Plumbagin, a Natural Compound with Several Biological Effects and Anti-Inflammatory Properties
Abstract
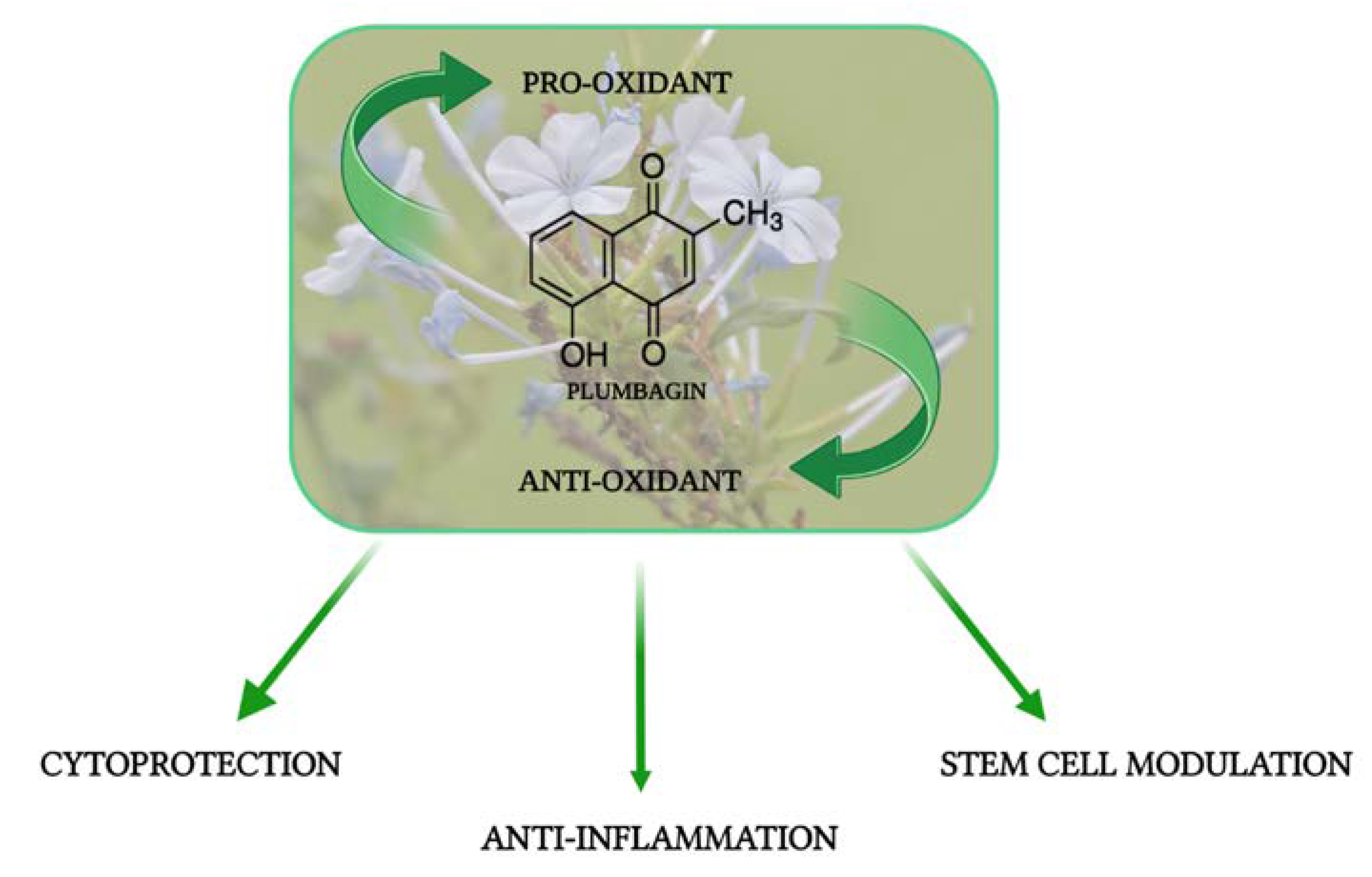
:1. Introduction
2. The Cytoprotective Activity of PB
3. The Anti-Inflammatory Activity of PB
3.1. PB in Osteoarthritis, Rheumatoid Arthritis, and Osteoporosis
3.2. The Antifibrotic Activity of PB
3.3. Antibacterial and Antiparasitic Activity of PB
4. The Role of PB in Stem Cells and in Cell Senescence
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef]
- Shivani, D.; Venkatesh; D’Souza, R.; Shenoy, B.D.; Udupi, R.H.; Udupa, N. Niosomal delivery of plumbagin ester for better anti-fertility activity. Indian Drugs 2002, 39, 163–165. [Google Scholar]
- Ahmad, I.; Mehmood, Z.; Mohammad, F.; Ahmad, S. Antimicrobial potency and synergistic activity of five traditionally used Indian medicinal plants. J. Med. Aromat. Plant Sci. 2000, 23, 173–176. [Google Scholar]
- Jeyachandran, R.; Mahesh, A.; Cindrella, L.; Sudhakar, S.; Pazhanichamy, K. Antibacterial activity of plumbagin and root extracts of Plumbago zeylanica L. Acta Biol. Crac. Ser. Bot. 2009, 51, 17–22. [Google Scholar]
- Oyedapo, O.O. Studies on Bioactivity of the Root Extract of Plumbago zeylanica. Int. J. Pharmacogn. 1996, 34, 365–369. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Ahmad, M.I. Plumbagin and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 229–246. [Google Scholar] [CrossRef]
- Shukla, B.; Saxena, S.; Usmani, S.; Kushwaha, P. Phytochemistry and pharmacological studies of Plumbago zeylanica L.: A medicinal plant review. Clin. Phytosci. 2021, 7, 34. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Zhong, W.; Fischer, J.W.; Mustafa, A.; Shi, X.; Meske, L.; Hong, H.; Cai, W.; Havighurst, T.; Kim, K.; et al. Plumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3M-luciferase cells in an orthotopic xenograft mouse model. Mol. Oncol. 2013, 7, 428–439. [Google Scholar] [CrossRef]
- Yadav, A.M.; Bagade, M.M.; Ghumnani, S.; Raman, S.; Saha, B.; Kubatzky, K.F.; Ashma, R. The phytochemical plumbagin reciprocally modulates osteoblasts and osteoclasts. Biol. Chem. 2021, 403, 211–229. [Google Scholar] [CrossRef]
- Hsieh, Y.J.; Lin, L.C.; Tsai, T.H. Measurement and pharmacokinetic study of plumbagin in a conscious freely moving rat using liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 844, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.A.; Snima, K.S.; Kamath, R.C.; Nair, S.V.; Lakshmanan, V.K. Plumbagin nanoparticles induce dose and pH dependent toxicity on prostate cancer cells. Curr. Drug Deliv. 2015, 12, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Thakor, N.; Janathia, B. Plumbagin: A potential candidate for future research and development. Curr. Pharm. Biotechnol. 2022, 23, 1800–1812. [Google Scholar] [CrossRef]
