment, inflammatory response | [93,94] | |
| SAG5 | Mz (A) | No | Elicitation humoral response, cell-mediated immunity impairment, inflammatory response | [93] | |
| SAG6 | Mz (A) | Yes | Immunoprotection | [95] | |
| SAG7 | Gam (S) | No | NR | [96] | |
| SAG8-9 | Mz (A) | No | Host-parasite interaction | [93] | |
| SAG10 | Sz, Mz, aSt (A) Gam (S) | No | Invasion, pathogenesis and immune evasion, drug resistance | [23,47] | |
| SAG12 | Mz (A) | No | Elicitation humoral response, cell-mediated immunity impairment, inflammatory response | [93] | |
| SAG13 | sOo, Sz, Mz (A) | Yes | Host-parasite interaction, possible drug resistance | [23,93] | |
| SAG15 | Mz (A) | Yes | Elicitation humoral response, immunoprotection | [93,95] | |
| SAG17 | Mz (A) | No | Host-parasite interaction | [45] | |
| SAG19 | Mz (A) | No | Elicitation humoral response | [97] | |
| SAG23 | Mz (A) | Yes | Elicitation humoral response | [45,93] | |
| AMA1 | Sz (A) | Yes | Invasion (moving junction formation), Immunoprotection | [81,98] | |
| AMA2 | Mz (A) | Yes | Not known, absence of immunoprotection | [81] | |
| AMA3 | sOo, Sz (A) | No | Invasion | [99] | |
| IMP-1 | Sz (A) | Yes | Immunogenicity and immunoprotection | [81] | |
| MIC1 | Sz, Mz (A) | Yes | Association with EtMIC2 during attachment | [100] | |
| MIC4 | Sz, Mz (A) | Yes | Adhesion, invasion, and immunogenicity | [100] | |
| MIC8 | Sz, Mz (A) | Yes | Attachment, and immunogenicity | [56] |
3.2. Eimeria brunetti
3.3. Eimeria maxima
3.4. Eimeria mitis
3.5. Eimeria necatrix
3.6. Eimeria tenella
4. GPI-Anchor Biosynthesis
5. Chicken Coccidiosis Vaccines
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adl, S.M.; Simpson, A.G.B.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The Revised Classification of Eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–514. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Worthing, K.; Jenkins, M.C. Exploring Eimeria Genomes to Understand Population Biology: Recent Progress and Future Opportunities. Genes 2020, 11, 1103. [Google Scholar] [CrossRef]
- FAO. Understanding and Integrating Gender Issues in Livestock Proyects and Programmes; FAO: Rome, Italy, 2013. [Google Scholar]
- Portillo Salgado, R.; Vazquez Martinez, I. Género y Seguridad Alimentaria: Rol e Importancia de La Mujer En La Avicultura de Traspatio En Tetela de Ocampo, Puebla, México. Temas Cienc. Tecnol. 2019, 23, 33–40. [Google Scholar]
- Ahmed, S.; Begum, M.; Khatun, A.; Gofur, M.R.; Azad, M.T.; Kabir, A.; Haque, T.S. Family Poultry (FP) as a Tool for Improving Gender Equity and Women’s Empowerment in Developing Countries: Evidence from Bangladesh. Eur. J. Agric. Food Sci. 2021, 3, 37–44. [Google Scholar] [CrossRef]
- Kumar, M.; Dahiya, S.P.; Ratwan, P. Backyard Poultry Farming in India: A Tool for Nutritional Security and Women Empowerment. Biol. Rhythm. Res. 2021, 52, 1476–1491. [Google Scholar] [CrossRef]
- Di Pillo, F.; Anríquez, G.; Alarcón, P.; Jimenez-Bluhm, P.; Galdames, P.; Nieto, V.; Schultz-Cherry, S.; Hamilton-West, C. Backyard Poultry Production in Chile: Animal Health Management and Contribution to Food Access in an Upper Middle-Income Country. Prev. Vet. Med. 2019, 164, 41–48. [Google Scholar] [CrossRef]
- Ashenafi, H.; Tadesse, S.; Medhin, G.; Tibbo, M. Study on Coccidiosis of Scavenging Indigenous Chickens in Central Ethiopia. Trop. Anim. Health Prod. 2004, 36, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Yadav, A.; Sofi, O.M.U.D.; Kushwaha, A.; Yadav, V.; Rafiqi, S.I.; Godara, R.; Katoch, R. Prevalence and Molecular Characterization of Eimeria Species Affecting Backyard Poultry of Jammu Region, North India. Trop. Anim. Health Prod. 2022, 54, 296. [Google Scholar] [CrossRef] [PubMed]
- Lawal, J.R.; Jajere, S.M.; Ibrahim, U.I.; Geidam, Y.A.; Gulani, I.A.; Musa, G.; Ibekwe, B.U. Prevalence of Coccidiosis among Village and Exotic Breed of Chickens in Maiduguri, Nigeria. Vet. World 2016, 9, 653–659. [Google Scholar] [CrossRef]
- Sharma, S.; Iqbal, A.; Azmi, S.; Mushtaq, I.; Wani, Z.A.; Ahmad, S. Prevalence of Poultry Coccidiosis in Jammu Region of Jammu & Kashmir State. J. Parasit. Dis. 2015, 39, 85–89. [Google Scholar] [CrossRef]
