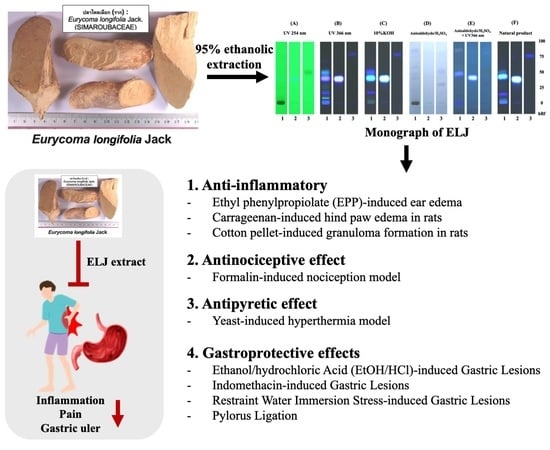
Anti-Inflammatory, Antinociceptive, Antipyretic, and Gastroprotective Effects of Eurycoma longifolia Jack Ethanolic Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extract Preparation
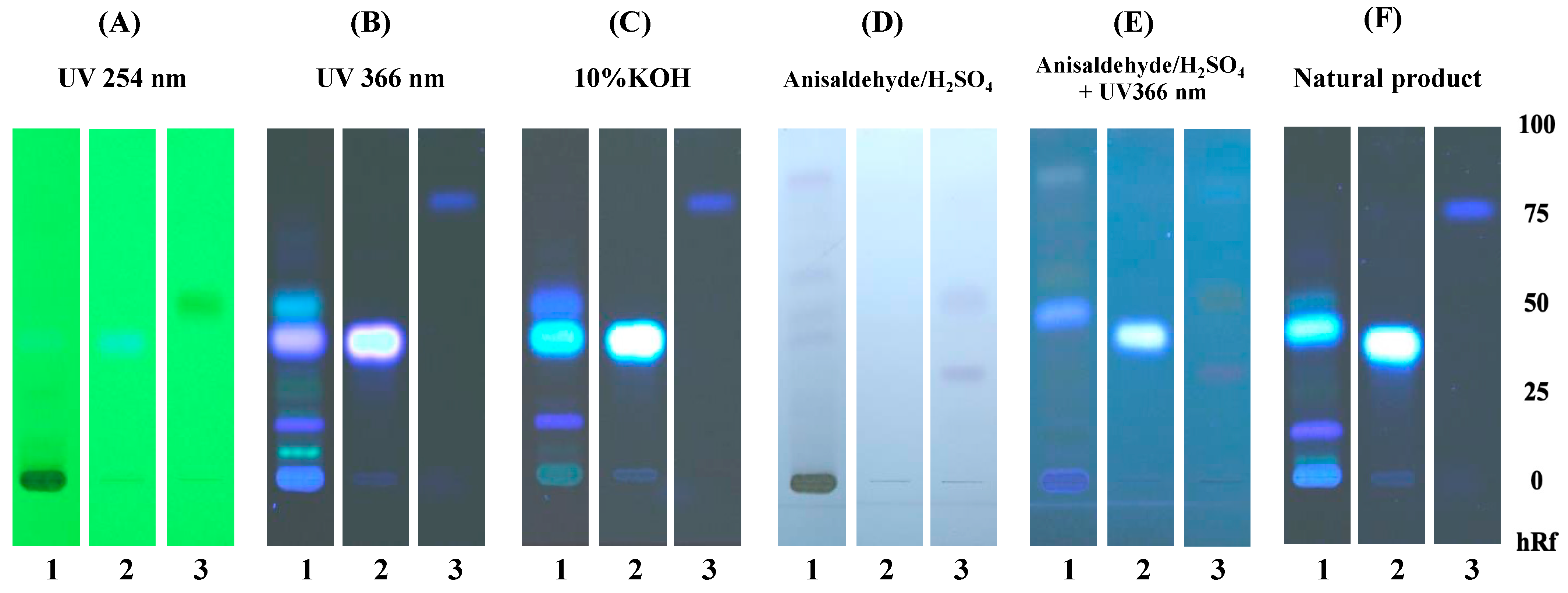
2.3. Phytochemical Constituents of E. longifolia as Determined by TLC
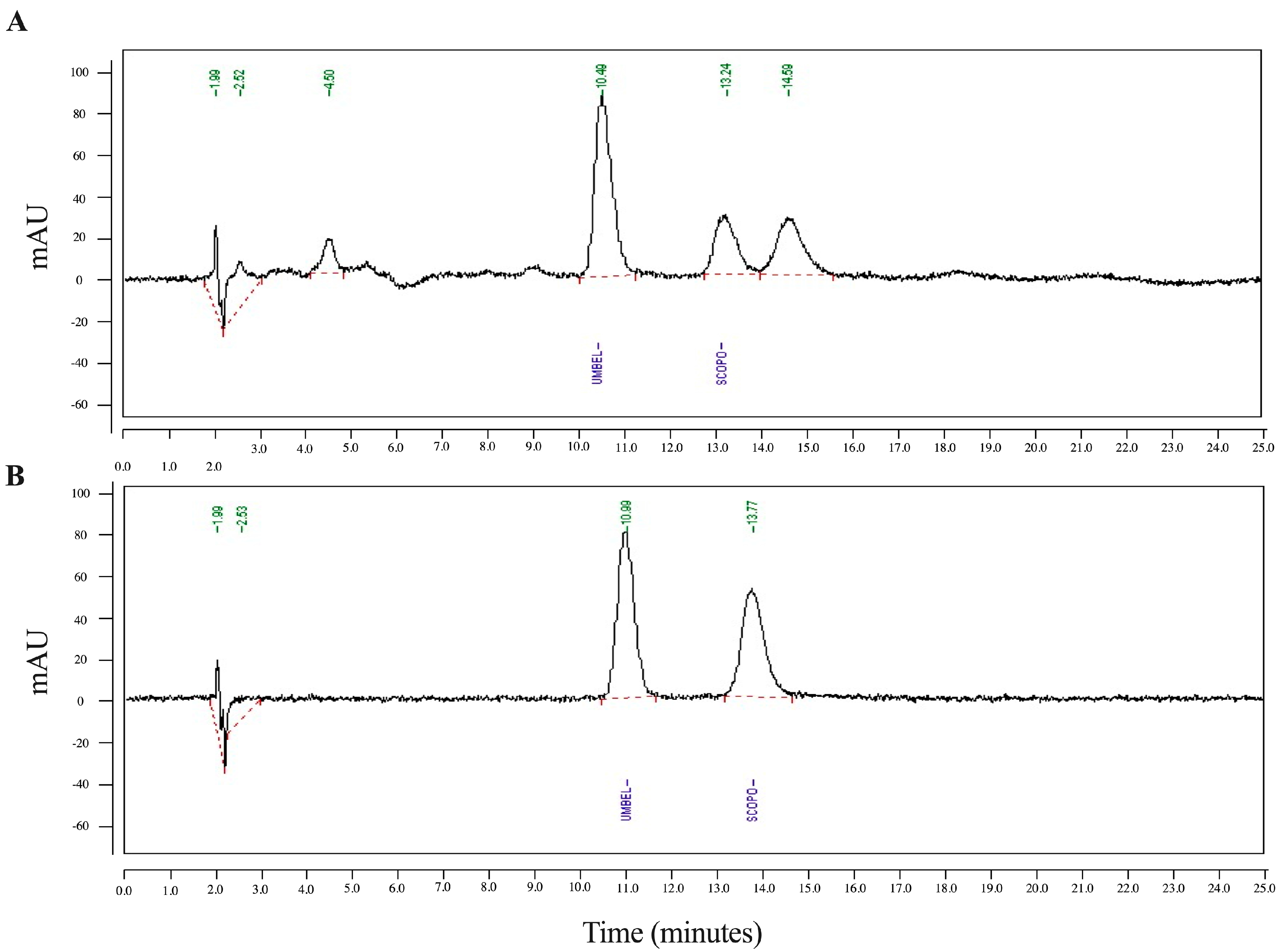
2.4. Identification of Phytochemicals by High-Pressure Liquid Chromatography (HPLC)
2.5. Animals
2.6. Test Substance Administration
2.7. Anti-Inflammatory Activity
2.7.1. Ethyl Phenylpropiolate (EPP)-Induced Ear Edema
2.7.2. Carrageenan-Induced Hind Paw Edema in Rats
