Inflammatory Mediators of Endothelial Dysfunction
Abstract
1. Introduction
Endothelial Dysfunction and Inflammation
2. NLRP3 Inflammasome and Atherosclerosis
3. Major Inflammation Modulators of NLRP3 Inflammasome
4. NLRP3 Inflammasome and Pyroptosis
5. Role of Hydrogen Sulfide in Endothelial Dysfunction
6. Contemporary Research
Potential Anti-Inflammatory Therapies Tested in Clinical Studies
7. The Role of Inflammatory Mediators in Cardiovascular Disease
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esper, R.J.; Nordaby, R.A.; Vilarino, J.O.; Paragano, A.; Cacharron, J.L.; Machado, R.A. Endothelial dysfunction: A comprehensive appraisal. Cardiovasc. Diabetol. 2006, 5, 4. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993; Volume 2. [Google Scholar]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; Hanghicel, P.; Sitar-Taut, A.; Suharoschi, R.; Vulturar, R.; et al. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets. Oxid. Med. Cell. Longev. 2021, 2021, 8671713. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Hansson, G.K. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Ministrini, S.; Carbone, F.; Montecucco, F. Updating concepts on atherosclerotic inflammation: From pathophysiology to treatment. Eur. J. Clin. Investig. 2021, 51, e13467. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Choudhury, R. Inflammation and atherosclerosis: What is on the horizon? Heart 2020, 106, 80–85. [Google Scholar] [CrossRef]
- Ridker, P.M.; Koenig, W.; Kastelein, J.J.; Mach, F.; Luscher, T.F. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur. Heart J. 2018, 39, 4109–4111. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Grosse-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef]
- Delles, C.; Dymott, J.A.; Neisius, U.; Rocchiccioli, J.P.; Bryce, G.J.; Moreno, M.U.; Carty, D.M.; Berg, G.A.; Hamilton, C.A.; Dominiczak, A.F. Reduced LDL-cholesterol levels in patients with coronary artery disease are paralleled by improved endothelial function: An observational study in patients from 2003 and 2007. Atherosclerosis 2010, 211, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.B.; Hammadah, M.; Kim, J.H.; Uphoff, I.; Shah, A.; Levantsevych, O.; Almuwaqqat, Z.; Moazzami, K.; Sullivan, S.; Ward, L.; et al. Association of Transient Endothelial Dysfunction Induced by Mental Stress with Major Adverse Cardiovascular Events in Men and Women With Coronary Artery Disease. JAMA Cardiol. 2019, 4, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, E.; Pirro, M. Endothelial injury and repair: A novel theory for atherosclerosis. Angiology 2008, 59, 69S–72S. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial Dysfunction and Cardiovascular Disease: History and Analysis of the Clinical Utility of the Relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Weber, C.; Lutgens, E. Regulation of atherosclerotic plaque inflammation. J. Intern. Med. 2015, 278, 462–482. [Google Scholar] [CrossRef]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Huang, L.H.; Randolph, G.J. Cytokine Circuits in Cardiovascular Disease. Immunity 2019, 50, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Tajfard, M.; Tavakoly Sany, S.B.; Avan, A.; Latiff, L.A.; Rahimi, H.R.; Moohebati, M.; Hasanzadeh, M.; Ghazizadeh, H.; Esmaeily, H.; Doosti, H.; et al. Relationship between serum high sensitivity C-reactive protein with angiographic severity of coronary artery disease and traditional cardiovascular risk factors. J. Cell. Physiol. 2019, 234, 10289–10299. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Mitre, L.S.; Wolf, D. Inflammatory Cell Recruitment in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 635527. [Google Scholar] [CrossRef] [PubMed]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Takahashi, M. NLRP3 inflammasome as a novel player in myocardial infarction. Int. Heart J. 2014, 55, 101–105. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome—A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Tabit, C.E.; Holbrook, M.; Shenouda, S.M.; Dohadwala, M.M.; Widlansky, M.E.; Frame, A.A.; Kim, B.H.; Duess, M.A.; Kluge, M.A.; Levit, A.; et al. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc. Med. 2012, 17, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Centola, M.; Wood, G.; Frucht, D.M.; Galon, J.; Aringer, M.; Farrell, C.; Kingma, D.W.; Horwitz, M.E.; Mansfield, E.; Holland, S.M.; et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood 2000, 95, 3223–3231. [Google Scholar] [CrossRef]
- Fang, L.; Wang, K.K.; Zhang, P.F.; Li, T.; Xiao, Z.L.; Yang, M.; Yu, Z.X. Nucleolin promotes Ang II-induced phenotypic transformation of vascular smooth muscle cells by regulating EGF and PDGF-BB. J. Cell. Mol. Med. 2020, 24, 1917–1933. [Google Scholar] [CrossRef]