- Sukkasem, N.; Chatuphonprasert, W.; Tatiya-Aphiradee, N.; Jarukamjorn, K. Imbalance of the antioxidative system by plumbagin and Plumbago indica L. extract induces hepatotoxicity in mice. J. Intercult. Ethnopharmacol. 2016, 24, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural plants compounds as modulators of epithelial-to-mesenchymal transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer effects and mechanisms of action of plumbagin: Review of research advances. Biomed. Res. Int. 2020, 2020, 6940953. [Google Scholar] [CrossRef]
- Luo, P.; Wong, Y.F.; Ge, L.; Zhang, Z.F.; Liu, Y.; Liu, L.; Zhou, H. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor-κB activation. J. Pharmacol. Exp. Ther. 2010, 335, 735–742. [Google Scholar] [CrossRef]
- Wang, T.; Wu, F.; Jin, Z.; Zhai, Z.; Wang, Y.; Tu, B.; Yan, W.; Tang, T. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem. Toxicol. 2014, 64, 177–183. [Google Scholar] [CrossRef]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M.; Sandur, S.K.; Sainis, K.B.; Poduval, T.B. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via NF-kappaB suppression. Inflammation 2014, 37, 542–554. [Google Scholar] [CrossRef]
- Guo, Y.X.; Liu, L.; Yan, D.Z.; Guo, J.P. Plumbagin prevents osteoarthritis in human chondrocytes through Nrf-2 activation. Mol. Med. Rep. 2017, 15, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Kuan-Hong, W.; Bai-Zhou, L. Plumbagin protects against hydrogen peroxide-induced neurotoxicity by modulating NF-κB and Nrf-2. Arch. Med. Sci. 2018, 14, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, L.; Hou, Y.; Si, M.; Zhao, Y.P.; Nie, L. Plumbagin protects against spinal cord injury-induced oxidative stress and inflammation in wistar rats through Nrf-2 upregulation. Drug Res. 2015, 65, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, D.; Yang, J.Y.; Yan, T.B. Plumbagin protects against glucocorticoid-induced osteoporosis through Nrf-2 pathway. Cell Stress Chaperones 2015, 20, 621–629. [Google Scholar] [CrossRef]
- Chu, H.; Yu, H.; Ren, D.; Zhu, K.; Huang, H. Plumbagin exerts protective effects in nucleus pulposus cells by attenuating hydrogen peroxide-induced oxidative stress, inflammation and apoptosis through NF-κB and Nrf-2. Int. J. Mol. Med. 2016, 37, 1669–1676. [Google Scholar] [CrossRef]
- Zaki, A.M.; El-Tanbouly, D.M.; Abdelsalam, R.M.; Zaki, H.F. Plumbagin ameliorates hepatic ischemia-reperfusion injury in rats: Role of high mobility group box 1 in inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2018, 106, 785–793. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, J.G.; Wu, L. Plumbagin inhibits neuronal apoptosis, intimal hyperplasia and also suppresses TNF-α/NF-κB pathway induced inflammation and matrix metalloproteinase-2/9 expression in rat cerebral ischemia. Saudi J. Biol. Sci. 2018, 25, 1033–1039. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, S.; Zheng, W.; Fu, H.; Wu, T.; Hu, F. Plumbagin attenuated oxygen-glucose deprivation/reoxygenation-induced injury in human SH-SY5Y cells by inhibiting NOX4-derived ROS-activated NLRP3 inflammasome. Biosci. Biotechnol. Biochem. 