2.7.3. Cotton-pellet-induced Granuloma Formation in Rats
2.8. Formalin-Induced Nociception Model
2.9. Antipyretic Activity
2.10. Gastric Ulcer Models
2.10.1. Ethanol/Hydrochloric Acid (EtOH/HCl)-Induced Gastric Lesions
2.10.2. Indomethacin-Induced Gastric Lesions
2.10.3. Restraint Water Immersion Stress-Induced Gastric Lesions
2.11. Pylorus Ligation
2.12. Statistical Analysis
3. Results
3.1. Specification of E. longifolia Jack by TLC and HPLC Determinations
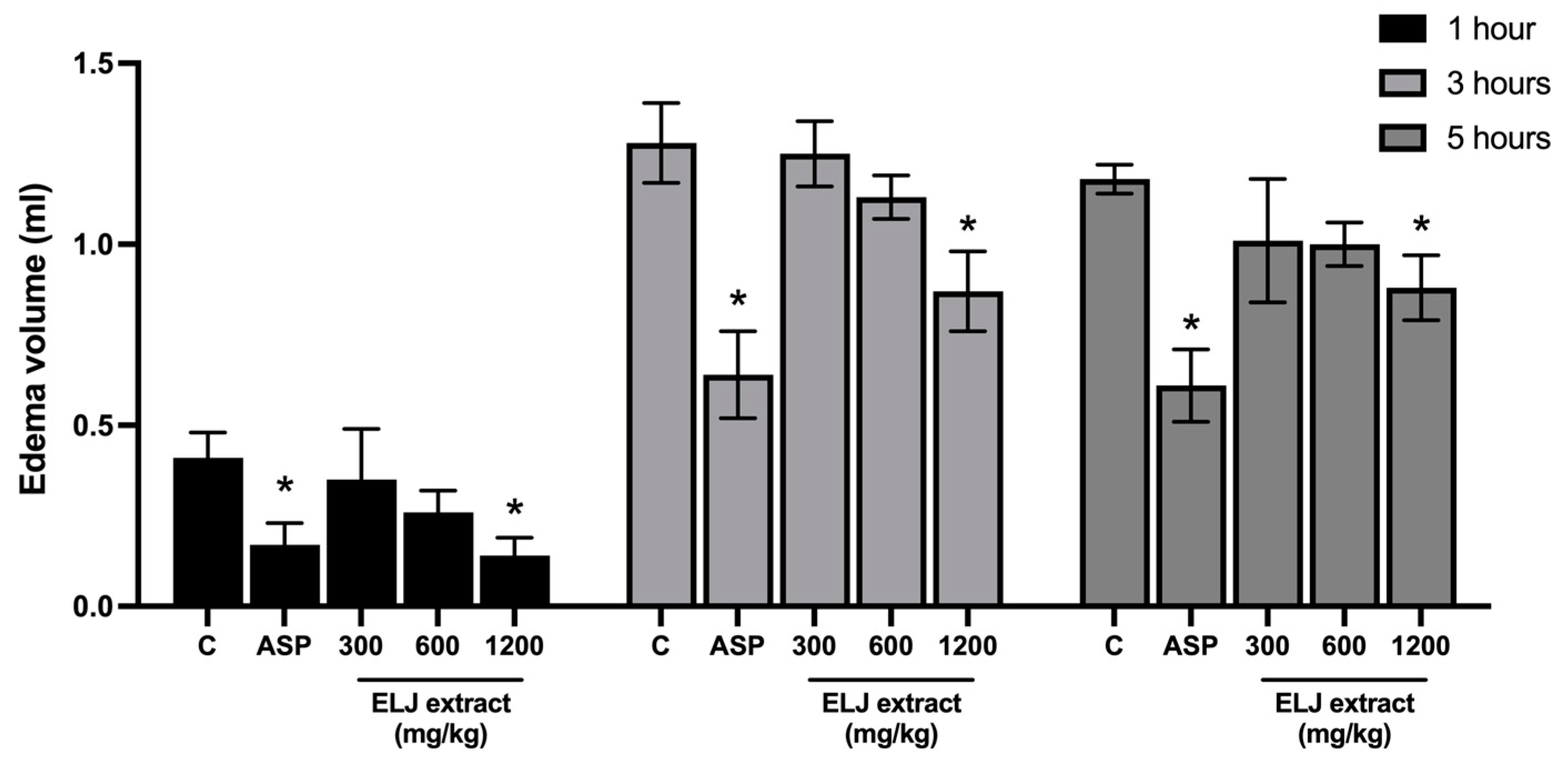
3.2. EPP-Induced Ear Edema
3.3. Carrageenan-Induced Hind Paw Edema
3.4. Cotton-Pellet-Induced Granuloma Formation
3.5. Formalin-Induced Nociception Model
3.6. Antipyretic Activity