- Liaqat, A.; Asad, M.; Shoukat, F.; Khan, A.U. A Spotlight on the Underlying Activation Mechanisms of the NLRP3 Inflammasome and its Role in Atherosclerosis: A Review. Inflammation 2020, 43, 2011–2020. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Shi, X.; Xie, W.L.; Kong, W.W.; Chen, D.; Qu, P. Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2455–2466. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Chang, C. NR3C2 mediates oxidised low-density lipoprotein-induced human coronary endothelial cells dysfunction via modulation of NLRP3 inflammasome activation. Autoimmunity 2023, 56, 2189135. [Google Scholar] [CrossRef]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Georgin-Lavialle, S.; Grateau, G.; Amselem, S.; Giurgea, I.; Karabina, S.A. Inflammasome biology, molecular pathology and therapeutic implications. Pharmacol. Ther. 2018, 187, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, G.; Han, D.H.; Lee, M.; Kim, I.; Kim, B.; Kim, K.H.; Song, Y.M.; Yoo, J.E.; Wang, H.J.; et al. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy 2017, 13, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Rivera, K.; Andia, M.E.; Martinez Rodriguez, G. The IL-1 Family and Its Role in Atherosclerosis. Int. J. Mol. Sci. 2022, 24, 17. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Wan, Z.; Fan, Y.; Liu, X.; Xue, J.; Han, Z.; Zhu, C.; Wang, X. NLRP3 inflammasome promotes diabetes-induced endothelial inflammation and atherosclerosis. Diabetes Metab. Syndr. Obes. 2019, 12, 1931–1942. [Google Scholar] [CrossRef]
- Zheng, F.; Xing, S.; Gong, Z.; Mu, W.; Xing, Q. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediat. Inflamm. 2014, 2014, 507208. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Paramel Varghese, G.; Folkersen, L.; Strawbridge, R.J.; Halvorsen, B.; Yndestad, A.; Ranheim, T.; Krohg-Sorensen, K.; Skjelland, M.; Espevik, T.; Aukrust, P.; et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Heart Assoc. 2016, 5, e003031. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xing, S.; Gong, Z.; Xing, Q. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ. 2013, 22, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Mu, N.; Lou, X.; Li, W.; Chen, Y.; Fan, D.; Tan, H. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab. Investig. 2017, 97, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S. Role of innate immunity in diabetes and metabolism: Recent progress in the study of inflammasomes. Immune Netw. 2011, 11, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef]
- Arend, W.P.; Palmer, G.; Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008, 223, 20–38. [Google Scholar] [CrossRef]
- Cassel, S.L.; Joly, S.; Sutterwala, F.S. The NLRP3 inflammasome: A sensor of immune danger signals. Semin. Immunol. 2009, 21, 194–198. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Melnichenko, A.A.; Wetzker, R.; Gerasimova, E.V.; Orekhov, A.N. NLPR3 Inflammasomes and Their Significance for Atherosclerosis. Biomedicines 2020, 8, 205. [Google Scholar] [CrossRef]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef]
- Takahashi, M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 2022, 118, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ikeda, U.; Masuyama, J.; Kitagawa, S.; Kasahara, T.; Saito, M.; Kano, S.; Shimada, K. Involvement of adhesion molecules in human monocyte adhesion to and transmigration through endothelial cells in vitro. Atherosclerosis 1994, 108, 73–81. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Grebe, A.; Latz, E. Danger signaling in atherosclerosis. Circ. Res. 2015, 116, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Pretre, V.; Papadopoulos, D.; Regard, J.; Pelletier, M.; Woo, J. Interleukin-1 (IL-1) and the inflammasome in cancer. Cytokine 2022, 153, 155850. [Google Scholar] [CrossRef]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef]
- Chi, H.; Messas, E.; Levine, R.A.; Graves, D.T.; Amar, S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: Pharmacotherapeutic implications. Circulation 2004, 110, 1678–1685. [Google Scholar] [CrossRef]
- Herman, W.H.; Holcomb, J.M.; Hricik, D.E.; Simonson, M.S. Interleukin-1 beta induces endothelin-1 gene by multiple mechanisms. Transplant. Proc. 1999, 31, 1412–1413. [Google Scholar] [CrossRef]
- Libby, P.; Warner, S.J.; Friedman, G.B. Interleukin 1: A mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J. Clin. Investig. 1988, 81, 487–498. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, S.; Shimpo, M.; Naito, A.; Ogata, Y.; Kobayashi, E.; Ikeda, U.; Shimada, K. beta-very low density lipoprotein enhances inducible nitric oxide synthase expression in cytokine-stimulated vascular smooth muscle cells. Atherosclerosis 2002, 162, 307–313. [Google Scholar] [CrossRef]
- Wang, X.; Feuerstein, G.Z.; Gu, J.L.; Lysko, P.G.; Yue, T.L. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 1995, 115, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, N.; Sukhova, G.K.; Libby, P.; Reynolds, R.S.; Young, J.L.; Schonbeck, U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for atherogenesis. J. Exp. Med. 2002, 195, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Sethwala, A.M.; Goh, I.; Amerena, J.V. Combating Inflammation in Cardiovascular Disease. Heart Lung Circ. 2021, 30, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Kuida, K.; Tsutsui, H.; Ku, G.; Hsiao, K.; Fleming, M.A.; Hayashi, N.; Higashino, K.; Okamura, H.; Nakanishi, K.; et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997, 275, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Walters, S.; Maringe, C.; Coleman, M.P.; Peake, M.D.; Butler, J.; Young, N.; Bergstrom, S.; Hanna, L.; Jakobsen, E.; Kolbeck, K.; et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax 2013, 68, 551–564. [Google Scholar] [CrossRef]
- Kim, S.H.; Azam, T.; Novick, D.; Yoon, D.Y.; Reznikov, L.L.; Bufler, P.; Rubinstein, M.; Dinarello, C.A. Identification of amino acid residues critical for biological activity in human interleukin-18. J. Biol. Chem. 2002, 277, 10998–11003. [Google Scholar] [CrossRef]