- Andreopoulou, M.; Chaligiannis, I.; Sotiraki, S.; Daugschies, A.; Bangoura, B. Prevalence and Molecular Detection of Eimeria Species in Different Types of Poultry in Greece and Associated Risk Factors. Parasitol. Res. 2022, 121, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Godwin, R.M.; Morgan, J.A.T. A Molecular Survey of Eimeria in Chickens across Australia. Vet. Parasitol. 2015, 214, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rochell, S.J.; Parsons, C.M.; Dilger, R.N. Effects of Eimeria acervulina Infection Severity on Growth Performance, Apparent Ileal Amino Acid Digestibility, and Plasma Concentrations of Amino Acids, Carotenoids, and A1-Acid Glycoprotein in Broilers. Poult. Sci. 2016, 95, 1573–1581. [Google Scholar] [CrossRef]
- Dalibard, P.; Hess, V.; Tutour, L.; Peisker, M.; Peris, S.; Gutierrez, A.P.; Redshaw, M. (Eds.) Amino Acid in Animal Nutrition; EU Feed Additives and Premixtures Association (FEFANA): Brussels, Belgium, 2014; ISBN 9782960128932. [Google Scholar]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef]
- Hauck, R.; Carrisosa, M.; Mccrea, B.A.; Dormitorio, T.; Macklin, K.S. Evaluation of Next-Generation Amplicon Sequencing to Identify Eimeria spp. of Chickens. Avian Dis. 2019, 63, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hinsu, A.T.; Thakkar, J.R.; Koringa, P.G.; Vrba, V.; Jakhesara, S.J.; Psifidi, A.; Guitian, J.; Tomley, F.M.; Rank, D.N.; Raman, M.; et al. Illumina Next Generation Sequencing for the Analysis of Eimeria Populations in Commercial Broilers and Indigenous Chickens. Front. Vet. Sci. 2018, 5, 176. [Google Scholar] [CrossRef]
- Venkatas, J.; Adeleke, M.A. Emerging Threat of Eimeria Operational Taxonomic Units (OTUs) on Poultry Production. Parasitology 2019, 146, 1615–1619. [Google Scholar] [CrossRef]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and Biological Characterisation of Three Cryptic Eimeria Operational Taxonomic Units That Infect Chickens (Gallus gallus domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef]
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O.; Nduka, F.O.; Abah, A.E. Populations of Eimeria tenella Express Resistance to Commonly Used Anticoccidial Drugs in Southern Nigeria. Int. J. Vet. Sci. Med. 2018, 6, 192–200. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; Yang, X.; Ke, Q.; Lei, W.; Mughal, M.N.; Fang, R.; Zhou, Y.; Shen, B.; Zhao, J. Genetic Diversity and Drug Sensitivity Studies on Eimeria tenella Field Isolates from Hubei Province of China. Parasit. Vectors 2017, 10, 137. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, B.; Xu, L.; Zhao, Q.; Zhu, S.; Zhao, H.; Dong, H.; Han, H. Comparative Transcriptome Analyses of Drug-Sensitive and Drug-Resistant Strains of Eimeria tenella by RNA-Sequencing. J. Eukaryot. Microbiol. 2020, 67, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.C.; Joyner, L.P. The Development of Drug-Resistant Strains of Eimeria maxima in the Laboratory. Parasitology 1975, 71, 153–165. [Google Scholar] [CrossRef]
- Blake, D.P. Eimeria Genomics: Where Are We Now and Where Are We Going? Vet. Parasitol. 2015, 212, 68–74. [Google Scholar] [CrossRef]
- Soutter, F.; Werling, D.; Tomley, F.M.; Blake, D.P. Poultry Coccidiosis: Design and Interpretation of Vaccine Studies. Front. Vet. Sci. 2020, 7, 101. [Google Scholar] [CrossRef]
- Williams, R.B. Historical Article: Fifty Years of Anticoccidial Vaccines for Poultry (1952–2002). Avian Dis. 2002, 46, 775–802. [Google Scholar] [CrossRef]
- Wallach, M.G.; Ashash, U.; Michael, A.; Smith, N.C. Field Application of a Subunit Vaccine against an Enteric Protozoan Disease. PLoS ONE 2008, 3, e3948. [Google Scholar] [CrossRef]
- Gumina, E.; Hall, J.W.; Vecchi, B.; Hernandez-Velasco, X.; Lumpkins, B.; Mathis, G.; Layton, S. Evaluation of a Subunit Vaccine Candidate (Biotech Vac Cox) against Eimeria spp. in Broiler Chickens. Poult. Sci. 2021, 100, 101329. [Google Scholar] [CrossRef]
- Tomazic, M.L.; Marugan-Hernandez, V.; Rodriguez, A.E. Next-Generation Technologies and Systems Biology for the Design of Novel Vaccines Against Apicomplexan Parasites. Front. Vet. Sci. 2022, 8, 800361. [Google Scholar] [CrossRef]
- Heitlinger, E.; Spork, S.; Lucius, R.; Dieterich, C. The Genome of Eimeria falciformis—Reduction and Specialization in a Single Host Apicomplexan Parasite. BMC Genom. 2014, 15, 696. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Blake, D.P.; Ansari, H.R.; Billington, K.; Browne, H.P.; Bryant, J.; Dunn, M.; Hung, S.S.; Kawahara, F.; Miranda-Saavedra, D.; et al. Genomic Analysis of the Causative Agents of Coccidiosis in Domestic Chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, V.; Shirley, M.W.; Millard, B.J. A Comparative Study of Two Lines of Eimeria tenella Attenuated Either by Selection for Precocious Development in the Chicken or by Growth in Chicken Embryos. Avian Pathol. 1986, 15, 323–335. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.; Wisher, M.H.; Rose, M.E.; Jeffers, T.K. Eimeria tenella: Immunological Diversity between Asexual Generations. Parasite Immunol. 1988, 10, 649–660. [Google Scholar] [CrossRef]