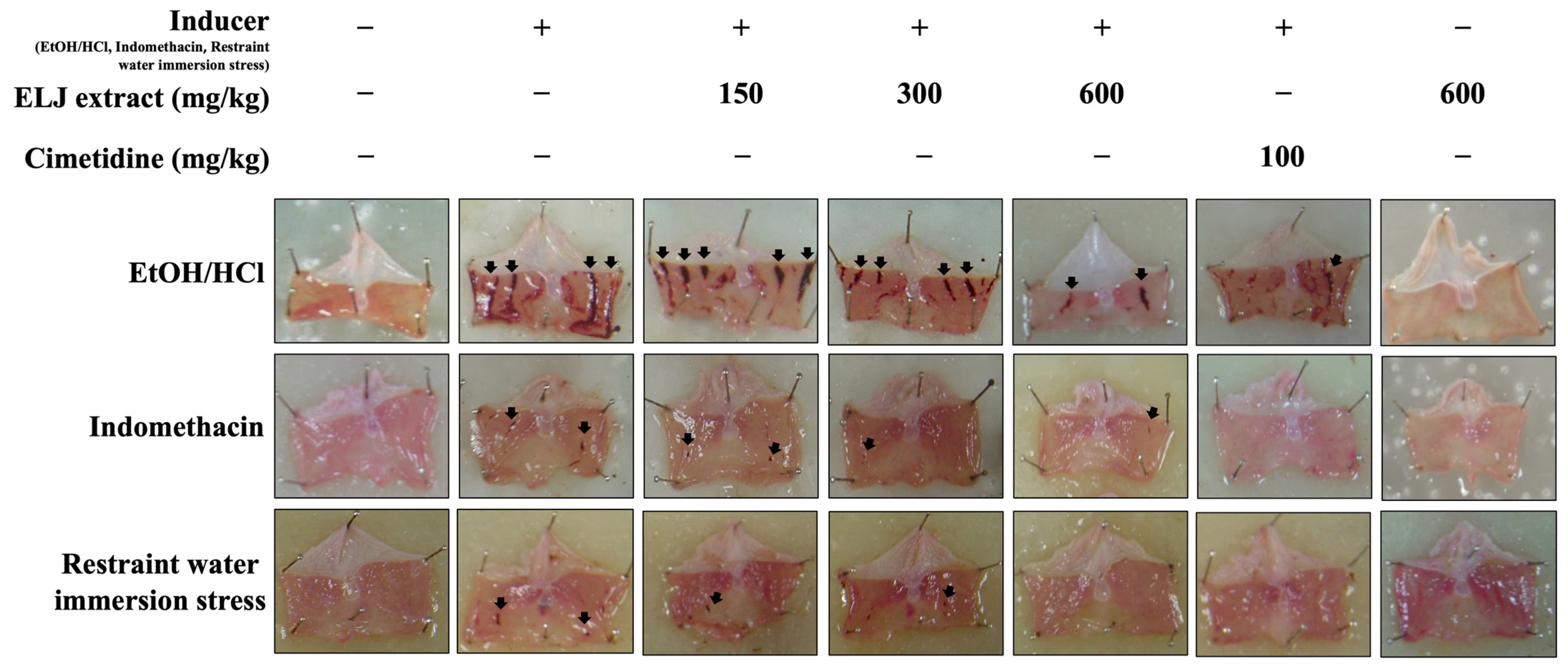
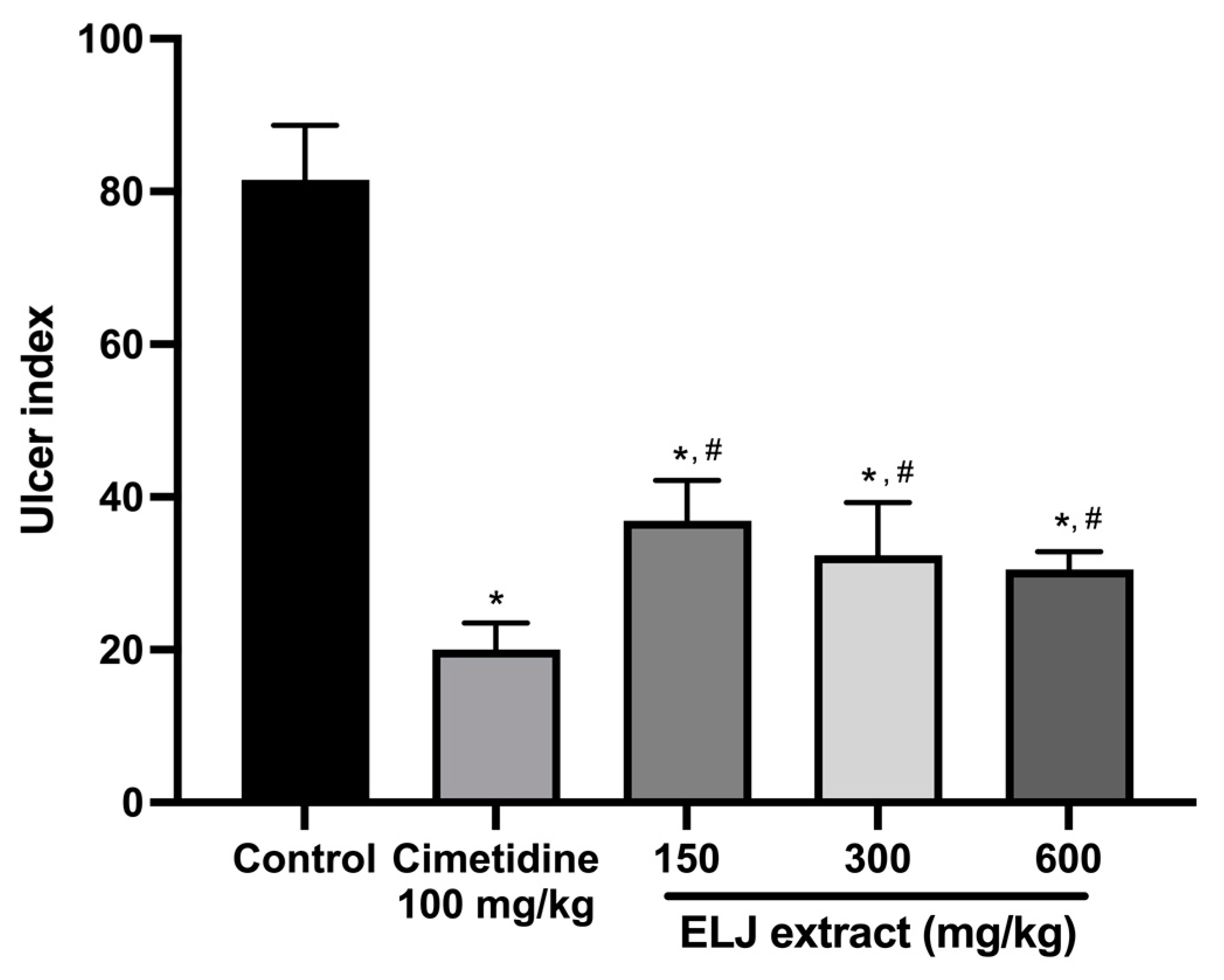
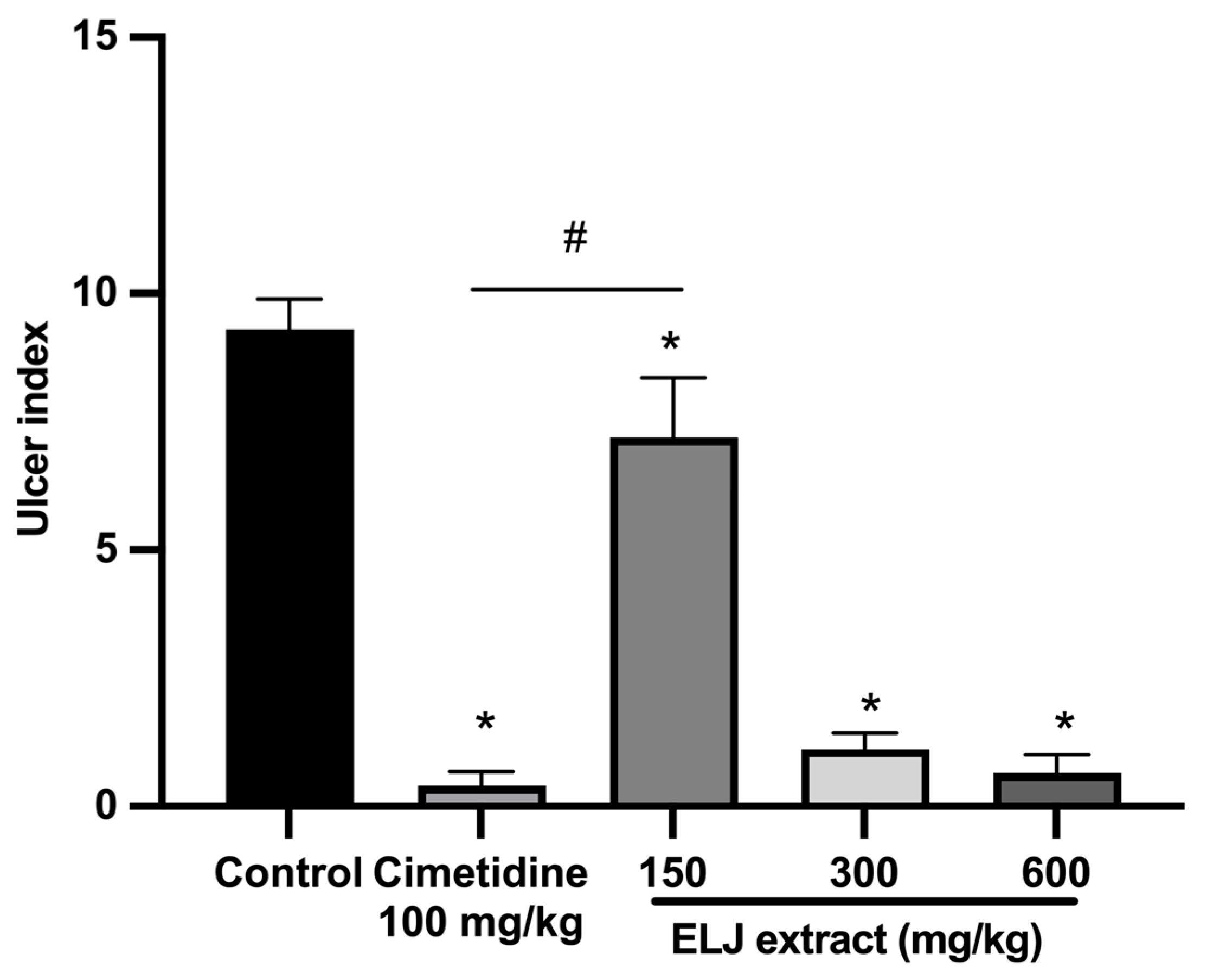
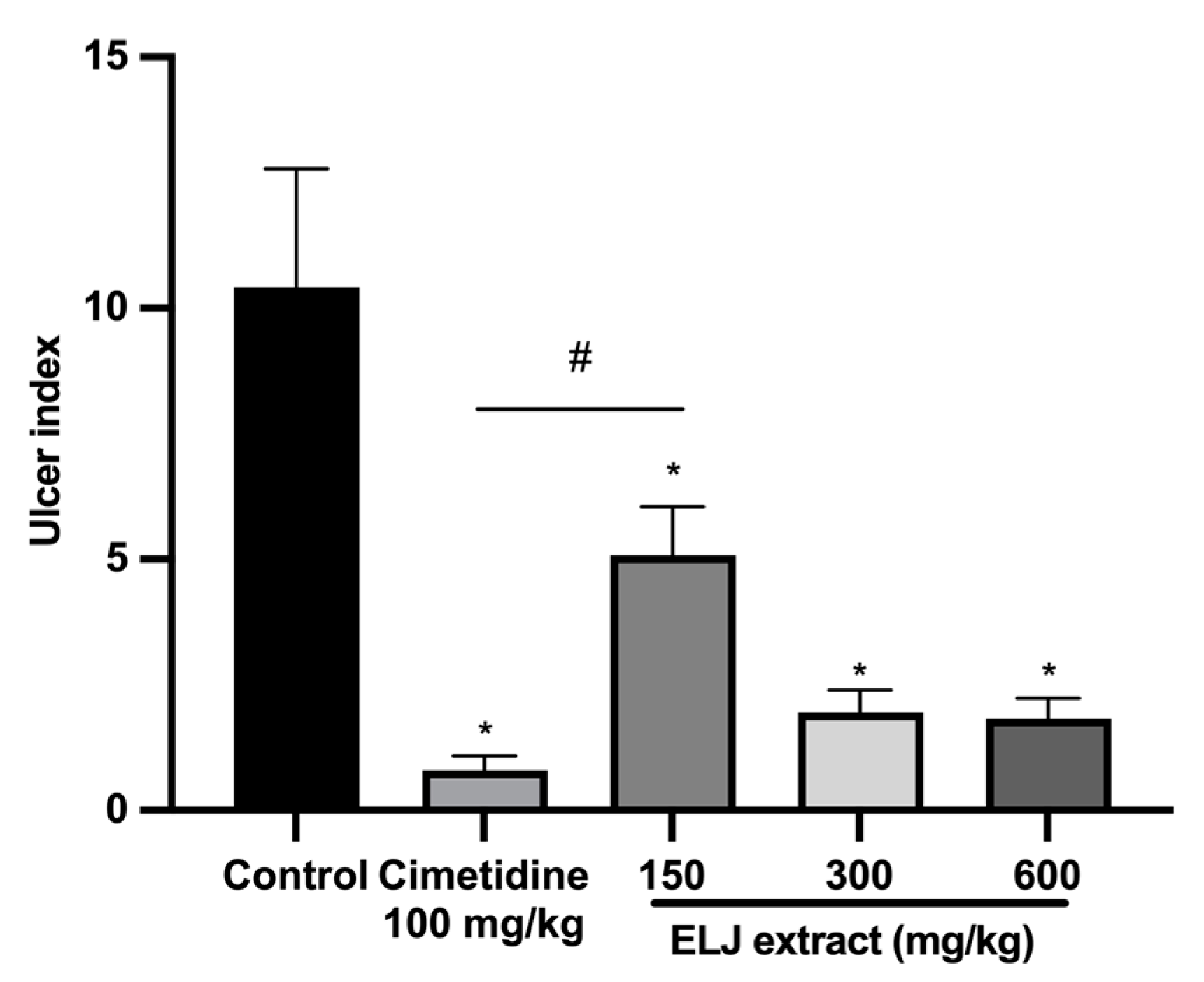
3.7. Anti-Ulcerogenic Activities of ELJ Extract in Gastric Ulcer Models
3.7.1. Effect of ELJ on the EtOH/HCl-Induced Gastric Ulcer in Rats
3.7.2. Effect of ELJ and Cimetidine on Indomethacin-Induced Gastric Ulcer in Rats
3.7.3. Effect of ELJ on Restrained Water Immersion Stress-Induced Gastric Ulcer in Rats
3.8. Pylorus Ligation Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–131. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-inflammatory Agents: An Update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef]