- Kim, S.H.; Eisenstein, M.; Reznikov, L.; Fantuzzi, G.; Novick, D.; Rubinstein, M.; Dinarello, C.A. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc. Natl. Acad. Sci. USA 2000, 97, 1190–1195. [Google Scholar] [CrossRef]
- Bresnihan, B.; Roux-Lombard, P.; Murphy, E.; Kane, D.; FitzGerald, O.; Dayer, J.M. Serum interleukin 18 and interleukin 18 binding protein in rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 726–729. [Google Scholar] [CrossRef]
- Ludwiczek, O.; Kaser, A.; Novick, D.; Dinarello, C.A.; Rubinstein, M.; Vogel, W.; Tilg, H. Plasma levels of interleukin-18 and interleukin-18 binding protein are elevated in patients with chronic liver disease. J. Clin. Immunol. 2002, 22, 331–337. [Google Scholar] [CrossRef]
- Mazodier, K.; Marin, V.; Novick, D.; Farnarier, C.; Robitail, S.; Schleinitz, N.; Veit, V.; Paul, P.; Rubinstein, M.; Dinarello, C.A.; et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 2005, 106, 3483–3489. [Google Scholar] [CrossRef]
- Whitman, S.C.; Ravisankar, P.; Daugherty, A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(-/-) mice through release of interferon-gamma. Circ. Res. 2002, 90, E34–E38. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Liu, X.; Bi, X.P.; Xing, S.S.; Li, L.; Gong, H.P.; Zhong, M.; Wang, Z.H.; Zhang, Y.; Zhang, W. IL-18 overexpression promotes vascular inflammation and remodeling in a rat model of metabolic syndrome. Atherosclerosis 2010, 208, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Elhage, R.; Jawien, J.; Rudling, M.; Ljunggren, H.G.; Takeda, K.; Akira, S.; Bayard, F.; Hansson, G.K. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 2003, 59, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Tiret, L.; Godefroy, T.; Lubos, E.; Nicaud, V.; Tregouet, D.A.; Barbaux, S.; Schnabel, R.; Bickel, C.; Espinola-Klein, C.; Poirier, O.; et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation 2005, 112, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, B.; Matthews, V.B.; Brandt, C.; Hojman, P.; Allen, T.L.; Estevez, E.; Watt, M.J.; Bruce, C.R.; Mortensen, O.H.; Syberg, S.; et al. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes 2013, 62, 3064–3074. [Google Scholar] [CrossRef]
- Jung, C.; Gerdes, N.; Fritzenwanger, M.; Figulla, H.R. Circulating levels of interleukin-1 family cytokines in overweight adolescents. Mediat. Inflamm. 2010, 2010, 958403. [Google Scholar] [CrossRef]
- Opstad, T.B.; Pettersen, A.A.; Arnesen, H.; Seljeflot, I. Circulating levels of IL-18 are significantly influenced by the IL-18 +183 A/G polymorphism in coronary artery disease patients with diabetes type 2 and the metabolic syndrome: An observational study. Cardiovasc. Diabetol. 2011, 10, 110. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Mishra, P.K.; Adameova, A.; Hill, J.A.; Baines, C.P.; Kang, P.M.; Downey, J.M.; Narula, J.; Takahashi, M.; Abbate, A.; Piristine, H.C.; et al. Guidelines for evaluating myocardial cell death. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H891–H922. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Aachoui, Y.; Leaf, I.A.; Hagar, J.A.; Fontana, M.F.; Campos, C.G.; Zak, D.E.; Tan, M.H.; Cotter, P.A.; Vance, R.E.; Aderem, A.; et al. Caspase-11 protects against bacteria that escape the vacuole. Science 2013, 339, 975–978. [Google Scholar] [CrossRef]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Aizawa, E.; Karasawa, T.; Watanabe, S.; Komada, T.; Kimura, H.; Kamata, R.; Ito, H.; Hishida, E.; Yamada, N.; Kasahara, T.; et al. GSDME-Dependent Incomplete Pyroptosis Permits Selective IL-1alpha Release under Caspase-1 Inhibition. iScience 2020, 23, 101070. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Singh, S.; Banerjee, R. PLP-dependent H(2)S biogenesis. Biochim. Biophys. Acta 2011, 1814, 1518–1527. [Google Scholar] [CrossRef]
- Xu, W.; Cui, C.; Cui, C.; Chen, Z.; Zhang, H.; Cui, Q.; Xu, G.; Fan, J.; Han, Y.; Tang, L.; et al. Hepatocellular cystathionine gamma lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor. Hepatology 2022, 76, 1794–1810. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Da Settimo, F.; Calderone, V. Hydrogen sulphide: Biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Curr. Med. Chem. 2012, 19, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Szabo, C.; Ichinose, F.; Ahmed, A.; Whiteman, M.; Papapetropoulos, A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol. Sci. 2015, 36, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches. J. Adv. Res. 2021, 27, 99–113. [Google Scholar] [CrossRef]
- Emerson, M. Hydrogen Sulfide and Platelets: A Possible Role in Thrombosis. Handb. Exp. Pharmacol. 2015, 230, 153–162. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Marino, A.; Martelli, A.; Citi, V.; Fu, M.; Wang, R.; Calderone, V.; Levi, R. The novel H2S donor 4-carboxy-phenyl isothiocyanate inhibits mast cell degranulation and renin release by decreasing intracellular calcium. Br. J. Pharmacol. 2016, 173, 3222–3234. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liang, X.; Wu, J.; Dong, S.; Li, H.; Jin, M.; Sun, D.; Zhang, W.; Zhong, X. Inhibition of hydrogen sulfide on the proliferation of vascular smooth muscle cells involved in the modulation of calcium sensing receptor in high homocysteine. Exp. Cell Res. 2016, 347, 184–191. [Google Scholar] [CrossRef]
- Weber, G.J.; Pushpakumar, S.; Tyagi, S.C.; Sen, U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol. Res. 2016, 113 Pt A, 300–312. [Google Scholar] [CrossRef]