- Belli, S.I.; Ferguson, D.J.P.; Katrib, M.; Slapetova, I.; Mai, K.; Slapeta, J.; Flowers, S.A.; Miska, K.B.; Tomley, F.M.; Shirley, M.W.; et al. Conservation of Proteins Involved in Oocyst Wall Formation in Eimeria maxima, Eimeria tenella and Eimeria acervulina. Int. J. Parasitol. 2009, 39, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Burrell, A.; Tomley, F.M.; Vaughan, S.; Marugan-Hernandez, V. Life Cycle Stages, Specific Organelles and Invasion Mechanisms of Eimeria Species. Parasitology 2020, 147, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.S.; Boothroyd, J.C. The C-Terminus of Toxoplasma RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex. PLoS Pathog. 2011, 7, e1001282. [Google Scholar] [CrossRef]
- Bangoura, B.; Daugschies, A. Eimeria. In Parasitic Protozoa of Farm Animals and Pets; Springer: Cham, Switzerland, 2018; pp. 55–92. ISBN 9783319701325. [Google Scholar]
- Liu, S.; Wang, L.; Zheng, H.; Xu, Z.; Roellig, D.M.; Li, N.; Frace, M.A.; Tang, K.; Arrowood, M.J.; Moss, D.M.; et al. Comparative Genomics Reveals Cyclospora cayetanensis Possesses Coccidia-like Metabolism and Invasion Components but Unique Surface Antigens. BMC Genom. 2016, 17, 316. [Google Scholar] [CrossRef]
- Tachado, S.D.; Mazhari-Tabrizi, R.; Schofield, L. Specificity in Signal Transduction among Glycosylphosphatidylinositols of Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. Parasite Immunol. 1999, 21, 609–617. [Google Scholar] [CrossRef]
- Gowda, D.C. TLR-Mediated Cell Signaling by Malaria GPIs. Trends Parasitol. 2007, 23, 596–604. [Google Scholar] [CrossRef]
- Debierre-Grockiego, F.; Campos, M.A.; Azzouz, N.; Schmidt, J.; Bieker, U.; Resende, M.G.; Mansur, D.S.; Weingart, R.; Schmidt, R.R.; Golenbock, D.T.; et al. Activation of TLR2 and TLR4 by Glycosylphosphatidylinositols Derived from Toxoplasma gondii. J. Immunol. 2007, 179, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, S. Major Surface Antigens in Zoonotic Babesia. Pathogens 2022, 11, 99. [Google Scholar] [CrossRef]
- Tabarés, E.; Ferguson, D.; Clark, J.; Soon, P.E.; Wan, K.L.; Tomley, F. Eimeria tenella Sporozoites and Merozoites Differentially Express Glycosylphosphatidylinositol-Anchored Variant Surface Proteins. Mol. Biochem. Parasitol. 2004, 135, 123–132. [Google Scholar] [CrossRef]
- Jahn, D.; Matros, A.; Bakulina, A.Y.; Tiedemann, J.; Schubert, U.; Giersberg, M.; Haehnel, S.; Zoufal, K.; Mock, H.P.; Kipriyanov, S.M. Model Structure of the Immunodominant Surface Antigen of Eimeria tenella Identified as a Target for Sporozoite-Neutralizing Monoclonal Antibody. Parasitol. Res. 2009, 105, 655–668. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.; Zhao, Q.; Dong, H.; Huang, B.; Zhao, H.; Li, Z.; Wang, L.; Han, H. Molecular Characterization of Surface Antigen 10 of Eimeria tenella. Parasitol. Res. 2019, 118, 2989–2999. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Tomley, F.M. Microneme Proteins in Apicomplexans. Subcell. Biochem. 2008, 47, 33–45. [Google Scholar] [CrossRef]
- Lovett, J.L.; Marchesini, N.; Moreno, S.N.J.; Sibley, L.D. Toxoplasma gondii Microneme Secretion Involves Intracellular Ca2+ Release from Inositol 1,4,5-Triphosphate (IP3)/Ryanodine-Sensitive Stores. J. Biol. Chem. 2002, 277, 25870–25876. [Google Scholar] [CrossRef]
- Donahue, C.; Carruthers, V.B.; Gilk, S.D.; Ward, G.E. The Toxoplasma Homolog of Plasmodium Apical Membrane Antigen-1 (AMA-1) Is a Microneme Protein Secreted in Response to Elevated Intracellular Calcium Levels. Mol. Biochem. Parasitol. 2000, 111, 15–30. [Google Scholar] [CrossRef]
- Tomley, F.M.; Bumstead, J.M.; Billington, K.J.; Dunn, P.P. Molecular Cloning and Characterization of a Novel Acidic Microneme Protein (Etmic-2) from the Apicomplexan Protozoan Parasite, Eimeria tenella. Mol. Biochem. Parasitol. 1996, 79, 195–206. [Google Scholar] [CrossRef]
- Tomley, F.M.; Billington, K.J.; Bumstead, J.M.; Clark, J.D.; Monaghan, P. EtMIC4: A Microneme Protein from Eimeria tenella That Contains Tandem Arrays of Epidermal Growth Factor-like Repeats and Thrombospondin Type-I Repeats. Int. J. Parasitol. 2001, 31, 1303–1310. [Google Scholar] [CrossRef]