- Marinaccio, L.; Zengin, G.; Pieretti, S.; Minosi, P.; Szucs, E.; Benyhe, S.; Novellino, E.; Masci, D.; Stefanucci, A.; Mollica, A. Food-inspired peptides from spinach Rubisco endowed with antioxidant, antinociceptive and anti-inflammatory properties. Food Chem. 2023, 18, 100640. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Sinan, K.I.; Marletta, M.; Pieretti, S.; Stefanucci, A.; Etienne, O.K.; Jekő, J.; Cziáky, Z.; Bahadori, M.B.; et al. A Study on Chemical Characterization and Biological Abilities of Alstonia boonei Extracts Obtained by Different Techniques. Antioxidants 2022, 11, 2171. [Google Scholar] [CrossRef]
- Rehman, S.U.; Choe, K.; Yoo, H.H. Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules 2016, 21, 331. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Damu, A.G.; Lee, K.-H.; Wu, T.-S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. 2004, 12, 537–544. [Google Scholar] [CrossRef]
- Herbal Medicine Reference Textbook Preparation Subcommittee. Monograph of selected Thai materia medica: PLA LAI PUEAK-RAK. J. Thai Trad. Alt. Med. 2018, 16, 158–162. [Google Scholar]
- Office of Resource and Information Technology Kamphaeng Phet Rajabhat University. PLA LAI PUEAK. Available online: https://arit.kpru.ac.th/ap2/local/?nu=pages&page_id=1660&code_db=610010&code_type=01 (accessed on 29 December 2022).