- Zavaczki, E.; Jeney, V.; Agarwal, A.; Zarjou, A.; Oros, M.; Katko, M.; Varga, Z.; Balla, G.; Balla, J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011, 80, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Bibli, S.I.; Hu, J.; Sigala, F.; Wittig, I.; Heidler, J.; Zukunft, S.; Tsilimigras, D.I.; Randriamboavonjy, V.; Wittig, J.; Kojonazarov, B.; et al. Cystathionine gamma Lyase Sulfhydrates the RNA Binding Protein Human Antigen R to Preserve Endothelial Cell Function and Delay Atherogenesis. Circulation 2019, 139, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Sepe, I.; Lanza, D.; Capasso, R.; Zappavigna, S.; Capasso, G.; Caraglia, M.; Ingrosso, D. Hydrogen sulfide reduces cell adhesion and relevant inflammatory triggering by preventing ADAM17-dependent TNF-alpha activation. J. Cell. Biochem. 2013, 114, 1536–1548. [Google Scholar] [CrossRef]
- Hu, H.J.; Jiang, Z.S.; Zhou, S.H.; Liu, Q.M. Hydrogen sulfide suppresses angiotensin II-stimulated endothelin-1 generation and subsequent cytotoxicity-induced endoplasmic reticulum stress in endothelial cells via NF-kappaB. Mol. Med. Rep. 2016, 14, 4729–4740. [Google Scholar] [CrossRef]
- Feng, S.; Bowden, N.; Fragiadaki, M.; Souilhol, C.; Hsiao, S.; Mahmoud, M.; Allen, S.; Pirri, D.; Ayllon, B.T.; Akhtar, S.; et al. Mechanical Activation of Hypoxia-Inducible Factor 1alpha Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2087–2101. [Google Scholar] [CrossRef]
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019, 30, 184–197. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Tian, X.; Zhang, L.; Yang, G.; Tao, Y.; Liang, C.; Li, K.; Yu, X.; Tang, X.; et al. The Increased Endogenous Sulfur Dioxide Acts as a Compensatory Mechanism for the Downregulated Endogenous Hydrogen Sulfide Pathway in the Endothelial Cell Inflammation. Front. Immunol. 2018, 9, 882. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Andreis, A.; Imazio, M.; Avondo, S.; Casula, M.; Paneva, E.; Piroli, F.; De Ferrari, G.M. Adverse events of colchicine for cardiovascular diseases: A comprehensive meta-analysis of 14 188 patients from 21 randomized controlled trials. J. Cardiovasc. Med. 2021, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chetrit, E.; Levy, M. Colchicine prophylaxis in familial Mediterranean fever: Reappraisal after 15 years. Semin. Arthritis Rheum. 1991, 20, 241–246. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Gniadecki, R.; Iversen, L.; Bryld, L.E.; Lasthein, S.; Lindhardsen, J.; Kristensen, S.L.; Torp-Pedersen, C.; et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Jacobsson, L.T.; Turesson, C.; Gulfe, A.; Kapetanovic, M.C.; Petersson, I.F.; Saxne, T.; Geborek, P. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1213–1218. [Google Scholar] [PubMed]
- Bouabdallaoui, N.; Tardif, J.C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef] [PubMed]
- Rungapiromnan, W.; Mason, K.J.; Lunt, M.; McElhone, K.; Burden, A.D.; Rutter, M.K.; Warren, R.B.; Griffiths, C.E.M.; Ashcroft, D.M.; Group, B.S. Risk of major cardiovascular events in patients with psoriasis receiving biologic therapies: A prospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T.; ATTACH Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef]
- Mann, D.L.; McMurray, J.J.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Morton, A.C.; Rothman, A.M.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: The MRC-ILA Heart Study. Eur. Heart J. 2015, 36, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, R.; Stahli, B.E.; Heg, D.; Denegri, A.; Manka, R.; Kapos, I.; von Eckardstein, A.; Carballo, D.; Hamm, C.W.; Vietheer, J.; et al. Controlled-Level EVERolimus in Acute Coronary Syndrome (CLEVER-ACS)—A phase II, randomized, double-blind, multi-center, placebo-controlled trial. Am. Heart J. 2022, 247, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Sriranjan, R.; Lu, Y.; Hubsch, A.; Kaloyirou, F.; Vamvaka, E.; Helmy, J.; Kostapanos, M.; Klatzmann, D.; Tedgui, A. Low dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndrome (LILACS). Eur. Heart J. 2020, 41, ehaa946.1735. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tollefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damas, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients With Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Wohlford, G.F.; Van Tassell, B.W.; Billingsley, H.E.; Kadariya, D.; Canada, J.M.; Carbone, S.; Mihalick, V.L.; Bonaventura, A.; Vecchie, A.; Chiabrando, J.G.; et al. Phase 1B, Randomized, Double-Blinded, Dose Escalation, Single-Center, Repeat Dose Safety and Pharmacodynamics Study of the Oral NLRP3 Inhibitor Dapansutrile in Subjects With NYHA II-III Systolic Heart Failure. J. Cardiovasc. Pharmacol. 2020, 77, 49–60. [Google Scholar] [CrossRef]
- Mihaila, R.; Ruhela, D.; Xu, L.; Joussef, S.; Geng, X.; Shi, J.; Kim, A.S.; Yares, W.; Furstoss, K.; Iverson, K. Analytical comparability demonstrated for an IgG4 molecule, inclacumab, following transfer of manufacturing responsibility from Roche to Global Blood Therapeutics. Expert Opin. Biol. Ther. 2022, 22, 1417–1428. [Google Scholar] [CrossRef]
- Rinaldi, R.; Brugaletta, S. Myocardial Infarction with Non-obstructed Coronary Arteries: A Yet Barely Investigated Field with Several Unmet Clinical Needs. EMJ Int. Cardiol. 2023. [Google Scholar] [CrossRef]
- Effects of Curcumin on Markers of Cardiovascular Risk in Patients with CAD. ClinicalTrials.gov Identifier: NCT04458116. Available online: clinicaltrials.gov/ct2/show/NCT04458116 (accessed on 4 May 2023).