- Gras, S.; Jackson, A.; Woods, S.; Pall, G.; Whitelaw, J.; Leung, J.M.; Ward, G.E.; Roberts, C.W.; Meissner, M. Parasites Lacking the Micronemal Protein MIC2 Are Deficient in Surface Attachment and Host Cell Egress, but Remain Virulent in Vivo. Wellcome Open Res. 2017, 2, 32. [Google Scholar] [CrossRef]
- Marugan-Hernandez, V.; Fiddy, R.; Nurse-Francis, J.; Smith, O.; Pritchard, L.; Tomley, F.M. Characterization of Novel Microneme Adhesive Repeats (MAR) in Eimeria tenella. Parasit. Vectors 2017, 10, 491. [Google Scholar] [CrossRef]
- Periz, J.; Gill, A.C.; Hunt, L.; Brown, P.; Tomley, F.M. The Microneme Proteins EtMIC4 and EtMIC5 of Eimeria tenella Form a Novel, Ultra-High Molecular Mass Protein Complex That Binds Target Host Cells. J. Biol. Chem. 2007, 282, 16891–16898. [Google Scholar] [CrossRef]
- Zhao, N.; Ming, S.; Sun, L.; Wang, B.; Li, H.; Zhang, X.; Zhao, X. Identification and Characterization of Eimeria tenella Microneme Protein (EtMIC8). Microbiol. Spectr. 2021, 9, e0022821. [Google Scholar] [CrossRef]
- Deans, J.A.; Alderson, T.; Thomas, A.W.; Mitchell, G.H.; Lennox, E.S.; Cohen, S. Rat Monoclonal Antibodies Which Inhibit the in Vitro Multiplication of Plasmodium knowlesi. Clin. Exp. Immunol. 1982, 49, 297–309. [Google Scholar]
- Macraild, C.A.; Anders, R.F.; Foley, M.; Norton, R.S. Apical Membrane Antigen. 1 as an Anti-Malarial Drug. Curr. Top. Med. Chem. 2011, 11, 2039–2047. [Google Scholar] [CrossRef]
- Gaffar, F.R.; Yatsuda, A.P.; Franssen, F.F.J.; de Vries, E. Erythrocyte Invasion by Babesia bovis Merozoites Is Inhibited by Polyclonal Antisera Directed against Peptides Derived from a Homologue of Plasmodium falciparum Apical Membrane Antigen 1. Infect. Immun. 2004, 72, 2947–2955. [Google Scholar] [CrossRef]
- Triglia, T.; Healer, J.; Caruana, S.R.; Hodder, A.N.; Anders, R.F.; Crabb, B.S.; Cowman, A.F. Apical Membrane Antigen 1 Plays a Central Role in Erythrocyte Invasion by Plasmodium Species. Mol. Microbiol. 2000, 38, 706–718. [Google Scholar] [CrossRef]
- Tonkin, M.L.; Crawford, J.; Lebrun, M.L.; Boulanger, M.J. Babesia divergens and Neospora caninum Apical Membrane Antigen 1 Structures Reveal Selectivity and Plasticity in Apicomplexan Parasite Host Cell Invasion. Protein Sci. 2013, 22, 114–127. [Google Scholar] [CrossRef]
- Blake, D.P.; Billington, K.J.; Copestake, S.L.; Oakes, R.D.; Quail, M.A.; Wan, K.L.; Shirley, M.W.; Smith, A.L. Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite. PLoS Pathog. 2011, 7, e1001279. [Google Scholar] [CrossRef]
- Yin, G.; Lin, Q.; Wei, W.; Qin, M.; Liu, X.; Suo, X.; Huang, Z. Protective Immunity against Eimeria tenella Infection in Chickens Induced by Immunisation with a Recombinant C-Terminal Derivative of EtIMP1. Vet. Immunol. Immunopathol. 2014, 162, 117–121. [Google Scholar] [CrossRef]
- Cui, X.; Lei, T.; Yang, D.; Hao, P.; Li, B.; Liu, Q. Toxoplasma gondii Immune Mapped Protein-1 (TgIMP1) Is a Novel Vaccine Candidate against Toxoplasmosis. Vaccine 2012, 30, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lei, T.; Yang, D.Y.; Hao, P.; Liu, Q. Identification and Characterization of a Novel Neospora caninum Immune Mapped Protein 1. Parasitology 2012, 139, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Olajide, J.S.; Qu, Z.; Yang, S.; Oyelade, O.J.; Cai, J. Eimeria Proteins: Order amidst Disorder. Parasit. Vectors 2022, 15, 38. [Google Scholar] [CrossRef]
- Jenkins, M.C.; Danforth, H.D.; Lillehoj, H.S.; Fetterer, R.H. CDNA Encoding an Immunogenic Region of a 22 Kilodalton Surface Protein of Eimeria acervulina Sporozoites. Mol. Biochem. Parasitol. 1989, 32, 153–161. [Google Scholar] [CrossRef]
- Jenkins, M.; Lillehoj, H.S.; Dame, A.B.; Jenkins, M.C. Eimeria acervulina: DNA Cloning and Characterization of Recombinant Sporozoite and Merozoite Antigens. Exp. Parasitol. 1988, 66, 96–107. [Google Scholar] [CrossRef]
- Laurent, F.; Bourdieu, C.; Kazanji, M.; Yvore, P.; Pery, P. The Immunodominant Eimer acervulina Sporozoite Antigen Previously Described as P160/P240 Is a 19-Kilodalton Antigen Present in Several Eimeria Species. Mol. Biochem. Parasitol. 1994, 63, 79–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Lu, Y.; Amer, S.; Xu, P.; Wang, J.; Lu, J.; Bao, Y.; Deng, B.; He, H.; et al. Prokaryotic Expression and Identification of 3-1E Gene of Merozoite Surface Antigen of Eimeria acervulina. Parasitol. Res. 2011, 109, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Choi, K.D.; Jenkins, M.C.; Vakharia, V.N.; Song, K.D.; Han, J.Y.; Lillehoj, E.P. A Recombinant Eimeria Protein Inducing Interferon-Gamma Production: Comparison of Different Gene Expression Systems and Immunisation Strategies for Vaccination against Coccidiosis. Avian Dis. 2000, 44, 379–389. [Google Scholar] [CrossRef]