2020, 84, 134–142. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Hrelia, S. Combined treatment with three natural antioxidants enhances neuroprotection in a SH-SY5Y 3D culture model. Antioxidants 2019, 8, 420. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, Y.; Wang, D.; Cheng, X.; Yang, F.; Zhang, Q.; Xue, Z.; Li, Y.; Zhang, L.; et al. Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production. Oncotarget 2016, 7, 82864–82875. [Google Scholar] [CrossRef]
- Pan, P.H.; Wang, Y.Y.; Lin, S.Y.; Liao, S.L.; Chen, Y.F.; Huang, W.C.; Chen, C.J.; Chen, W.Y. Plumbagin ameliorates bile duct ligation-induced cholestatic liver injury in rats. Biomed. Pharmacother. 2022, 151, 113133. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; MalcolmWalker, J.; Yellon, D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chinnathambi, A.; Ali Alharbi, S.; Yin, F. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats. Environ. Toxicol. 2020, 35, 1374–1385. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, H.; Gong, W.; Cao, F.; Wu, T.; Hu, F. Plumbagin protects H9c2 cardiomyocytes against TBHP-induced cytotoxicity by alleviating ROS-induced apoptosis and modulating autophagy. Exp. Ther. Med. 2022, 24, 501. [Google Scholar] [CrossRef]
- Haddad, J.J.; Harb, H.L. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: A signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 2005, 42, 987–1014. [Google Scholar] [CrossRef]
- Checker, R.; Sharma, D.; Sandur, S.K.; Subrahmanyam, G.; Krishnan, S.; Poduval, T.B.; Sainis, K.B. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J. Cell. Biochem. 2010, 110, 1082–1093. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, W.; Kang, R.; Xie, M.; Billiar, T.; Wang, H.; Cao, L.; Tang, D. Plumbagin protects mice from lethal sepsis by modulating immunometabolism upstream of PKM2. Mol. Med. 2016, 22, 162–172. [Google Scholar] [CrossRef]
- Arruri, V.; Komirishetty, P.; Areti, A.; Dungavath, S.K.N.; Kumar, A. Nrf2 and NF-κB modulation by Plumbagin attenuates functional, behavioural and biochemical deficits in rat model of neuropathic pain. Pharmacol. Rep. 2017, 69, 625–632. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Mendonca, P.; Kolta, M.G.; Soliman, K.F.A. The attenuating effects of plumbagin on pro-inflammatory cytokine expression in LPS-activated BV-2 microglial cells. J. Neuroimmunol. 2017, 313, 129–137. [Google Scholar] [CrossRef]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and spiny burs of Castanea Sativa from an experimental chestnut grove: Metabolomic analysis and anti-neuroinflammatory activity. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Mandrone, M.; Chiocchio, I.; Zambonin, L.; Barbalace, M.C.; Zalambani, C.; Angeloni, C.; Malaguti, M.; Prata, C.; Poli, F.; et al. By-product extracts from Castanea sativa counteract hallmarks of neuroinflammation in a microglial model. Antioxidants 2023, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, I.S.; Hatmal, M.M.; Abuarqoub, D.; Esawi, E.; Zalloum, H.; Wehaibi, S.; Nsairat, H.; Alshaer, W. 1,4-naphthoquinone is a potent inhibitor of IRAK1 kinases and the production of inflammatory cytokines in THP-1 differentiated macrophages. ACS Omega 2021, 6, 25299–25310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Osteoarthritis year in review 2021: Biology. Osteoarthr. Cartil. 