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Moreira, D.M.; Lueneberg, M.E.; da Silva, R.L.; Fattah, T.; Gottschall, C.A.M. MethotrexaTE THerapy in ST-Segment Elevation MYocardial InfarctionS: A Randomized Double-Blind, Placebo-Controlled Trial (TETHYS Trial). J. Cardiovasc. Pharmacol. Ther. 2017, 22, 538–545. [Google Scholar] [CrossRef]
- Moreira, D.M.; Vieira, J.L.; Gottschall, C.A. The effects of METhotrexate therapy on the physical capacity of patients with ISchemic heart failure: A randomized double-blind, placebo-controlled trial (METIS trial). J. Card. Fail. 2009, 15, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Pillinger, M.; Zhong, H.; Cronstein, B.; Xia, Y.; Lorin, J.D.; Smilowitz, N.R.; Feit, F.; Ratnapala, N.; Keller, N.M.; et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020, 13, e008717. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.P.; Quinn, S.; Tong, D.; Boyle, A.; Hamilton-Craig, C.; Adams, H.; Layland, J. Colchicine and Quality of Life in Patients with Acute Coronary Syndromes: Results from the COPS Randomized Trial. Cardiovasc. Revasc. Med. 2022, 44, 53–59. [Google Scholar] [CrossRef]
- Bresson, D.; Roubille, F.; Prieur, C.; Biere, L.; Ivanes, F.; Bouleti, C.; Dubreuil, O.; Rioufol, G.; Boutitie, F.; Sideris, G.; et al. Colchicine for Left Ventricular Infarct Size Reduction in Acute Myocardial Infarction: A Phase II, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Study Protocol—The COVERT-MI Study. Cardiology 2021, 146, 151–160. [Google Scholar] [CrossRef]
- Hays, A.G.; Schar, M.; Bonanno, G.; Lai, S.; Meyer, J.; Afework, Y.; Steinberg, A.; Stradley, S.; Gerstenblith, G.; Weiss, R.G. Randomized Trial of Anti-inflammatory Medications and Coronary Endothelial Dysfunction in Patients With Stable Coronary Disease. Front. Cardiovasc. Med. 2021, 8, 728654. [Google Scholar] [CrossRef]
- Marquis-Gravel, G.; Goodman, S.G.; Anderson, T.J.; Bell, A.D.; Bewick, D.; Cox, J.; Gregoire, J.C.; Gupta, A.; Huynh, T.; Kertland, H.; et al. Colchicine for Prevention of Atherothrombotic Events in Patients With Coronary Artery Disease: Review and Practical Approach for Clinicians. Can. J. Cardiol. 2021, 37, 1837–1845. [Google Scholar] [CrossRef]
- Mackenzie, I.S.; Hawkey, C.J.; Ford, I.; Greenlaw, N.; Pigazzani, F.; Rogers, A.; Struthers, A.D.; Begg, A.G.; Wei, L.; Avery, A.J.; et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): A multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 1195–1205. [Google Scholar] [CrossRef]
- Cung, T.T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guerin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Ottani, F.; Latini, R.; Staszewsky, L.; La Vecchia, L.; Locuratolo, N.; Sicuro, M.; Masson, S.; Barlera, S.; Milani, V.; Lombardi, M.; et al. Cyclosporine A in Reperfused Myocardial Infarction: The Multicenter, Controlled, Open-Label CYCLE Trial. J. Am. Coll. Cardiol. 2016, 67, 365–374. [Google Scholar] [CrossRef]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J.; Investigators, A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.K.; Jhund, P.S.; Perez, A.C.; Bohm, M.; Cleland, J.G.; Gullestad, L.; Kjekshus, J.; van Veldhuisen, D.J.; Wikstrand, J.; Wedel, H.; et al. Effect of rosuvastatin on repeat heart failure hospitalizations: The CORONA Trial (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail. 2014, 2, 289–297. [Google Scholar] [CrossRef]
- Available online: clinicaltrials.gov/ct2/show/NCT05211401 (accessed on 4 May 2023).
- Available online: clinicaltrials.gov/ct2/show/NCT04762472 (accessed on 4 May 2023).
- Zheng, Z.; Zhu, S.; Lv, M.; Gu, Z.; Hu, H. Harnessing nanotechnology for cardiovascular disease applications-a comprehensive review based on bibliometric analysis. Nano Today 2022, 44, 101453. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Miller, A.; Agarwal, C.; Zakin, E.; Acholonu, M.; Gidwani, U.; Sharma, A.; Kulbak, G.; Shani, J.; Chen, O. Imaging Modalities to Identity Inflammation in an Atherosclerotic Plaque. Radiol. Res. Pract. 2015, 2015, 410967. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, M.; Liu, Y.; Li, H.; Shang, L.; Xu, T.; Chen, Z.; Wang, F.; Qiao, T.; Li, K. Iron accumulation in macrophages promotes the formation of foam cells and development of atherosclerosis. Cell Biosci. 2020, 10, 137. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84, 104802. [Google Scholar] [CrossRef]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [Google Scholar] [CrossRef]
