Clinical, Dermoscopic, and Histological Characteristics of Melanoma Patients According to the Age Groups: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
- -
- Epidemiologic data (gender, age at the moment of melanoma diagnosis, and location of melanoma), family or personal history of melanoma, and previous non-melanoma skin cancer (NMSC).
- -
- The topography of melanoma was described as head and neck, trunk, upper limb and lower limb (including acral melanomas), and special locations (nail apparatus, mucous membrane of the oral or genital area, and eye).
- -
- Melanoma risk factors that manifested clinically, including multiple acquired melanocytic nevi (above 50 melanocytic nevi), atypical nevus syndrome, skin phototype I or II, solar lentiginosis (as a marker for sun burn episodes), previous/coexisting non-melanoma skin cancer (NMSC), and genetic syndromes.
- -
- Presence of histopathological report of melanoma. The histopathological subtypes of melanoma were evaluated as lentigo maligna (facial and extrafacial), lentigo maligna melanoma (facial), superficial spreading melanoma, nodular melanoma, spitzoid melanoma, nevoid melanoma, and desmoplastic melanoma. The melanoma invasiveness was described according to the TNM staging system/8th AJCC classification as pTis, pT1, pT2, pT3, and pT4 [10].
- -
- Videodermoscopic documentation of melanoma. The dermoscopic pattern of melanoma was allocated to one of the following subtypes: multicomponent asymmetric, spitzoid, melanoma on sun damaged skin, hypomelanotic/amelanotic, homogenous, nodular, melanoma on face, and melanoma in special location (nail apparatus/acral/mucous membranes). The dermoscopic regression structures were regarded as present or absent.
Statistical Analysis
3. Results
3.1. The Characteristics of the Melanoma Patients
3.2. The Analysis between the Individual Age Groups
3.3. Crude (Unadjusted) Odds Ratios for the Age Groups (Table 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer 2022, 22, 836. [Google Scholar] [CrossRef]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Dermato-Venereol. 2020, 100, adv00136. [Google Scholar] [CrossRef]
- Nagore, E.; Hueso, L.; Botella Estrada, R.; Alfaro Rubio, A.; Serna, I.; Guallar, J.; González, I.; Ribes, I.; Guillen, C. Smoking, sun ex-posure, number of nevi and previous neoplasias are risk factors for melanoma in older patients (60 years and over). J. Eur. Acad. Dermatol. Venereol. 2010, 24, 50–57. [Google Scholar] [CrossRef]
- Massone, C.; Hofman-Wellenhof, R.; Chiodi, S.; Sola, S. Dermoscopic Criteria, Histopathological Correlates and Genetic Findings of Thin Melanoma on Non-Volar Skin. Genes 2021, 12, 1288. [Google Scholar] [CrossRef]
- Zhang, T.; Dutton-Regester, K.; Brown, K.M.; Hayward, N.K. The genomic landscape of cutaneous melanoma. Pigment. Cell Melanoma Res. 2016, 29, 266–283. [Google Scholar] [CrossRef]
- Djavid, A.R.; Stonesifer, C.; Fullerton, B.T.; Wang, S.W.; Tartaro, M.A.; Kwinta, B.D.; Grimes, J.M.; Geskin, L.J.; Saenger, Y.M. Etiologies of Melanoma Development and Prevention Measures: A Review of the Current Evidence. Cancers 2021, 13, 4914. [Google Scholar] [CrossRef]
- Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=53&data_type=1&graph_type=3&compareBy=race&chk_race_1=1&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_race_9=9&chk_race_8=8&chk_race_3=3&chk_race_2=2&rate_type=2&sex=1&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0 (accessed on 22 May 2023).
- Available online: https://onkologia.org.pl/pl/raporty (accessed on 22 May 2023).