2022, 30, 207–215. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’Leary, C. Repositioning natural antioxidants for therapeutic applications in tissue engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Agarwal, S.; Misra, R.; Aggarwal, A. Synovial fluid RANKL and matrix metalloproteinase levels in enthesitis related arthritis subtype of juvenile idiopathic arthritis. Rheumatol. Int. 2009, 29, 907–911. [Google Scholar] [CrossRef]
- Chemel, M.; Le Goff, B.; Brion, R.; Cozic, C.; Berreur, M.; Amiaud, J.; Bougras, G.; Touchais, S.; Blanchard, F.; Heymann, M.F.; et al. Interleukin 34 expression is associated with synovitis severity in rheumatoid arthritis patients. Ann. Rheum. Dis. 2012, 71, 150–154. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Ainola, M.; Mandelin, J.; Liljeström, M.; Konttinen, Y.T.; Salo, J. Imbalanced expression of RANKL and osteoprotegerin mRNA in pannus tissue of rheumatoid arthritis. Clin. Exp. Rheumatol. 2008, 26, 240–246. [Google Scholar]
- Hensvold, A.H.; Joshua, V.; Li, W.; Larkin, M.; Qureshi, F.; Israelsson, L.; Padyukov, L.; Lundberg, K.; Defranoux, N.; Saevarsdottir, S.; et al. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res. Ther. 2015, 17, 239. [Google Scholar] [CrossRef]
- Abimannan, T.; Peroumal, D.; Parida, J.R.; Barik, P.K.; Padhan, P.; Devadas, S. Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radic. Biol. Med. 2016, 99, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Y.; Li, X.; Lei, Y.M.; Xia, L.P.; Lu, J.; Shen, H. Effects of IL-34 on the secretion of RANKL/OPG by fibroblast-like synoviocytes and peripheral blood mononuclear cells in rheumatoid arthritis. Eur. Cytokine Netw. 2019, 30, 67–73. [Google Scholar] [CrossRef]
- Weinstein, R.S. Clinical practice. Glucocorticoid-induced bone disease. N. Engl. J. Med. 2011, 365, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.; Alhawagri, M.; Hagen-Stapleton, A.; Kitaura, H.; Kanagawa, O.; Novack, D.V. NF-(kappa)B-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J. Clin. Investig. 2005, 115, 1848–1854. [Google Scholar] [CrossRef]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of TNF-induced osteoclast differentiation. Cells 2021, 11, 132. [Google Scholar] [CrossRef]
- Shen, G.; Liu, X.; Lei, W.; Duan, R.; Yao, Z. Plumbagin is a NF-κB-inducing kinase inhibitor with dual anabolic and antiresorptive effects that prevents menopausal-related osteoporosis in mice. J. Biol. Chem. 2022, 298, 101767. [Google Scholar] [CrossRef]