- Nishi, K.; Imamura, T.; Kitamura, K.; Ogawa, T.; Fujimoto, S.; Kakitsubata, Y.; Ishikawa, T.; Asada, Y.; Kodama, T. Associations of plasma pentraxin 3 and monocyte chemoattractant protein-1 concentrations with cardiovascular disease in patients with chronic kidney disease. Ren. Fail. 2011, 33, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Temelli, B.; Yetkin Ay, Z.; Savas, H.B.; Aksoy, F.; Kumbul Doguc, D.; Uskun, E.; Varol, E. Circulation levels of acute phase proteins pentraxin 3 and serum amyloid A in atherosclerosis have correlations with periodontal inflamed surface area. J. Appl. Oral Sci. 2018, 26, e20170322. [Google Scholar] [CrossRef] [PubMed]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Associations of pentraxin 3 with cardiovascular disease and all-cause death: The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Napoleone, E.; di Santo, A.; Peri, G.; Mantovani, A.; de Gaetano, G.; Donati, M.B.; Lorenzet, R. The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: Another link between inflammation and clotting activation. J. Leukoc. Biol. 2004, 76, 203–209. [Google Scholar] [CrossRef]
- Befekadu, R.; Grenegard, M.; Larsson, A.; Christensen, K.; Ramstrom, S. Dynamic Changes in Pentraxin-3 and Neprilysin in ST Segment Elevation Myocardial Infarction. Biomedicines 2022, 10, 275. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Antiaging Vaccines Targeting Senescent Cells. Rejuvenation Res. 2022, 25, 39–45. [Google Scholar] [CrossRef]
- Kanno, Y. The uPA/uPAR System Orchestrates the Inflammatory Response, Vascular Homeostasis, and Immune System in Fibrosis Progression. Int. J. Mol. Sci. 2023, 24, 1796. [Google Scholar] [CrossRef]
- Kozlov, S.; Okhota, S.; Avtaeva, Y.; Melnikov, I.; Matroze, E.; Gabbasov, Z. Von Willebrand factor in diagnostics and treatment of cardiovascular disease: Recent advances and prospects. Front. Cardiovasc. Med. 2022, 9, 1038030. [Google Scholar] [CrossRef]
- Scherpereel, A.; Depontieu, F.; Grigoriu, B.; Cavestri, B.; Tsicopoulos, A.; Gentina, T.; Jourdain, M.; Pugin, J.; Tonnel, A.B.; Lassalle, P. Endocan, a new endothelial marker in human sepsis. Crit. Care Med. 2006, 34, 532–537. [Google Scholar] [CrossRef]
- Nirala, B.K.; Perumal, V.; Gohil, N.K. Glycated serum albumin stimulates expression of endothelial cell specific molecule-1 in human umbilical vein endothelial cells: Implication in diabetes mediated endothelial dysfunction. Diab. Vasc. Dis. Res. 2015, 12, 290–297. [Google Scholar] [CrossRef]
- Perkins, J.M.; Joy, N.G.; Tate, D.B.; Davis, S.N. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E168–E176. [Google Scholar] [CrossRef] [PubMed]
- Noflatscher, M.; Schreinlechner, M.; Sommer, P.; Kerschbaum, J.; Theurl, M.; Kirchmair, R.; Bauer, A.; Marschang, P. Effect of chronic kidney disease and sex on carotid and femoral plaque volume as measured by three-dimensional ultrasound. Clin. Nephrol. 2021, 96, 199. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.A.; Register, T.C.; Riley, R.F.; D’Agostino, R.B., Jr.; Stopyra, J.P.; Miller, C.D. Monocyte Chemoattractant Protein-1 as a Predictor of Coronary Atherosclerosis in Patients Receiving Coronary Angiography. Crit. Pathw. Cardiol. 2018, 17, 105–110. [Google Scholar] [CrossRef]
- Available online: clinicaltrials.gov/ct2/show/record/NCT00329069 (accessed on 4 May 2023).
- Latini, R.; Maggioni, A.P.; Peri, G.; Gonzini, L.; Lucci, D.; Mocarelli, P.; Vago, L.; Pasqualini, F.; Signorini, S.; Soldateschi, D.; et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2004, 110, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
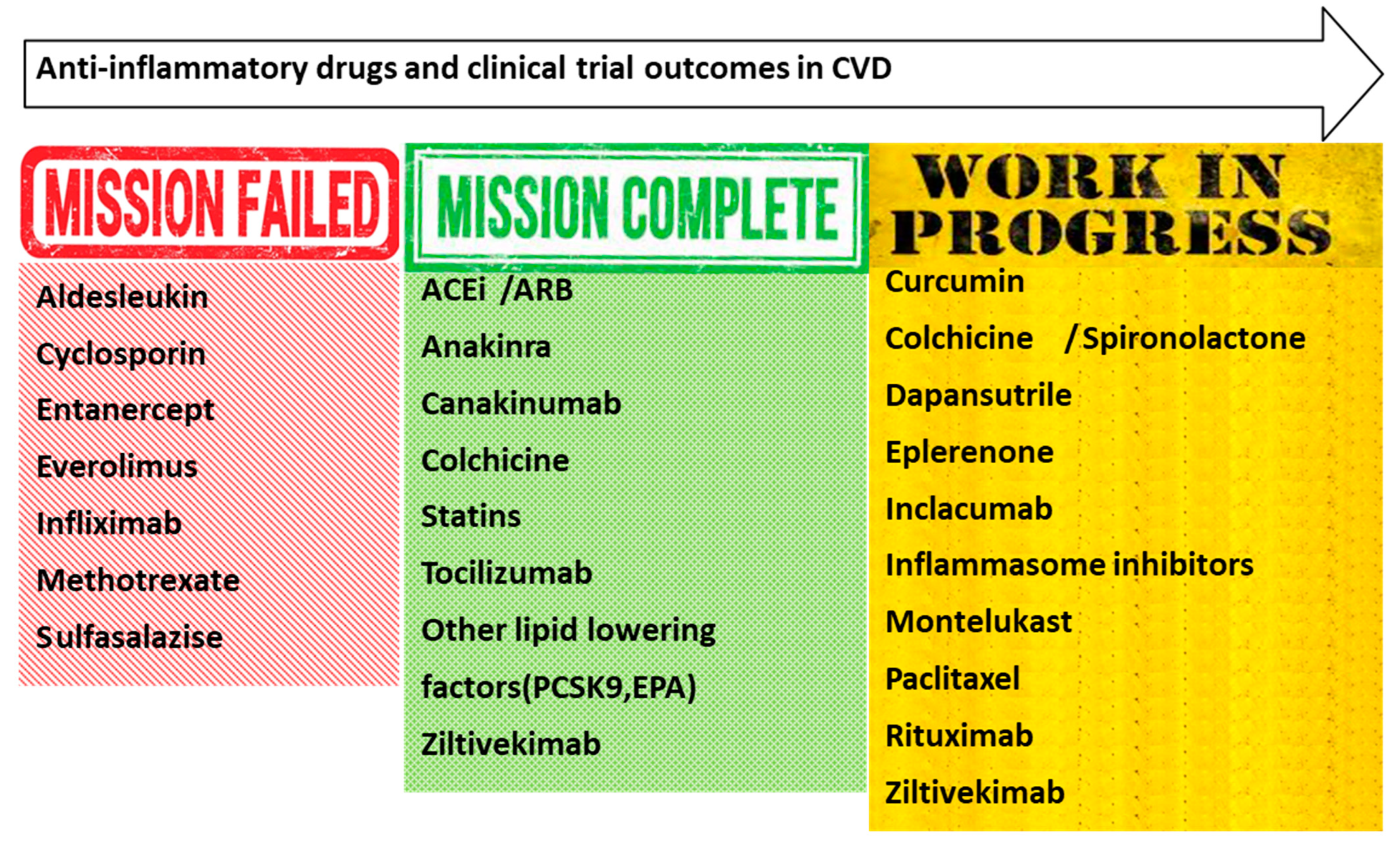
| Trial | Drug | Target Molecule-Signaling Pathways | Study Population Primary Outcome | Refs. |
|---|---|---|---|---|
| Cantos | Canakinumab | IL-1β inhibition | Previous MI, nonfatal MI, nonfatal stroke, CV death, reduced hsCRP, IL-6−17% in primary endpoints/higher incidence of fatal infections | [27] |
| ATTACH | Infliximab | NF-κB/TNF-α | Chronic heart failure ↑ mortality and hospitalization/no improvement in the clinical status of NYHA III–IV HF patients | [129] |
| Sulfasalazine and Endothelial Function (NCT00554203) | Sulfasalazine | ↓ NF-κB activation ↓ inflammatory TNF-α-induced genes | History of CAD/no amelioration of ED in patients with CAD, no effects on systemic inflammatory biomarkers | [34] |
| RENEWAL trial | Etanercept | TNF-α soluble antagonist | No clinically relevant benefit in congestive heart failure | [130] |