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma of the skin. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 563–585. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Sacchetto, L.; Zanetti, R.; Comber, H.; Bouchardy, C.; Brewster, D.H.; Broganelli, P.; Chirlaque, M.D.; Coza, D.; Galceran, J.; Gavin, A.; et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur. J. Cancer 2018, 92, 108–118. [Google Scholar] [CrossRef]
- Boada, A.; Tejera-Vaquerizo, A.; Requena, C.; Manrique-Silva, E.; Traves, V.; Nagore, E. Association between melanoma thickness and clinical and demographic characteristics. Eur. J. Dermatol. 2021, 31, 514–520. [Google Scholar] [CrossRef]
- Dubbini, N.; Puddu, A.; Salimbeni, G.; Malloggi, S.; Gandini, D.; Massei, P.; Ferraùto, G.; Rubino, T.; Ricci, L.; Menchini, G.; et al. Melanoma Prevention: Comparison of Different Screening Methods for the Selection of a High Risk Population. Int. J. Environ. Res. Public Health 2021, 18, 1953. [Google Scholar] [CrossRef]
- Johansson, M.; Brodersen, J.; Gøtzsche, P.C.; Jørgensen, K.J. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst. Rev. 2019, 6, CD012352. [Google Scholar] [CrossRef]
- Argenziano, G.; Zalaudek, I.; Hofmann-Wellenhof, R.; Bakos, R.M.; Bergman, W.; Blum, A.; Broganelli, P.; Cabo, H.; Caltagirone, F.; Catricalà, C.; et al. Total body skin examination for skin cancer screening in patients with focused symptoms. J. Am. Acad. Dermatol. 2012, 66, 212–219. [Google Scholar] [CrossRef]
- Omara, S.; Wen, D.; Ng, B.; Anand, R.; Matin, R.N.; Taghipour, K.; Esdaile, B. Identification of Incidental Skin Cancers Among Adults Referred to Dermatologists for Suspicious Skin Lesions. JAMA Netw. Open 2020, 3, e2030107. [Google Scholar] [CrossRef]
- Guitera, P.; Menzies, S.W.; Coates, E.; Azzi, A.; Fernandez-Penas, P.; Lilleyman, A.; Badcock, C.; Schmid, H.; Watts, C.G.; Collgros, H.; et al. Efficiency of Detecting New Primary Melanoma among Individuals Treated in a High-Risk Clinic for Skin Surveillance. JAMA Dermatol. 2021, 157, 521–530. [Google Scholar] [CrossRef]
- Argenziano, G.; Albertini, G.; Castagnetti, F.; De Pace, B.; Di Lernia, V.; Longo, C.; Pellacani, G.; Piana, S.; Ricci, C.; Zalaudek, I. Early diagnosis of melanoma: What is the impact of dermoscopy? Dermatol. Ther. 2012, 25, 403–409. [Google Scholar] [CrossRef]
- Pizarro, A.; Arranz, D.; Villeta, M.; Valencia, J. Absence of thick, nodular melanomas during long-term surveillance with total body photography and digital dermatoscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e341–e342. [Google Scholar] [CrossRef]