- Sultanli, S.; Ghumnani, S.; Ashma, R.; Kubatzky, K.F. Plumbagin, a biomolecule with (anti)osteoclastic properties. Int. J. Mol. Sci. 2021, 22, 2779. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, M.; Liu, X.; Yuan, Z.; Peng, Y.; Huang, Z.; Duan, X.; Zhao, T. Anti-fibrotic effect of plumbagin on CCl₄-lesioned rats. Cell. Physiol. Biochem. 2015, 35, 1599–1608. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Chen, B.; Cai, Y.J.; Zou, Z.L.; Wang, J.G.; Lin, Z.; Wang, X.D.; Fu, L.Y.; Hu, Y.R.; et al. Plumbagin ameliorates CCl4-induced hepatic fibrosis in rats via the epidermal growth factor receptor signaling pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 645727. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Liu, X.; Wu, G.; Zhong, J.; Zhao, T.; Li, J.; Lin, Y.; Zhou, Y.; Wei, Y. Plumbagin ameliorates liver fibrosis via a ROS-mediated NF-кB signaling pathway in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 108923. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.J.; Jang, S.; Lee, G.E.; Hwang, S.Y.; Kwon, Y.; Hong, J.Y.; Sohn, M.H.; Park, S.Y.; Yoon, H.G. Plumbagin suppresses pulmonary fibrosis via inhibition of p300 histone acetyltransferase activity. J. Med. Food 2020, 23, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, S.; Taherian, M.; Bayati, P.; Mousavizadeh, K.; Pashangzadeh, S.; Anisian, A.; Mojtabavi, N. Plumbagin attenuates bleomycin-induced lung fibrosis in mice. Allergy Asthma Clin. Immunol. 2022, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fang, Y.; Jiang, Y.; Jiang, S.; Li, Y.; Li, W.; Xu, M.; Aschner, M.; Liu, G. Plumbagin attenuates traumatic tracheal stenosis in rats and inhibits lung fibroblast proliferation and differentiation via TGF-β1/Smad and Akt/mTOR pathways. Bioengineered 2021, 12, 4475–4488. [Google Scholar] [CrossRef] [PubMed]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Țincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial resistance to antibiotics and effective antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef]
- Marrazzo, P.; Pizzuti, V.; Zia, S.; Sargenti, A.; Gazzola, D.; Roda, B.; Bonsi, L.; Alviano, F. Microfluidic tools for enhanced characterization of therapeutic stem cells and prediction of their potential antimicrobial secretome. Antibiotics 2021, 10, 750. [Google Scholar] [CrossRef]
- Kapoor, N.; Kandwal, P.; Sharma, G.; Gambhir, L. Redox ticklers and beyond: Naphthoquinone repository in the spotlight against inflammation and associated maladies. Pharmacol. Res. 2021, 174, 105968. [Google Scholar] [CrossRef]
- Singh, A.P.; Sharma, A. Structural insights and pharmaceutical relevance of plumbagin in parasitic disorders: A comprehensive review. Recent Adv. Antiinfect. Drug Discov. 2022, 17, 187–198. [Google Scholar] [CrossRef]
- Hassan, S.T.; Berchová-Bímová, K.; Petráš, J. Plumbagin, a plant-derived compound, exhibits antifungal combinatory effect with amphotericin B against Candida albicans clinical isolates and anti-hepatitis C virus activity. Phytother. Res. 2016, 30, 1487–1492. [Google Scholar] [CrossRef]