| VCUART3 (NCT01950299) | Anakinra | IL-1R blocker | STEMI patients/ lower incidence of death or new-onset heart failure or of death and hospitalization for heart failure/significantly reduces the systemic inflammatory response compared with placebo | [131] |
| MRC-ILA | Anakinra | As above | NSTEMI/49% reduction in CRP levels over first 7 days/significant increase in MACE at 1-year follow-up | [132] |
| CLEVER-ACS | Everolimus | mTOR pathway | STEMI PATIENTS No reduction in MI size No improvement of microvascular obstruction | [133] |
| LILACS | Aldesleukin (recombinant human IL-2) | IL-2 receptor Treg cells | Stable ischemic heart disease and ACS/increase in Tregs but not CD4+ effector T cells | [134] |
| ASSAIL-MI | Tocilizumab | IL-6 | STEMI within 6 h/5.6% increase in myocardial salvage index/no significant difference in infarct size at 6 months/significant reduction in CRP | [135] |
| RESCUE | Ziltivekimab | IL-6 | Chronic kidney disease, CRP ≥2 mg/L/significantly greater reduction in CRP from baseline at 12 weeks, a significant reduction in lipoprotein A, but no change in LDL/HDL ratio | [136] |
| Study of Dapansutrile Capsules in Heart Failure | Dapansutrile | NLRP3 inflammasome inhibitor | Stable patients with HFrEF/ changes in left ventricular ejection fraction will be analyzed | [137] |
| NCT04927247 | Inclacumab | Monoclonal antibody targeting P-selectin | Sickle cell disease vaso-occlusive crisis/vaso-occlusive pain episode in sickle cell disease | [138] |
| StratMed-MINOCA | Eplerenone | Reduces blood vessel injury and is used to treat heart failure | MI, AMI with nonobstructive coronary artery myocardial injury-COVID-19 to test the use of eplerenone in patients with heart attack/heart injury who have small vessel disease, including patients with COVID-19 | [139] |
| Curcumin Supplementation Effects on Markers of Cardiovascular Risk, Inflammation, Oxidative Stress, and Functional Capacity in Patients with CAD | Curcumin is produced by turmeric root (Curcuma longa) | Promoting the activation of inflammasome (NLPR3) | The effects of curcumin supplementation on inflammatory cytokines | [140] |
| CIRT | Methotrexate | Replication inhibition of B cells, T cells neutrophils, monocytes | Previous MI and T2 diabetes metabolic syndrome, nonfatal MI, nonfatal stroke, CV death, no change in hs-CRP, IL-6, IL-1β, no reduction in primary endpoints | [141] |
| TETHYS | Methotrexate | As above | STEMI within 12 h, no effect on creatine kinase release over first 72 h/no difference in CRP levels/significantly worse LVEF in the methotrexate group at 3 months/no effect on the incidence of MACE | [142] |
| METIS trial | Methotrexate | Ischemic chronic heart failure | No difference in 6 min walk time before vs. after treatment/no effect on CRP levels/no effect on the incidence of MACE | [143] |
| COLCOT | Colchicine | Inhibition of microtubule polymerization reduced IL-1β, IL-6 | 1 month after MI, CV death, MI stroke −23% in primary endpoints | [127] |
| LoDoCo2 | Low-dose colchicine | As above | Acute and chronic CAD/as above, −31% of primary endpoints | [121,144] |
| COLCHICINE-PCI | Colchicine | As above | Patients referred for PCI/no significant difference in PCI-related myocardial injury or MACE within 30 days/elevation of plasma IL-6 and CRP from baseline—24 h s significantly reduced in the colchicine group/increased adverse gastrointestinal symptoms in the colchicine group | [145] |
| COPS | Colchicine | As above | ACS/no significant difference in MACE/increased total death incidence in the colchicine group, mostly related to sepsis | [146] |
| COVERT-MI | Colchicine | As above | STEMI within 12 h/no significant reduction in infarct size at 5 days/no significant reduction in MACE at 3 months’ follow-up/no difference in inflammatory marker (WBC count, CRP) at 48 h | [147] |
| Randomized Trial of Anti-inflammatory Medications and Coronary Endothelial Dysfunction in Patients with Stable Coronary Disease | Colchicine/methotrexate | As above | Patients with stable CAD and either elevated hsCRP or diabetes/metabolic syndrome on stable statin therapy failed to improve coronary endothelial function | [148] |
| CLEAR SYNERGY | Colchicine/spironolactone | As above | Patients with STEMI/evaluation of markers of neutrophil activity at randomization (baseline) and 3 months’ follow-up in the colchicine versus placebo groups, and examination of clinical and genetic factors that determine the heterogeneity of treatment response and distinguish colchicine responders from nonresponders | [149] |