- Pellacani, G.; Farnetani, F.; Chester, J.; Kaleci, S.; Ciardo, S.; Bassoli, S.; Casari, A.; Longo, C.; Manfredini, M.; Cesinaro, A.M.; et al. Cutaneous Melanoma Systematic Diagnostic Workflows and Integrated Reflectance Confocal Microscopy Assessed with a Retrospective, Comparative Longitudinal (2009–2018) Study. Cancers 2022, 14, 838. [Google Scholar] [CrossRef]
- Kalloniati, E.; Cavouras, D.; Plachouri, K.M.; Geropoulou, E.; Sakellaropoulos, G.; Georgiou, S. Clinical, dermoscopic and histological assessment of melanocytic lesions: A comparative study of the accuracy of the diagnostic methods. Hippokratia 2021, 25, 156–161. [Google Scholar]
- Nazzaro, G.; Passoni, E.; Pozzessere, F.; Maronese, C.A.; Marzano, A.V. Dermoscopy Use Leads to Earlier Cutaneous Melanoma Diagnosis in Terms of Invasiveness and Size? A Single-Center, Retrospective Experience. J. Clin. Med. 2022, 11, 4912. [Google Scholar] [CrossRef]
- Dessinioti, C.; Geller, A.; Whiteman, D.; Garbe, C.; Grob, J.; Kelly, J.; Scolyer, R.; Rawson, R.; Lallas, A.; Pellacani, G.; et al. Not all melanomas are created equal: A review and call for more research into nodular melanoma. Br. J. Dermatol. 2021, 185, 700–710. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Peternel, S.; Mully, T.W.; North, J.P.; Pincus, L.B.; LeBoit, P.E.; McCalmont, T.H.; Bastian, B.C.; Yeh, I. Faculty Opinions recommendation of Spitz melanoma is a distinct subset of spitzoid melanoma. Mod. Pathol. 2020, 33, 1122–1134. [Google Scholar] [CrossRef]
- Pusiol, T.; Piscioli, F.; Speziali, L.; Zorzi, M.G.; Morichetti, D.; Roncati, L. Clinical Features, Dermoscopic Patterns, and Histological Diagnostic Model for Melanocytic Tumors of Uncertain Malignant Potential (MELTUMP). J. Natl. Cancer Inst. 2015, 23, 185–194. [Google Scholar]
- Shaikh, W.R.; Dusza, S.W.; Weinstock, M.A.; Oliveria, S.A.; Geller, A.C.; Halpern, A.C. Melanoma Thickness and Survival Trends in the United States, 1989 to 2009. J. Natl. Cancer Inst. 2016, 108, djv294. [Google Scholar] [CrossRef]
- Hübner, J.; Waldmann, A.; Eisemann, N.; Noftz, M.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Association between risk factors and detection of cutaneous melanoma in the setting of a population-based skin cancer screening. Eur. J. Cancer Prev. 2018, 27, 563–569. [Google Scholar] [CrossRef]
- Gimotty, P.A.; Elder, D.E.; Fraker, D.L.; Botbyl, J.; Sellers, K.; Elenitsas, R.; Ming, M.E.; Schuchter, L.; Spitz, F.R.; Czerniecki, B.J.; et al. Identification of High-Risk Patients Among Those Diagnosed With Thin Cutaneous Melanomas. J. Clin. Oncol. 2007, 25, 1129–1134. [Google Scholar] [CrossRef]
- Stracci, F.; Fabrizi, V.; D’alò, D.; La Rosa, F.; Papini, M. Risk of multiple primary cancers following melanoma and non-melanoma skin cancer. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1384–1388. [Google Scholar] [CrossRef]