- Chen, X.; Yin, L.; Peng, L.; Liang, Y.; Lv, H.; Ma, T. Synergistic effect and mechanism of plumbagin with gentamicin against carbapenem-resistant Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 2751–2759. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, J.; Zhang, X.; Liu, Y.; Huang, Z.; Yuan, L.; Zhang, Y.; Cao, J.; Chen, L.; Liu, Y.; et al. Plumbagin resurrect colistin susceptible against colistin-resistant Pseudomonas aeruginosa in vitro and in vivo. Front. Microbiol. 2022, 13, 1020652. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, H.; Iswarya, S.; Pavithra, N.; Senthilnathan, S.; Gnanamani, A. In vitro antibacterial activity of plumbagin isolated from Plumbago zeylanica L. against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2019, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.M.I.H.; Perera, D.D.B.D.; Keerthirathna, L.R.; Heendeniya, S.; Anderson, R.J.; Williams, D.E.; Peiris, L.D.C. Antimicrobial activity of Plumbago indica and ligand screening of plumbagin against methicillin-resistant Staphylococcus aureus. J. Biomol. Struct. Dyn. 2022, 40, 3273–3284. [Google Scholar] [CrossRef]
- Reddy, P.J.; Ray, S.; Sathe, G.J.; Prasad, T.S.; Rapole, S.; Panda, D.; Srivastava, S. Proteomics analyses of Bacillus subtilis after treatment with plumbagin, a plant-derived naphthoquinone. OMICS 2015, 19, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, S.; Shaw, R.; Patra, M.M.; Calcuttawala, F.; Mukherjee, N.; Das Gupta, S.K. Mycobacterium tuberculosis thymidylate synthase (ThyX) is a target for plumbagin, a natural product with antimycobacterial activity. PLoS ONE 2020, 15, e0228657. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, B.P.; Kathuria, M.; Pant, G.; Kumari, N.; Mitra, K. Plumbagin, a plant-derived naphthoquinone metabolite induces mitochondria mediated apoptosis-like cell death in Leishmania donovani: An ultrastructural and physiological study. Apoptosis 2016, 21, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Chaweeborisuit, P.; Suriyonplengsaeng, C.; Suphamungmee, W.; Sobhon, P.; Meemon, K. Nematicidal effect of plumbagin on Caenorhabditis elegans: A model for testing a nematicidal drug. Z. Naturforsch. C. J. Biosci. 2016, 71, 121–131. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Zamani, P.; Amiri, M.; Hassanzadeh, S.M.; Ramazani, A. Nanoincorporation of plumbagin in micelles increase its in vivo anti-plasmodial properties. Iran. J. Parasitol. 2022, 17, 202–213. [Google Scholar] [CrossRef]
- Roy, A. Plumbagin: A potential anti-cancer compound. Mini Rev. Med. Chem. 2021, 21, 731–737. [Google Scholar] [CrossRef]
- Liu, F.; Kong, X.; Lv, L.; Gao, J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015, 359, 288–298. [Google Scholar] [CrossRef]