- Ma, D.; Ma, C.; Gao, M.; Li, G.; Niu, Z.; Huang, X. Induction of Cellular Immune Response by DNA Vaccine Coexpressing, E. acervulina 3-1E Gene and Mature CHIl-15 Gene. J. Parasitol. Res. 2012, 2012, 654279. [Google Scholar] [CrossRef]
- Yuelan, Z.; Yiwei, L.; Liyuan, L.; Yue, Z.; Wenbo, C.; Yongzhan, B.; Jianhua, Q. Expression and Identification of the ADF-Linker-3-1E Gene of Eimeria acervulina of Chicken. Parasitol. Res. 2016, 115, 1641–1647. [Google Scholar] [CrossRef]
- Wang, P.; Jia, Y.; Han, Y.; Wang, W.; Zhu, Y.; Xu, J.; Guan, C.; Ying, J.; Deng, S.; Wang, J.; et al. Eimeria acervulina Microneme Protein 3 Inhibits Apoptosis of the Chicken Duodenal Epithelial Cell by Targeting the Casitas B-Lineage Lymphoma Protein. Front. Vet. Sci. 2021, 8, 636809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Huang, J.W.; Li, M.H.; Sui, Y.X.; Wang, S.; Liu, L.R.; Xu, L.X.; Yan, R.F.; Song, X.K.; Li, X.R. Identification and Molecular Characterization of Microneme 5 of Eimeria acervulina. PLoS ONE 2014, 9, e115411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Z.; Huang, J.; Sun, X.; Haseeb, M.; Ahmed, S.; Shah, M.A.A.; Yan, R.; Song, X.; Xu, L.; et al. Molecular Characterization of a Potential Receptor of Eimeria acervulina Microneme Protein 3 from Chicken Duodenal Epithelial Cells. Parasite 2020, 27, 18. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Huang, J.; Wang, S.; Lu, M.; Song, X.; Xu, L.; Yan, R.; Li, X. The Molecular Characterization and Immune Protection of Microneme 2 of Eimeria acervulina. Vet. Parasitol. 2016, 215, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, S.; Huang, J.; Liu, L.; Lu, M.; Li, M.; Sui, Y.; Xu, L.; Yan, R.; Song, X.; et al. Proteomic Analysis of Eimeria acervulina Sporozoite Proteins Interaction with Duodenal Epithelial Cells by Shotgun LC–MS/MS. Mol. Biochem. Parasitol. 2015, 202, 29–33. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Liu, J.; Li, W.; Ji, Y.; Tian, D.; Tian, L.; Yang, X.; Xu, L.; Yan, R.; et al. Identification of Common Immunodominant Antigens of Eimeria tenella, Eimeria acervulina and Eimeria maxima by Immunoproteomic Analysis. Oncotarget 2017, 8, 34935–34945. [Google Scholar] [CrossRef]
- Hoan, T.D.; Thao, D.T.; Gadahi, J.A.; Song, X.; Xu, L.; Yan, R.; Li, X. Analysis of Humoral Immune Response and Cytokines in Chickens Vaccinated with Eimeria brunetti Apical Membrane Antigen-1 (EbAMA1) DNA Vaccine. Exp. Parasitol. 2014, 144, 65–72. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Kim, S.; Billington, K.; Bumstead, J.; Marugán-Hernández, V.; Küster, T.; Ferguson, D.J.P.; Vervelde, L.; Blake, D.P.; Tomley, F.M. Development of Cross-Protective Eimeria-Vectored Vaccines Based on Apical Membrane Antigens. Int. J. Parasitol. 2018, 48, 505–518. [Google Scholar] [CrossRef]
- Chen, H.; Huang, C.; Chen, Y.; Mohsin, M.; Li, L.; Lin, X.; Huang, Z.; Yin, G. Efficacy of Recombinant N- and C-Terminal Derivative of EmIMP1 against E. maxima Infection in Chickens. Br. Poult. Sci. 2020, 61, 518–522. [Google Scholar] [CrossRef]
- Jenkins, M.C.; Fetterer, R.; Miska, K.; Tuo, W.; Kwok, O.; Dubey, J.P. Characterization of the Eimeria maxima Sporozoite Surface Protein IMP1. Vet. Parasitol. 2015, 211, 146–152. [Google Scholar] [CrossRef]
- Yang, X.C.; Li, M.H.; Liu, J.H.; Ji, Y.H.; Li, X.R.; Xu, L.X.; Yan, R.F.; Song, X.K. Identification of Immune Protective Genes of Eimeria maxima through CDNA Expression Library Screening. Parasit. Vectors 2017, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, J.; Li, Y.; Ehsan, M.; Wang, S.; Zhou, Z.; Song, X.; Yan, R.; Xu, L.; Li, X. Molecular Characterisation and the Protective Immunity Evaluation of Eimeria maxima Surface Antigen Gene. Parasit. Vectors 2018, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, J.; Tian, D.; Li, W.; Zhou, Z.; Huang, J.; Song, X.; Xu, L.; Yan, R.; Li, X. The Molecular Characterization and Protective Efficacy of Microneme 3 of Eimeria mitis in Chickens. Vet. Parasitol. 2018, 258, 114–123. [Google Scholar] [CrossRef]
- Song, X.; Gao, Y.; Xu, L.; Yan, R.; Li, X. Partial Protection against Four Species of Chicken Coccidia Induced by Multivalent Subunit Vaccine. Vet. Parasitol. 2015, 212, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Xu, Z.; Tuo, W.; Li, C.; Lillehoj, H.; Wan, G.; Gong, H.; Huang, J.; Tian, G.; Li, S.; et al. Immunoproteomic Analysis of the Sporozoite Antigens of Eimeria necatrix. Vet. Parasitol. 2022, 301, 109642. [Google Scholar] [CrossRef]