- Neale, R.E.; Forman, D.; Murphy, M.F.G.; Whiteman, D.C. Site-specific occurrence of nonmelanoma skin cancers in patients with cutaneous melanoma. Br. J. Cancer 2005, 93, 597–601. [Google Scholar] [CrossRef]
- Ghiasvand, R.; Robsahm, T.E.; Green, A.C.; Rueegg, C.S.; Weiderpass, E.; Lund, E.; Veierød, M.B. Association of Phenotypic Characteristics and UV Radiation Exposure With Risk of Melanoma on Different Body Sites. JAMA Dermatol. 2019, 155, 39–49. [Google Scholar] [CrossRef]
- Kim, D.P.; Kus, K.J.; Ruiz, E. Basal Cell Carcinoma Review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Slowinska, M.; Kaminska-Winciorek, G.; Kowalska-Oledzka, E.; Czarnecka, I.; Czarnecki, R.; Nasierowska-Guttmejer, A.; Paluchowska, E.; Owczarek, W. Dermoscopy of Small Diameter Melanomas with the Diagnostic Feasibility of Selected Algorithms—A Clinical Retrospective Multicenter Study. Cancers 2021, 13, 6095. [Google Scholar] [CrossRef]
- Nazzaro, G.; Maronese, C.A.; Casazza, G.; Giacalone, S.; Spigariolo, C.B.; Roccuzzo, G.; Avallone, G.; Guida, S.; Brancaccio, G.; Broganelli, P.; et al. Dermoscopic predictors of melanoma in small diameter melanocytic lesions (mini-melanoma): A retrospective multicentric study of 269 cases. Int. J. Dermatol. 2023. [Google Scholar] [CrossRef]
- Pereira, A.R.; Corral-Forteza, M.; Collgros, H.; El Sharouni, M.; Ferguson, P.M.; Scolyer, R.A.; Guitera, P. Dermoscopic features and screening strategies for the detection of small-diameter melanomas. Clin. Exp. Dermatol. 2022, 47, 932–941. [Google Scholar] [CrossRef]
- Demierre, M.-F. Thin melanomas and regression, thick melanomas and older men: Prognostic implications and perspectives on secondary prevention. Arch. Dermatol. 2002, 138, 678–682. [Google Scholar] [CrossRef]
- Carrera, C.; Scope, A.; Dusza, S.W.; Argenziano, G.; Nazzaro, G.; Phan, A.; Tromme, I.; Rubegni, P.; Malvehy, J.; Puig, S.; et al. Clinical and dermoscopic characterization of pediatric and adolescent melanomas: Multicenter study of 52 cases. J. Am. Acad. Dermatol. 2018, 78, 278–288. [Google Scholar] [CrossRef]
- De Giorgi, V.; Magnaterra, E.; Zuccaro, B.; Magi, S.; Magliulo, M.; Medri, M.; Mazzoni, L.; Venturi, F.; Silvestri, F.; Tomassini, G.M.; et al. Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study. Cancers 2023, 15, 1835. [Google Scholar] [CrossRef]
- Lallas, A.; Moscarella, E.; Longo, C.; Kyrgidis, A.; de Mestier, Y.; Vale, G.; Guida, S.; Pellacani, G.; Argenziano, G. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J. Am. Acad. Dermatol. 2015, 72, 47–53. [Google Scholar] [CrossRef]