- Bedir, E.; Abou-Gazar, H.; Ngwendson, J.N.; Khan, I.A. Eurycomaoside: A new quassinoid-type glycoside from the roots of Eurycoma longifolia. Chem. Pharm. Bull. 2003, 51, 1301–1303. [Google Scholar] [CrossRef]
- Guo, Z.; Vangapandu, S.; Sindelar, R.W.; Walker, L.A.; Sindelar, R.D. Biologically active quassinoids and their chemistry: Potential leads for drug design. Curr. Med. Chem. 2005, 12, 173–190. [Google Scholar] [CrossRef]
- Ang, H.H.; Cheang, H.S.; Yusof, A.P.M. Effects of Eurycoma longifolia Jack (Tongkat Ali) on the initiation of sexual performance of inexperienced castrated male rats. Exp. Anim. 2000, 49, 35–38. [Google Scholar] [CrossRef]
- Low, B.S.; Choi, S.B.; Abdul Wahab, H.; Das, P.K.; Chan, K.L. Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J Ethnopharmacol. 2013, 149, 201–207. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia 2010, 81, 669–679. [Google Scholar] [CrossRef]
- Han, Y.M.; Woo, S.U.; Choi, M.S.; Park, Y.N.; Kim, S.H.; Yim, H.; Yoo, H.H. Antiinflammatory and analgesic effects of Eurycoma longifolia extracts. Arch. Pharm. Res. 2016, 39, 421–428. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Khonsung, P.; Piyabhan, P.; Nanna, U.; Soonthornchareonnon, N.; Jaijoy, K. Anti-inflammatory and anti-ulcerogenic activities of Chantaleela recipe. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 485–494. [Google Scholar] [CrossRef]
- Qodriyah, H.; Asmadi, A. Eurycoma longifolia in Radix for the treatment of ethanol-induced gastric lesion in rats. Pak. J. Biol. Sci. 2013, 16, 1815–1818. [Google Scholar] [CrossRef]
- Bureau of Drug and Narcotic, Department of Medicine Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia Volume IV; Department of Medical Sciences: Bangkok, Thailand, 2014.
- Tung, N.H.; Uto, T.; Hai, N.T.; Li, G.; Shoyama, Y. Quassinoids from the root of Eurycoma longifolia and their antiproliferative activity on human cancer cell lines. Pharmacogn. Mag. 2017, 13, 459. [Google Scholar]
- Bao, Y.; Ji, W.H.; Ma, Y.H.; Ji, L.J. Simultaneous determination of six main constituents in Swertia of Qinghai Province and Sichuan Province by HPLC. Zhongguo Zhong Yao Za Zhi 2006, 31, 2036–2038. [Google Scholar]
- Nie, Y.; Lin, P. Determination of five active constituents in aerial part of Tibetan medicine Gentiana straminea by HPLC. Zhongguo Zhong Yao Za Zhi 2010, 35, 1276–1279. [Google Scholar] [CrossRef]
- Brattsand, R.; Thalén, A.; Roempke, K.; Källström, L.; Gruvstad, E. Influence of 16 alpha, 17 alpha-acetal substitution and steroid nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J. Steroid. Biochem. 1982, 16, 779–786. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Swingle, K.F.; Shideman, F.E. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J. Pharmacol. Exp. Ther. 1972, 183, 226–234. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Teotino, U.M.; Friz, L.P.; Gandini, A.; Dellabella, D. Thio Derivatives of 2,3-Dihydro-4h-1,3-Benzoxazin-4-One. Synthesis And Pharmacological Properties. J. Med. Chem. 1963, 6, 248–250. [Google Scholar] [CrossRef]
- Mizui, T.; Doteuchi, M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats. Jpn. J. Clin. Pharmacol. Ther. 1983, 33, 939–945. [Google Scholar] [CrossRef]
- Djahanguiri, B. The production of acute gastric ulceration by indomethacin in the rats. Scand J. Gastroenterol. 1969, 4, 265–267. [Google Scholar]
- Hayden, L.J.; Thomas, G.; West, G. Inhibitors of gastric lesions in the rat. J. Pharm. Pharmacol. 1978, 30, 244–246. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, B.-W.; Kwon, H.-J.; Nam, S.-W. Curative effect of selenium against indomethacin-induced gastric ulcers in rats. J. Microbiol. Biotechnol. 2011, 21, 400–404. [Google Scholar] [CrossRef]
- Takagi, K.; Kasuya, Y.; Watanabe, K. Studies on the Drugs for Peptic Ulcer. A Reliable Method for Producing Stress Ulcer in Rats. Chem. Pharm. Bull. 1964, 12, 465–472. [Google Scholar] [CrossRef]
- Lu, C.-L.; Li, Z.-P.; Zhu, J.-P.; Zhao, D.-Q.; Ai, H.-B. Studies on functional connections between the supraoptic nucleus and the stomach in rats. J. Physiol. Sci. 2011, 61, 191–199. [Google Scholar] [CrossRef]
- SHAY, H. A simple method for the uniform production of gastric ulceration in the rats. Gastroenterology 1945, 5, 143–149. [Google Scholar]
- Chaingam, J.; Juengwatanatrakul, T.; Yusakul, G.; Kanchanapoom, T.; Putalun, W. HPLC-UV-Based Simultaneous Determination of Canthin-6-One Alkaloids, Quassinoids, and Scopoletin: The Active Ingredients in Eurycoma Longifolia Jack and Eurycoma Harmandiana Pierre, and Their Anti-Inflammatory Activities. J. AOAC Int. 2021, 104, 802–810. [Google Scholar] [CrossRef]
- Miyake, K.; Tezuka, Y.; Awale, S.; Li, F.; Kadota, S. Quassinoids from Eurycoma longifolia. J. Nat. Prod. 2009, 72, 2135–2140. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Cicero, A.F.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Della Cuna, F.S.R.; Russo, G.I.; Stamatiou, K. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91. [Google Scholar] [CrossRef]