- Zhi, W.; Chen, H.; Bai, B.; Jia, Z.; Pan, X.; Wang, B.; Kong, R.; Liu, Q.; Ma, C.; Ma, D. Combined Oral Immunisation with Probiotics Entercoccus Faecalis Delivering Surface-Anchored Eimeria tenella Proteins Provide Protective Efficacies against Homologous Infection in Chickens. Front. Immunol. 2022, 13, 1042143. [Google Scholar] [CrossRef]
- Huang, J.; Chen, S.; Zhou, Z.; Sun, X.; Haseeb, M.; Lakho, S.A.; Zhang, Y.; Liu, J.; Shah, M.A.A.; Song, X.; et al. Poly (D, L-Lactide-Co-Glycolide) Delivery System Improve the Protective Efficacy of Recombinant Antigen TA4 against Eimeria tenella Infection. Poult. Sci. 2021, 100, 101083. [Google Scholar] [CrossRef]
- Han, H.Y.; Lin, J.J.; Zhao, Q.P.; Dong, H.; Jiang, L.L.; Xu, M.Q.; Zhu, S.H.; Huang, B. Identification of Differentially Expressed Genes in Early Stages of Eimeria tenella by Suppression Subtractive Hybridization and CDNA Microarray. J. Parasitol. 2010, 96, 95–102. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Zhang, T.; Liu, J.; Liu, Q. Evaluation of 4 Merozoite Antigens as Candidate Vaccines against Eimeria tenella Infection. Poult. Sci. 2021, 100, 100888. [Google Scholar] [CrossRef]
- Chow, Y.P.; Wan, K.L.; Blake, D.P.; Tomley, F.; Nathan, S. Immunogenic Eimeria tenella Glycosylphosphatidylinositol-Anchored Surface Antigens (SAGs) Induce Inflammatory Responses in Avian Macrophages. PLoS ONE 2011, 6, e25233. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Y.; Zhou, Y.; Zhao, J.; Fang, R. In Vivo Immunoprotective Comparison between Recombinant Protein and DNA Vaccine of Eimeria tenella Surface Antigen 4. Vet. Parasitol. 2020, 278, 109032. [Google Scholar] [CrossRef]
- Geng, T.; Luo, L.; Wang, Y.; Shen, B.; Fang, R.; Hu, M.; Zhao, J.; Zhou, Y. Evaluation of Immunoprotective Effects of Recombinant Proteins and DNA Vaccines Derived from Eimeria tenella Surface Antigen 6 and 15 in Vivo. Parasitol. Res. 2022, 121, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B.; et al. RNA Seq Analysis of the Eimeria tenella Gametocyte Transcriptome Reveals Clues about the Molecular Basis for Sexual Reproduction and Oocyst Biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef]
- Ramly, N.Z.; Dix, S.R.; Ruzheinikov, S.N.; Sedelnikova, S.E.; Baker, P.J.; Chow, Y.P.; Tomley, F.M.; Blake, D.P.; Wan, K.L.; Nathan, S.; et al. The Structure of a Major Surface Antigen SAG19 from Eimeria tenella Unifies the Eimeria SAG Family. Commun. Biol. 2021, 4, 376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Q.; Zhu, S.; Huang, B.; Yu, S.; Liang, S.; Wang, H.; Zhao, H.; Han, H.; Dong, H. Further Investigation of the Characteristics and Biological Function of Eimeria tenella Apical Membrane Antigen 1. Parasite 2020, 27, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, S.; Zhao, Q.; Huang, B.; Yu, S.; Yu, Y.; Liang, S.; Wang, H.; Zhao, H.; Han, H.; et al. Identification and Characterization of a Novel Apical Membrane Antigen 3 in Eimeria tenella. J. Eukaryot. Microbiol. 2021, 68, e12836. [Google Scholar] [CrossRef] [PubMed]
- Tomley, F.M.; Soldati, D.S. Mix and Match Modules: Structure and Function of Microneme Proteins in Apicomplexan Parasites. Trends Parasitol. 2001, 17, 81–88. [Google Scholar] [CrossRef]
- Hoan, T.D.; Zhang, Z.; Huang, J.; Yan, R.; Song, X.; Xu, L.; Li, X. Identification and Immunogenicity of Microneme Protein 2 (EbMIC2) of Eimeria brunetti. Exp. Parasitol. 2016, 162, 7–17. [Google Scholar] [CrossRef]
- da Silva, J.T.; Alvares, F.B.V.; de Lima, E.F.; Filho, G.M.d.S.; da Silva, A.L.P.; Lima, B.A.; Feitosa, T.F.; Vilela, V.L.R. Prevalence and Diversity of Eimeria spp. in Free-Range Chickens in Northeastern Brazil. Front. Vet. Sci. 2022, 9, 1031330. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, Q.; Li, M.; Sun, X.; Lu, M.; Yan, R.; Song, X.; Li, X. Pathogenic Effects of Single or Mixed Infections of Eimeria mitis, Eimeria necatrix, and Eimeria tenella in Chickens. Vet. Sci. 2022, 9, 657. [Google Scholar] [CrossRef]
- Su, S.; Hou, Z.; Liu, D.; Jia, C.; Wang, L.; Xu, J.; Tao, J. Comparative Transcriptome Analysis of Second- and Third-Generation Merozoites of Eimeria necatrix. Parasit. Vectors 2017, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- 105. Basic Local Alignment Search Tool. National Library of Medicine. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 19 December 2022).