- Lallas, A.; Apalla, Z.; Ioannides, D.; Lazaridou, E.; Kyrgidis, A.; Broganelli, P.; Alfano, R.; Zalaudek, I.; Argenziano, G.; International Dermoscopy Society; et al. Update on dermoscopy of Spitz/Reed naevi and management guidelines by the International Dermoscopy Society. Br. J. Dermatol. 2017, 177, 645–655. [Google Scholar] [CrossRef]
- Costa, C.; Megna, M.; Cappello, M.; Napolitano, M.; Monfrecola, G.; Scalvenzi, M. Melanoma frequency among symmetrical Spitzoid-looking lesions: A retrospective study. G. Ital. Dermatol. Venereol. 2019, 154, 26–31. [Google Scholar] [CrossRef]
- Duarte, A.F.; Sousa-Pinto, B.; Azevedo, L.F.; Barros, A.M.; Puig, S.; Malvehy, J.; Haneke, E.; Correia, O. Clinical ABCDE rule for early melanoma detection. Eur. J. Dermatol. 2021, 31, 771–778. [Google Scholar] [CrossRef]
- Tiodorovic-Zivkovic, D.; Argenziano, G.; Lallas, A.; Thomas, L.; Ignjatovic, A.; Rabinovitz, H.; Moscarella, E.; Longo, C.; Hofmann-Wellenhof, R.; Zalaudek, I. Age, gender, and topography influence the clinical and dermoscopic appearance of lentigo maligna. J. Am. Acad. Dermatol. 2015, 72, 801–808. [Google Scholar] [CrossRef]
- Jaimes, N.; Marghoob, A.A.; Rabinovitz, H.; Braun, R.P.; Cameron, A.; Rosendahl, C.; Canning, G.; Keir, J. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damaged skin. J. Am. Acad. Dermatol. 2015, 72, 1027–1035. [Google Scholar] [CrossRef]
- DeWane, M.E.; Kelsey, A.; Oliviero, M.; Rabinovitz, H.; Grant-Kels, J.M. Melanoma on chronically sun-damaged skin: Lentigo maligna and desmoplastic melanoma. J. Am. Acad. Dermatol. 2019, 81, 823–833. [Google Scholar] [CrossRef]
- Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Metzler, G.; Moehrle, M.; Breuninger, H.; Garbe, C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008, 112, 1795–1804. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; López-Abente, G.; Aragones, N.; Pollán, M.; Pastor-Barriuso, R.; Sánchez, M.; Pérez-Gómez, B. Trends in mortality from cutaneous malignant melanoma in Spain (1982–2016): Sex-specific age-cohort-period effects. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1522–1528. [Google Scholar] [CrossRef]
- Porto, A.C.; Blumetti, T.P.; Calsavara, V.F.; Torrezan, G.T.; de Paula, C.A.A.; Lellis, R.; Neto, J.P.D.; Carraro, D.M.; Braga, J.C.T. A cross-sectional study of clinical, dermoscopic, histopathological, and molecular patterns of scalp melanoma in patients with or without androgenetic alopecia. Sci. Rep. 2022, 12, 15096. [Google Scholar] [CrossRef]

| Total | Age < 31 y | Age 31–60 y | Age > 60 y | p Value | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Within the Group | Within the Group | Within the Group | |||
| Gender | 12 | 113 | 64 | NS | |
| Female | 120 (63.5%) | 6 (50.0%) | 71 (62.8%) | 43 (67.2%) | |