- Majidi Wizneh, F.; Zaini Asmawi, M. Eurycoma longifolia Jack (Simarubaceae); Advances in Its Medicinal Potentials. Pharmacogn. J. 2014, 6, 1–9. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
- Dhawan, D.; Gupta, J. Research article comparison of different solvents for phytochemical extraction potential from datura metel plant leaves. Int. J. Biol. Chem. 2017, 11, 17–22. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 9. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Chang, J.I. Biodiesel production from residual oils recovered from spent bleaching earth. Rene. Energ. 2010, 35, 269–274. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, X.; Guo, W.; Zhang, T. Advances in Studies on Chemistry, Pharmacological Effect, and Pharmacokinetics of Eurycoma longifolia. Chin. Herb. Med. 2011, 3, 186–195. [Google Scholar]
- Varghese, C.P.; Ambrose, C.; Jin, S.; Lim, Y.; Keisaban, T. Antioxidant and anti-inflammatory activity of Eurycoma longifolia Jack, a traditional medicinal plant in Malaysia. Int. J. Pharm. Sci. Nanotech. 2013, 5, 1875–1878. [Google Scholar] [CrossRef]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines-A review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Tada, H.; Yasuda, F.; Otani, K.; Doteuchi, M.; Ishihara, Y.; Shiro, M. New antiulcer quassinoids from Eurycoma longifolia. Eur. J. Med. Chem. 1991, 26, 345–349. [Google Scholar] [CrossRef]
- Hajjouli, S.; Chateauvieux, S.; Teiten, M.H.; Orlikova, B.; Schumacher, M.; Dicato, M.; Choo, C.Y.; Diederich, M. Eurycomanone and eurycomanol from Eurycoma longifolia Jack as regulators of signaling pathways involved in proliferation, cell death and inflammation. Molecules 2014, 19, 14649–14666. [Google Scholar] [CrossRef]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. NF-κB Inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef]
- Solomon, M.; Erasmus, N.; Henkel, R. In vivo effects of Eurycoma longifolia Jack (Tongkat Ali) extract on reproductive functions in the rat. Andrologia 2014, 46, 339–348. [Google Scholar] [CrossRef]
- Ang, H.H.; Cheang, H.S. Effects of Eurycoma longifolia jack on laevator ani muscle in both uncastrated and testosterone-stimulated castrated intact male rats. Arch. Pharm. Res. 2001, 24, 437–440. [Google Scholar] [CrossRef]
- Ang, H.H.; Sim, M.K. Eurycoma longifolia JACK and orientation activities in sexually experienced male rats. Biol. Pharm. Bull. 1998, 21, 153–155. [Google Scholar] [CrossRef]
- Lee, S.P.; Tasman-Jones, C. Prevention of acute gastric ulceration in the rat by cimetidine, a histamine H2-receptor antagonist. Clin. Exp. Pharmacol. Physiol. 1978, 5, 61–66. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Eurycoma longifolia (Tongkat Ali) root extract as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06937. [Google Scholar] [CrossRef]
- Carlson, R.P.; O’Neill-Davis, L.; Chang, J.; Lewis, A.J. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 1985, 17, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.; Strayer, D.S.; Rubin, E. Rubin’s Pathology: Clinicopathologic Foundations of Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Borges, R.S.; Palheta, I.C.; Ota, S.S.B.; Morais, R.B.; Barros, V.A.; Ramos, R.S.; Silva, R.C.; Costa, J.D.S.; Silva, C.; Campos, J.M.; et al. Toward of Safer Phenylbutazone Derivatives by Exploration of Toxicity Mechanism. Molecules 2019, 24, 143. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Di Rosa, M. Biological properties of carrageenan. J. Pharm. Pharmacol. 1972, 24, 89–102. [Google Scholar] [CrossRef]
- Rosa, S.G.; Brüning, C.A.; Pesarico, A.P.; de Souza, A.C.G.; Nogueira, C.W. Anti-inflammatory and antinociceptive effects of 2, 2-dipyridyl diselenide through reduction of inducible nitric oxide synthase, nuclear factor-kappa B and c-Jun N-terminal kinase phosphorylation levels in the mouse spinal cord. J. Trace. Elem. Med. Biol. 2018, 48, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, P.; Sneller, M.C. Pathogenesis of Wegener’s granulomatosis: Current concepts. Expert Rev. Mol. Med. 2005, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Katzung, B.G.; Trevor, A. Chapter 36: Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout. In Basic & Clinical Pharmacology, 9th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.P.; Boominathan, R.; Mandal, S.C. Evaluation of antipyretic potential of Cleome viscosa Linn. (Capparidaceae) extract in rats. J. Ethnopharmacol. 2003, 87, 11–13. [Google Scholar] [CrossRef]
- Moltz, H. Fever: Causes and consequences. Neurosci. Biobehav. Rev. 1993, 17, 237–269. [Google Scholar] [CrossRef]
- Lovejoy, F.H., Jr. Aspirin and acetaminophen: A comparative view of their antipyretic and analgesic activity. Pediatrics 1978, 62, 904–909. [Google Scholar]
- Rawlins, M.; Postgrad, R. Mechanism of salicylate-induced antipyresis. In Proceedings of the Pharmacology Thermoregulatory Proceeding Satellite Symposium, San Francisco, CA, USA, 23–28 July 1973; pp. 311–324. [Google Scholar]