- Brothers, V.M.; Kuhn, I.; Paul, L.S.; Gabe, J.D.; Andrews, W.H.; Sias, S.R.; McCaman, M.T.; Dragon, E.A.; Files, J.G. Characterization of a Surface Antigen of Eimeria tenella Sporozoites and Synthesis from a Cloned CDNA in Escherichia coli. Mol. Biochem. Parasitol. 1988, 28, 235–247. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Grigg, M.E.; Boothroyd, J.C.; Garcia, K.C. Structure of the Immunodominant Surface Antigen from the Toxoplasma gondii SRS Superfamily. Nat. Struct. Biol. 2002, 9, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Võ, T.C.; Naw, H.; Flores, R.A.; Lê, H.G.; Kang, J.M.; Yoo, W.G.; Kim, W.H.; Min, W.; Na, B.K. Genetic Diversity of Microneme Protein 2 and Surface Antigen 1 of Eimeria tenella. Genes 2021, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, L.; Yan, F.; Yan, R.; Song, X.; Li, X. Immunoproteomic Analysis of the Second-Generation Merozoite Proteins of Eimeria tenella. Vet. Parasitol. 2009, 164, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Fritig, B.; Mosinger, E. Pathogenesis-Related PR-1 Proteins Are Antifungal (Isolation and Characterization of Three 14-Kilodalton Proteins of Tomato and of a Basic PR-1 of Tobacco with Inhibitory Activity against Phytophthora Infestans). Plant. Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P.; et al. Nitric Oxide and Salicylic Acid Signaling in Plant Defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, J.; Krätzschmar, J.; Haendler, B.; el-Hayek, R.; Mochca-Morales, J.; Martin, B.M.; Patel, J.R.; Moss, R.L.; Schleuning, W.D.; Coronado, R. Primary Structure and Properties of Helothermine, a Peptide Toxin That Blocks Ryanodine Receptors. Biophys. J. 1995, 68, 2280–2288. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Q.; Zhu, S.; Wang, Q.; Wang, H.; Yu, S.; Yu, Y.; Liang, S.; Zhao, H.; Huang, B.; et al. Eimeria tenella Eimeria-Specific Protein That Interacts with Apical Membrane Antigen 1 (EtAMA1) Is Involved in Host Cell Invasion. Parasit. Vectors 2020, 13, 373. [Google Scholar] [CrossRef]
- Karsten, V.; Qi, H.; Beckers, C.J.; Reddy, A.; Dubremetz, J.F.; Webster, P.; Joiner, K.A. The Protozoan Parasite Toxoplasma gondii Targets Proteins to Dense Granules and the Vacuolar Space Using Both Conserved and Unusual Mechanisms. J. Cell Biol. 1998, 141, 1323–1333. [Google Scholar] [CrossRef]
- Marugan-Hernandez, V.; Long, E.; Blake, D.; Crouch, C.; Tomley, F. Eimeria tenella Protein Trafficking: Differential Regulation of Secretion versus Surface Tethering during the Life Cycle. Sci. Rep. 2017, 7, 4557. [Google Scholar] [CrossRef] [PubMed]
- Delorenzi, M.; Sexton, A.; Shams-Eldin, H.; Schwarz, R.T.; Speed, T.; Schofield, L. Genes for Glycosylphosphatidylinositol Toxin Biosynthesis in Plasmodium falciparum. Infect. Immun. 2002, 70, 4510–4522. [Google Scholar] [CrossRef] [PubMed]
- Basagoudanavar, S.H.; Feng, X.; Krishnegowda, G.; Muthusamy, A.; Gowda, D.C. Plasmodium falciparum GPI Mannosyltransferase-III Has Novel Signature Sequence and Is Functional. Biochem. Biophys. Res. Commun. 2007, 364, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.E.; Couto, A.; Echaide, I.; Schnittger, L.; Florin-Christensen, M. Babesia bovis Contains an Abundant Parasite-Specific Protein-Free Glycerophosphatidylinositol and the Genes Predicted for Its Assembly. Vet. Parasitol. 2010, 167, 227–235. [Google Scholar] [CrossRef]
- Blake, D.P.; Pastor-Fernández, I.; Nolan, M.J.; Tomley, F.M. Recombinant Anticoccidial Vaccines—A Cup Half Full? Infect. Genet. Evol. 2017, 55, 358–365. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, S.; Huang, J.; Ding, W.; Chen, Y.; Su, J.; Yan, R.; Xu, L.; Song, X.; Li, X. A Multiepitope Vaccine Encoding Four Eimeria Epitopes with PLGA Nanospheres: A Novel Vaccine Candidate against Coccidiosis in Laying Chickens. Vet. Res. 2022, 53, 27. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, R.; Wang, S.; Song, X.; Xu, L.; Li, X. Identification and Molecular Characterization of a Novel Antigen of Eimeria acervulina. Mol. Biochem. Parasitol. 2012, 186, 21–28. [Google Scholar] [CrossRef]