| Male | 69 (36.5%) | 6 (50.0%) | 42 (37.2%) | 21 (32.8%) | |
| History of personal/familial melanoma | NS | ||||
| Yes | 24 (12.5%) | 0 (0%) | 18 (15.9%) | 6 (9.4%) | |
| No | 165 (87.3%) | 12 (100.00%) | 95 (84.1%) | 58 (90.6%) | |
| Multiple acquired nevi/Atypical nevus syndrome | <0.0005 | ||||
| Yes | 121 (64.0%) | 8 (66.7%) | 86(76.1%) | 27(42.2%) | |
| No | 68 (36%) | 4 (33.3%) | 27 (23.9%) | 37 (57.8%) | |
| Skin phototype | NS | ||||
| I | 6 (3.2%) | 3 (25.0%) | 2 (1.7%) | 1 (1.5%) | |
| II | 177 (93.6%) | 9 (75.0%) | 107 (94.7%) | 61 (95.3%) | |
| III | 6 (3.2%) | 0 (0%) | 4 (3.5%) | 2 (3.1%) | |
| IV–V | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Solar lentiginosis | <0.0005 | ||||
| Yes | 81 (42.9%) | 2 (16.7%) | 32 (28.3%) | 47 (73.4%) | |
| No | 108 (57.1%) | 10 (83.3%) | 81 (71.7%) | 17 (26.6%) | |
| Histopathological report: | <0.01 | ||||
| 25 (12.0%) | 0 (0%) | 10 (8.0%) | 15 (20.8%) | |
| 4 (1.9%) | 0 (0%) | 0 (0%) | 4 (5.5%) | |
| Melanoma: | |||||
| 47 (22.5%) | 2 (16.6%) | 28 (22.4%) | 17 (23.6%) | |
| 101 (48.3%) | 7 (58.3%) | 69 (55.2.%) | 25 (34.7%) | |
| 24 (11.5%) | 2 (16.6%) | 13 (10.4%) | 9 (12.5%) | |
| 3 (1.4%) | 1 (8.3%) | 1 (0.8%) | 1 (1.4%) | |
| 5 (2.4%) | 0 (0%) | 4(3.2%) | 1 (1.4%) | |
| Dermoscopic pattern of melanoma | <0.0005 | ||||
| Multicomponent asymmetric | 91 (43.5%) | 4 (33.3%) | 63 (50.4%) | 24 (33.3%) | |
| Spitzoid | 37 (17.7%) | 6 (50.0%) | 28 (22.4%) | 3 (4.2%) | |
| Melanoma on sun damaged skin | 25 (12.0%) | 0 (0%) | 9 (7.2%) | 16 (22.2%) | |
| Hypomelanotic/amelanotic | 10 (4.8%) | 0 (0%) | 7 (5.6%) | 3 (4.2%) | |
| Homogenous | 5 (2.4%) | 0 (0%) | 4 (3.2%) | 1 (1.4%) | |
| Nodular | 12 (5.7%) | 1 (8.3%) | 6 (4.8%) | 5 (6.9%) | |
| Melanoma on face | 22 (10.5%) | 0 (0%) | 3 (2.4%) | 19 (26.4%) | |
| Melanoma in special location (nail apparatus/acral/mucous membranes) | 7 (3.3%) | 1 (8.3%) | 5 (4.0%) | 1 (1.4%) | |
| Dermoscopic structures of regression | <0.001 | ||||
| Yes | 61 (29.2%) | 1 (8.3%) | 27 (21.6%) | 33 (45.8%) | |
| No | 148 (70.8%) | 11 (91.7%) | 98 (78.4%) | 39 (54.2%) | |
| Melanoma location: | <0.0005 | ||||
| 25 (11.9%) | 0 (0%) | 6 (4.8%) | 19 (26.%) | |
| 70 (33.5%) | 4 (33.3.%) | 46 (36.8%) | 20 (27.8%) | |
| 40 (19.1%) | 3 (25.0%) | 22 (17.6%) | 15 (20.8%) | |
| 71 (34.0%) | 4 (33.3%) | 49 (39.2%) | 18 (25.0%) | |
| 1 (0.5%) | 1 (8.3%) | 0 (0%) | 0 (0%) | |
| 2 (1%) | 0 (0%) | 2 (1.6%) | 0 (0%) | |
| Previous/concomitant NMSC | <0.0005 | ||||
| Yes | 41 (21.7%) | 0 (0%) | 10 (8.9%) | 31(48.4%) | |
| No | 148 (78.3%) | 12 (100%) | 103 (91.1%) | 33 (51.6%) |
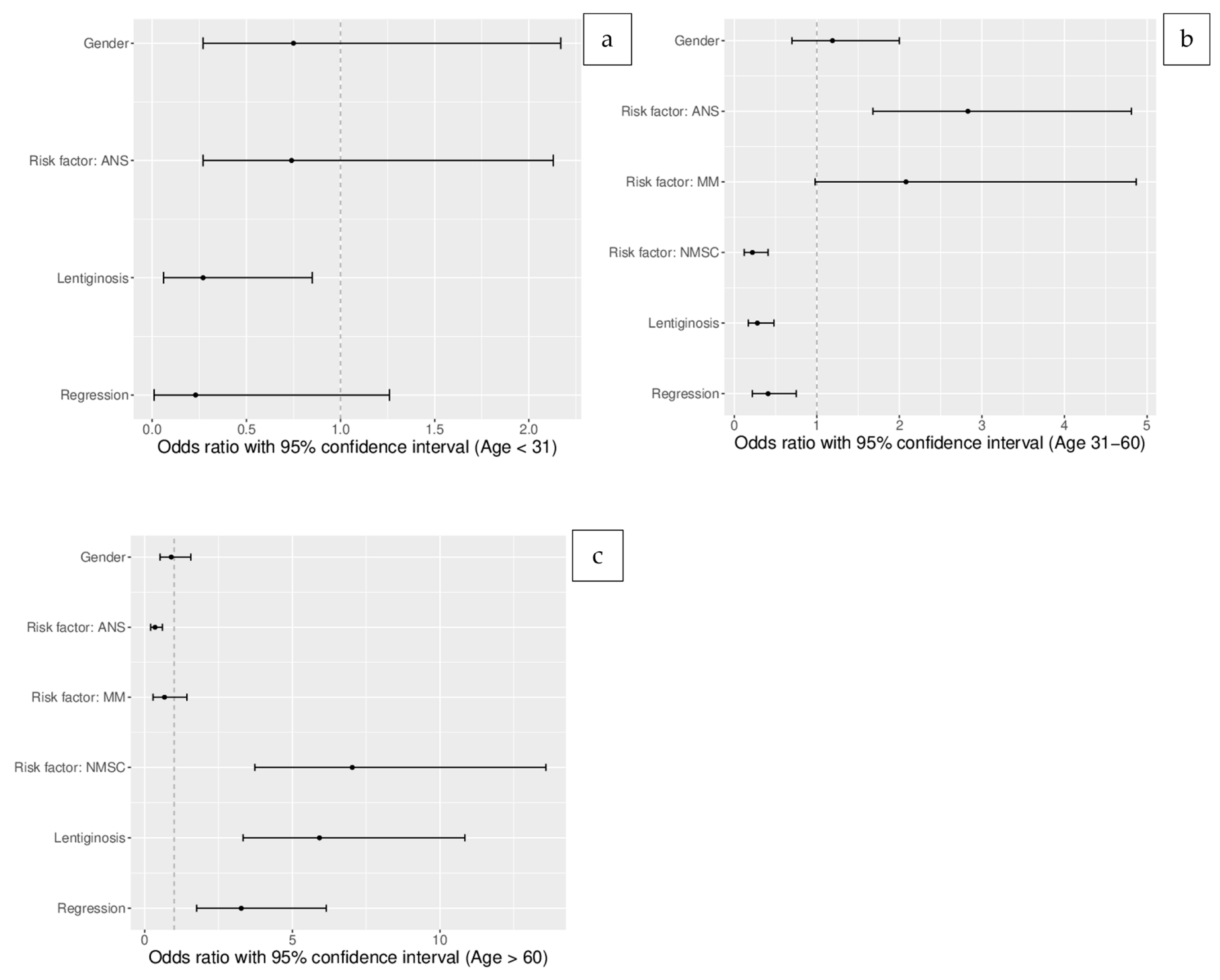
| Factor | OR | 95%CI | p-Value |
|---|---|---|---|
| Characteristics of melanoma patients below 31 years of age (unadjusted) | |||