- Donnenberg, V.S.; Donnenberg, A.D. Stem cell state and the epithelial to-mesenchymal transition: Implications for cancer therapy. J. Clin. Pharmacol. 2015, 55, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.T.; Qin, Y.; Zhou, Z.W.; He, Z.X.; Zhang, X.; Yang, T.; Yang, Y.X.; Wang, D.; Zhou, S.F.; Qiu, J.X. Plumbagin suppresses epithelial to mesenchymal transition and stemness via inhibiting Nrf2-mediated signaling pathway in human tongue squamous cell carcinoma cells. Drug Des. Dev. Ther. 2015, 9, 5511–5551. [Google Scholar] [CrossRef]
- Ma, I.; Allan, A.L. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. Rep. 2011, 7, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, V.; Hemalatha, S.K.; Pal, K.; Sinha, S.; Nair, A.S.; Mukhopadhyay, D.; Srinivas, P. Selective mode of action of plumbagin through BRCA1 deficient breast cancer stem cells. BMC Cancer 2016, 16, 336. [Google Scholar] [CrossRef]
- Reshma, R.S.; Sreelatha, K.H.; Somasundaram, V.; Satheesh Kumar, S.; Nadhan, R.; Nair, R.S.; Srinivas, P. Plumbagin, a naphthaquinone derivative induces apoptosis in BRCA 1/2 defective castrate resistant prostate cancer cells as well as prostate cancer stem-like cells. Pharmacol. Res. 2016, 105, 134–145. [Google Scholar] [CrossRef]
- Sakunrangsit, N.; Ketchart, W. Plumbagin inhibits cancer stem-like cells, angiogenesis and suppresses cell proliferation and invasion by targeting Wnt/β-catenin pathway in endocrine resistant breast cancer. Pharmacol. Res. 2019, 150, 104517. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Canaider, S.; Pizzuti, V.; Pampanella, L.; Casadei, R.; Facchin, F.; Ventura, C. Herb-Derived Products: Natural tools to delay and counteract stem cell senescence. Stem Cells Int. 2020, 2020, 8827038. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of epigallocatechin gallate and sulforaphane counteracts in vitro oxidative stress and delays stemness loss of amniotic fluid stem cells. Oxid. Med. Cell. Longev. 2018, 2018, 5263985. [Google Scholar] [CrossRef]
- Pizzuti, V.; Abruzzo, P.M.; Chatgilialoglu, A.; Zia, S.; Marrazzo, P.; Petrocelli, G.; Zannini, C.; Marchionni, C.; Poggi, P.; Simonazzi, G.; et al. A tailored lipid supplement restored membrane fatty acid composition and ameliorates in vitro biological features of human amniotic epithelial cells. J. Clin. Med. 2022, 11, 1236. [Google Scholar] [CrossRef]
- Guida, M.; Maraldi, T.; Resca, E.; Beretti, F.; Zavatti, M.; Bertoni, L.; La Sala, G.B.; De Pol, A. Inhibition of nuclear Nox4 activity by plumbagin: Effect on proliferative capacity in human amniotic stem cells. Oxid. Med. Cell. Longev. 2013, 2013, 680816. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Miki, K.; Yamaguchi, Y.; Takauji, Y.; Yamakami, Y.; Hossain, M.N.; Ayusawa, D.; Fujii, M. Extract of Plumbago zeylanica enhances the growth of hair follicle dermal papilla cells with down-regulation of 5α-reductase type II. J. Cosmet. Dermatol. 2020, 19, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Itami, S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J. Dermatol. Sci. 2011, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Cytoprotective Activity | |||
|---|---|---|---|
| Biological Effect | In Vitro | In Vivo | Ref. |