- Mesa, C.; Gómez-Osorio, L.M.; López-Osorio, S.; Williams, S.M.; Chaparro-Gutiérrez, J.J. Survey of Coccidia on Commercial Broiler Farms in Colombia: Frequency of Eimeria Species, Anticoccidial Sensitivity, and Histopathology. Poult. Sci. 2021, 100, 101239. [Google Scholar] [CrossRef]
- Jang, S.I.; Lillehoj, H.S.; Lee, S.H.; Lee, K.W.; Park, M.S.; Cha, S.R.; Lillehoj, E.P.; Subramanian, B.M.; Sriraman, R.; Srinivasan, V.A. Eimeria Maxima Recombinant Gam82 Gametocyte Antigen Vaccine Protects against Coccidiosis and Augments Humoral and Cell-Mediated Immunity. Vaccine 2010, 28, 2980–2985. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Tao, J. Efficacy of a DNA Vaccine Carrying Eimeria Maxima Gam56 Antigen Gene against Coccidiosis in Chickens. Korean J. Parasitol. 2013, 51, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Hou, Z.; Liu, D.; Jia, C.; Wang, L.; Xu, J.; Tao, J. Comparative Transcriptome Analysis of Eimeria Necatrix Third-Generation Merozoites and Gametocytes Reveals Genes Involved in Sexual Differentiation and Gametocyte Development. Vet. Parasitol. 2018, 252, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Fu, J.; Zhang, K.; Wang, X.; Liu, Y.; Zhang, H.; Fan, C.; Fei, C.; Xue, F. Proteomic Analysis of the Effect of Diclazuril on Second-Generation Merozoites of Eimeria tenella. Parasitol. Res. 2014, 113, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Belachew, E.B. Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites. J. Immunol. Res. 2018, 2018, 6529681. [Google Scholar] [CrossRef]
- Gordon, D.M.; Mc Govern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; La Chance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, Immunogenicity, and Efficacy of a Recombinantly Produced Plasmodium falciparum Circumsporozoite Protein-Hepatitis b Surface Antigen Subunit Vaccine. J. Infect. Dis. 1995, 171, 1576–1585. [Google Scholar] [CrossRef]
- Laurens, M.B. RTS, S/AS01 Vaccine (MosquirixTM): An Overview. Hum. Vaccin. Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef]
- Blake, D.P.; Clark, E.L.; Macdonald, S.E.; Thenmozhi, V.; Kundu, K.; Garg, R.; Jatau, I.D.; Ayoade, S.; Kawahara, F.; Moftah, A.; et al. Population, Genetic, and Antigenic Diversity of the Apicomplexan Eimeria tenella and Their Relevance to Vaccine Development. Proc. Natl. Acad. Sci. USA 2015, 112, E5343–E5350. [Google Scholar] [CrossRef]
- Kundu, K.; Garg, R.; Kumar, S.; Mandal, M.; Tomley, F.M.; Blake, D.P.; Banerjee, P.S. Humoral and Cytokine Response Elicited during Immunisation with Recombinant Immune Mapped Protein-1 (EtIMP-1) and Oocysts of Eimeria tenella. Vet. Parasitol. 2017, 244, 44–53. [Google Scholar] [CrossRef]
- Kim, S.-K.; Boothroyd, J.C. Stage-Specific Expression of Surface Antigens by Toxoplasma gondii as a Mechanism to Facilitate Parasite Persistence. J. Immunol. 2005, 174, 8038–8048. [Google Scholar] [CrossRef]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial Drugs of the Livestock Industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britez, J.D.; Rodriguez, A.E.; Di Ciaccio, L.; Marugán-Hernandez, V.; Tomazic, M.L. What Do We Know about Surface Proteins of Chicken Parasites Eimeria? Life 2023, 13, 1295. https://doi.org/10.3390/life13061295
Britez JD, Rodriguez AE, Di Ciaccio L, Marugán-Hernandez V, Tomazic ML. What Do We Know about Surface Proteins of Chicken Parasites Eimeria? Life. 2023; 13(6):1295. https://doi.org/10.3390/life13061295
Chicago/Turabian StyleBritez, Jesica Daiana, Anabel Elisa Rodriguez, Lucía Di Ciaccio, Virginia Marugán-Hernandez, and Mariela Luján Tomazic. 2023. "What Do We Know about Surface Proteins of Chicken Parasites Eimeria?" Life 13, no. 6: 1295. https://doi.org/10.3390/life13061295
APA StyleBritez, J. D., Rodriguez, A. E., Di Ciaccio, L., Marugán-Hernandez, V., & Tomazic, M. L. (2023). What Do We Know about Surface Proteins of Chicken Parasites Eimeria? Life, 13(6), 1295. https://doi.org/10.3390/life13061295