| Gender (male) | 1.8 | 0.53–6.15 | NS |
| Melanoma (previous/concomitant or in family history) | ND | ND | ND |
| Atypical nevus syndrome or multiple acquired nevi | 1.11 | 0.33–4.46 | NS |
| NMSC (previous/concomitant) | ND | ND | ND |
| Regression under dermoscopy | 0.23 | 0.01–1.26 | NS |
| Solar lentiginosis | 0.26 | 0.04–1.06 | NS |
| Characteristics of melanoma patients between 31 and 60 years of age (unadjusted) | |||
| Gender (male) | 0.7 | 0.59–1.98 | NS |
| Melanoma (previous/concomitant or in family history) | 2.17 | 0.85–6.34 | NS |
| Atypical nevus syndrome or multiple acquired nevi | 3.69 | 1.99–6.99 | <0.00001 |
| NMSC (previous/concomitant) | 0.14 | 0.06–0.31 | <0.000001 |
| Regression under dermoscopy | 0.41 | 0.22–0.75 | <0.005 |
| Solar lentiginosis | 0.22 | 0.12–0.41 | <0.000001 |
| Characteristics of melanoma patients 60 years old (unadjusted) | |||
| Gender (male) | 0.79 | 0.41–1.48 | NS |
| Melanoma (previous/concomitant or in family history) | 0.63 | 0.21–1.6 | NS |
| Atypical nevus syndrome or multiple acquired nevi | 0.24 | 0.13–0.46 | 0.00001 |
| NMSC (previous/concomitant) | 10.53 | 4.81–24.94 | <0.000001 |
| Regression under dermoscopy | 3.27 | 1.76–6.15 | <0.0001 |
| Solar lentiginosis | 7.27 | 3.74– 14.74 | <0.000001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słowińska, M.; Czarnecka, I.; Czarnecki, R.; Tatara, P.; Nasierowska-Guttmejer, A.; Lorent, M.; Cierniak, S.; Owczarek, W. Clinical, Dermoscopic, and Histological Characteristics of Melanoma Patients According to the Age Groups: A Retrospective Observational Study. Life 2023, 13, 1369. https://doi.org/10.3390/life13061369
Słowińska M, Czarnecka I, Czarnecki R, Tatara P, Nasierowska-Guttmejer A, Lorent M, Cierniak S, Owczarek W. Clinical, Dermoscopic, and Histological Characteristics of Melanoma Patients According to the Age Groups: A Retrospective Observational Study. Life. 2023; 13(6):1369. https://doi.org/10.3390/life13061369
Chicago/Turabian StyleSłowińska, Monika, Iwona Czarnecka, Robert Czarnecki, Paulina Tatara, Anna Nasierowska-Guttmejer, Małgorzata Lorent, Szczepan Cierniak, and Witold Owczarek. 2023. "Clinical, Dermoscopic, and Histological Characteristics of Melanoma Patients According to the Age Groups: A Retrospective Observational Study" Life 13, no. 6: 1369. https://doi.org/10.3390/life13061369
APA StyleSłowińska, M., Czarnecka, I., Czarnecki, R., Tatara, P., Nasierowska-Guttmejer, A., Lorent, M., Cierniak, S., & Owczarek, W. (2023). Clinical, Dermoscopic, and Histological Characteristics of Melanoma Patients According to the Age Groups: A Retrospective Observational Study. Life, 13(6), 1369. https://doi.org/10.3390/life13061369