| ALL HEART | Allopurinol | Inhibitor of xanthine oxidase, ROS pathway | ≥60 years old with ischemic heart disease but no history of gout/no difference in the primary outcome of nonfatal MI, nonfatal stroke, or cardiovascular death | [150] |
| CIRCUS | Cyclosporine | T cell activation, macrophage ROS/cytokine production | STEMI within 12 h/no effect on any cause of death/No effect on remodeling or MACE incidence at 6 months | [151] |
| CYCLE | Cyclosporine | As above | STEMI within 6 h/no effect on ST-segment resolution at 1 h/no effect on LV remodeling or incidence of MACE at 6 months | [152] |
| Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin [153] | Rosuvastatin | Inhibits NF-κB, TNF-α, and IL-6 | Intensive lipid lowering using rosuvastatin 40 mg daily on the progression of AS/↓ CRP levels ↓ LDL cholesterol | [153] |
| GISSI-HF (Gruppo Italiano Per Lo Studio Della Sopravvivenza Nell’Insufficienza Cardiaca) | Rosuvastatin | As above | Heart failure/↓ CRP levels ↓ LDL cholesterol | [154] |
| CORONA | Rosuvastatin | As above | Heart failure/↓ CRP levels ↓ LDL cholesterol | [155] |
| anaRITA MI2 | Rituximab | B-cell depletion with CD20 | Patients with STEMI and LVEF at 6 months with CMR | [156] |
| Air Pollution (PM2.5) on Accelerated Atherosclerosis: A Montelukast Interventional Study in Modernizing China | Montelukast | Leukotriene receptor antagonist | Subclinical atherosclerosis defined as changes in brachial flow-mediated dilation and carotid intima–media thickness | [157] |
| PAC-MAN | Paclitaxel | Blocks cellular proliferation (antimicrotubule agents) | Patients with stable CAD/reduction in plaque size measured by CCTA from baseline to 6–8 months | [158] |
| Target Molecules | Signaling Pathways | CVD Primary Endpoint Outcome | Refs. |
|---|---|---|---|
| ICAM-1 (CD54) | Leukocyte adhesion | Associates with the incidence of CAD and carotid atherosclerosis independent of known CVRF. | [163] |
| VCAM-1 (CD106) | Leukocyte adhesion | Baseline VCAM-1 is increased in initially healthy middle-aged men who develop cardiovascular disease. | [163] |
| E-selectin (CD62E) | Leukocyte adhesion | Associates with the incidence of CAD and carotid atherosclerosis independent of known CVRF. | [175] |
| P-selectin (CD62) | Leukocyte adhesion | Elevated levels predict early adverse events in patients with presumed CAD. | [175] |
| Correlation of atherosclerotic plaque volume and intima–media thickness with soluble P-selectin | Leukocyte adhesion | Correlation between P-selectin and the progression of atherosclerosis as measured by plaque volume and IMT in the carotid and femoral arteries, respectively. Secondary endpoints will include the correlation of established (hypertension, smoking, diabetes, dyslipidemia) and novel risk factors (hs-CRP, P-selectin, CETP, ICAM-1. | [176] |
| MCP-1 | Leukocyte adhesion | Correlation with the risk of incident PAD and CAD independent of traditional cardiovascular risk factors. | [177] |
| Vascular endothelial receptor activity in patients with CAD on medication with statins/MCP-1 | Leukocyte adhesion | MCP-1-induced monocyte chemotaxis after 1-month treatment with atorvastatin 40 mg or a placebo once a day. | [178] |
| PTX3/Lipid Assessment Trial Italian Network (LATIN) | NF-κB | Patients with MI with ST elevation PTX3 prognostic tool: 3-month mortality in patients with MI and ST elevation. | [27,179] |
| Dynamic changes in pentraxin 3 and neprilysin in ST-segment elevation myocardial infarction | PTX3 and neurolysin | Confirmation of the differences in kinetics between the two pentraxins CRP and PTX3, with PTX3 levels being high already in the acute samples while the peak for CRP came 1–3 days after PCI. Neprilysin is not generally elevated during STEMI, although a few patients showed very high levels. | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. https://doi.org/10.3390/life13061420
Dri E, Lampas E, Lazaros G, Lazarou E, Theofilis P, Tsioufis C, Tousoulis D. Inflammatory Mediators of Endothelial Dysfunction. Life. 2023; 13(6):1420. https://doi.org/10.3390/life13061420
Chicago/Turabian StyleDri, Eirini, Evangelos Lampas, George Lazaros, Emilia Lazarou, Panagiotis Theofilis, Costas Tsioufis, and Dimitris Tousoulis. 2023. "Inflammatory Mediators of Endothelial Dysfunction" Life 13, no. 6: 1420. https://doi.org/10.3390/life13061420
APA StyleDri, E., Lampas, E., Lazaros, G., Lazarou, E., Theofilis, P., Tsioufis, C., & Tousoulis, D. (2023). Inflammatory Mediators of Endothelial Dysfunction. Life, 13(6), 1420. https://doi.org/10.3390/life13061420