| ↑ Cell viability ↑ SOD, CAT, GST, GPx activity ↑ Nrf-2 expression ↓ Cas3, 8, 9 | ✓ | [21,22,23,24] | |
| ↓ Bax ↑ Bcl2 | ✓ | ✓ | [26,27] |
| ↑ Mitochondrial membrane potential ↓ Apoptosis | ✓ | [24,28,29] | |
| ↑ Liver regeneration ↑ Autophagy | ✓ | ✓ | [30] |
| ↓ LDH, AST, CK ↑ pTEN ↓ PI3K/Akt | ✓ | [34] | |
| ↓ ROS levels ↑ LC3A II/LC3 I ratio | ✓ | [35] | |
| Anti-inflammatory activity | |||
| ↑ ROS levels Inactivating Nf-kB | ✓ | [19,20,37] | |
| ↑ Cell viability ↓ IL6, IL1 β, NO | ✓ | [18,20,39] | |
| Blocking PKM2 ↓ IL1 β, NO, HMGB1 | ✓ | [38] | |
| ↑ iNOS, IL1 α, G-CSF, IL12, MCP1, MCP5 Inactivating NLRP3-induced inflammasome | ✓ | [28,40,42] | |
| ↑ GSH, GPx | ✓ | [26] | |
| Inactivating IRAK1 | ✓ | [43] | |
| ↓ ROS levels, lipid peroxidation ↓ iNOS, IL6, IL18, TNF α ↑ Nrf2 | ✓ | [21,45] | |
| ↓ RANKL ↑ OPG | ✓ | [52] | |
| Inhibiting NIF ↑ Runx2, OCN, OPN, ALP → Decreasing osteoporosis | ✓ | ✓ | [24,57] |
| Antifibrotic activity | |||
| ↓ ALT, AST, IL6, TNF α ↑ SOD, GSH, GPx activity ↓ TGF1- β, Collagen III, α-SMA | ✓ | ✓ | [30,31,59,61,62] |
| Inactivating EGFR/STAT3 signaling | ✓ | [60] | |
| Inhibiting TGF1- β/mTOR/Akt pathways | ✓ | ✓ | [64] |
| Antibacterial and antiparasitic activity | |||
| ↑ Intracellular uptake of gentamicin | ✓ | [70] | |
| Inhibiting DNA gyrase activity | ✓ | [73] | |
| ↑ Membrane permeability and drug effect | ✓ | [71,74] | |
| Inhibiting ThyX activity | ✓ | [75] | |
| ↓ Mitochondrial number Altering mitochondrial membrane potential | ✓ | [76,77] | |
| ↓ Plasmodial infection and propagation | ✓ | ✓ | [78] |
| Stem cells and cell senescence | |||
| ↑ ROS levels 1 ↓ Oct4, Sox2, Nanog, BMI-1 1 | ✓ | [82] | |
| ↓ Proliferation 1 ↓ FGF2, Oct4, Nanog, ALDH1 1 | ✓ | ✓ | [84,85,86] |
| ↓ ROS levels 2 ↑ Proliferation 2 Inhibiting NOX4 activity | ✓ | [91] | |
| ↑ Proliferation 2 ↓ SRD5A2 → Decreasing senescence 2 | ✓ | [92] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrocelli, G.; Marrazzo, P.; Bonsi, L.; Facchin, F.; Alviano, F.; Canaider, S. Plumbagin, a Natural Compound with Several Biological Effects and Anti-Inflammatory Properties. Life 2023, 13, 1303. https://doi.org/10.3390/life13061303
Petrocelli G, Marrazzo P, Bonsi L, Facchin F, Alviano F, Canaider S. Plumbagin, a Natural Compound with Several Biological Effects and Anti-Inflammatory Properties. Life. 2023; 13(6):1303. https://doi.org/10.3390/life13061303
Chicago/Turabian StylePetrocelli, Giovannamaria, Pasquale Marrazzo, Laura Bonsi, Federica Facchin, Francesco Alviano, and Silvia Canaider. 2023. "Plumbagin, a Natural Compound with Several Biological Effects and Anti-Inflammatory Properties" Life 13, no. 6: 1303. https://doi.org/10.3390/life13061303
APA StylePetrocelli, G., Marrazzo, P., Bonsi, L., Facchin, F., Alviano, F., & Canaider, S. (2023). Plumbagin, a Natural Compound with Several Biological Effects and Anti-Inflammatory Properties. Life, 13(6), 1303. https://doi.org/10.3390/life13061303