- Szelenyi, I.; Brune, K. Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig. Dis. Sci. 1988, 33, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M. Effects of ethanol on the gastric mucosa. Dig. Dis. 1987, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Ogle, C.W. The pharmacological differences and similarities between stress-and ethanol-induced gastric mucosal damage. Life Sci. 1992, 51, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Drini, M. Peptic ulcer disease and non-steroidal anti-inflammatory drugs. Aust. Prescr. 2017, 40, 91. [Google Scholar] [CrossRef]
- Wallace, J.L.; McKnight, W.; Reuter, B.K.; Vergnolle, N. NSAID-induced gastric damage in rats: Requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterol 2000, 119, 706–714. [Google Scholar] [CrossRef]
- Guo, S.; Gao, Q.; Jiao, Q.; Hao, W.; Gao, X.; Cao, J.-M. Gastric mucosal damage in water immersion stress: Mechanism and prevention with GHRP-6. World. J. Gastroenterol. 2012, 18, 3145. [Google Scholar] [CrossRef]
- Simões, S.; Lopes, R.; Campos, M.C.D.; Marruz, M.J.; da Cruz, M.E.M.; Corvo, L. Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. AMEM 2019, 2, 121–126. [Google Scholar] [CrossRef]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.; Nyarko, A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013, 2013, 796405. [Google Scholar] [CrossRef]
- Jayachitra, C.; Jamuna, S.; Ali, M.A.; Paulsamy, S.; Al-Hemaid, F.M. Evaluation of traditional medicinal plant, Cissus setosa Roxb.(Vitaceae) for antiulcer property. Saudi J. Biol. Sci. 2018, 25, 293–297. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Ridtitid, W.; Nima, S.; Phdoongsombut, N.; Ratanasuwon, P.; Kasiwong, S. Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J. Ethnopharmacol. 2011, 134, 243–250. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Suzuki, N.; Abas, A.B.; Mohri, K.; Utsuyama, M.; Hirokawa, K.; Takara, T. Immunomodulation in Middle-Aged Humans Via the Ingestion of Physta® Standardized Root Water Extract of Eurycoma longifolia Jack—A Randomized, Double-Blind, Placebo-Controlled, Parallel Study. Phytother. Res. 2016, 30, 627–635. [Google Scholar] [CrossRef] [PubMed]

| Test | Result |
|---|---|
| Foreign matter (%w/w) | Not found |
| Hexane extractive (%w/w) | 0.66 |
| Dichloromethane extractive (%w/w) | 0.94 |
| Ethanol extractive content (%w/w) | 1.51 |
| Water extractive content (%w/w) | 10.57 |
| Loss on drying (%v/w) | 9.58 |
| Total ash (%w/w) | 2.54 |
| Acid-insoluble ash (%w/w) | 0.90 |
| Chemical composition | saponin, terpenoids |
| Groups | Dose (mg/kg) | Granuloma Wet Weight (mg) | Granuloma Dry Weight (mg) | Transudative Weight (mg) | Granuloma Weight (mg/mg Cotton) | GI (%) |
|---|---|---|---|---|---|---|
| Control | - | 450.0 ± 27.0 | 81.8 ± 4.4 | 368.2 ± 23.4 | 3.1 ± 0.2 | - |
| Prednisolone | 5 | 291.0 ± 12.4 * | 58.6 ± 2.4 * | 232.5 ± 10.9 * | 1.9 ± 0.1 * | 37 |
| Aspirin | 300 | 415.8 ± 33.1 | 79.5 ± 4.9 | 336.3 ± 30.1 | 3.0 ± 0.2 | 4 |
| ELJ extract | 1200 | 347.4 ± 25.6 * | 66.2 ± 3.0 * | 281.2 ± 21.7 | 2.3 ± 0.3 | 25 |
| Groups | Dose (mg/kg) | Body Weight (g) | Dry Thymus Weight (mg/100 g) | ||
|---|---|---|---|---|---|
| Initial | Final | Gain | |||
| Control | - | 387.50 ± 13.34 | 394.17 ± 11.06 | 6.67 ± 3.07 | 21.91 ± 1.97 |
| Prednisolone | 5 | 365.00 ± 9.83 | 353.33 ± 10.14 * | −11.67 ± 6.54 * | 17.24 ± 1.79 |
| Aspirin | 300 | 360.83 ± 12.74 | 361.67 ± 11.38 * | 0.83 ± 4.36 | 16.96 ± 1.27 |
| ELJ extract | 1200 | 383.33 ± 3.80 | 380.00 ± 5.16 | −3.33 ± 4.22 | 16.99 ± 1.82 |
| Groups | Dose | Early Phase | Late Phase | ||
|---|---|---|---|---|---|
| (mg/kg) | Licking Time (s) | % Inhibition of Licking Response | Licking Time (s) | % Inhibition of Licking Response | |
| Control | - | 67.8 ± 4.5 | - | 93.8 ± 7.1 | - |
| Aspirin | 300 | 45.0 ± 5.3 * | 34 | 0.0 ± 0.0 * | 100 |
| Morphine | 10 | 0.0 ± 0.0 * | 100 | 1.5 ± 1.5 * | 98 |
| ELJ extract | 300 | 44.8 ± 5.0 * | 34 | 11.2 ± 9.5 * | 88 |
| 600 | 41.8 ± 3.3 * | 38 | 4.8 ± 3.4 * | 95 | |
| 1200 | 38.7 ± 3.2 * | 43 | 3.2 ± 3.2 * | 97 | |
| Groups | Dose (mg/kg) | Rectal Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 18 h after Yeast Injection | Time after Drug Administration (min) | |||||
| 30 min | 60 min | 90 min | 120 min | ||||
| Control | - | 37.98 ± 0.22 | 39.20 ± 0.23 | 39.17 ± 0.30 | 39.03 ±0.23 | 39.08 ± 0.21 | 39.03 ± 0.22 |
| Aspirin | 300 | 38.03 ± 0.21 | 39.07 ± 0.20 | 38.30 ± 0.25 * | 37.98 ± 0.22 * | 37.87 ± 0.18 * | 37.75 ± 0.20 * |
| ELJ extract | 300 | 38.03 ± 0.23 | 39.23 ± 0.09 | 39.02 ± 0.15 | 38.85 ± 0.17 | 38.85 ± 0.17 | 38.83 ± 0.20 |
| 600 | 37.88 ± 0.16 | 39.23 ± 0.10 | 38.83 ± 0.20 | 38.60 ± 0.13 | 38.65 ± 0.10 | 38.73 ± 0.07 | |
| 1200 | 38.07 ± 0.15 | 39.00 ± 0.13 | 38.30 ± 0.27 * | 38.18 ± 0.25 * | 38.27 ± 0.27 * | 38.08 ± 0.31 * | |
| Group | Gastric Volume (mL) | Secretory Rate (mL/100 g/5 h) | pH | Total Acidity (mEq/100 g/5 h) |
|---|---|---|---|---|
| Control | 3.25 ± 0.35 | 1.54 ± 0.19 | 1.95 ± 0.32 | 29.20 ± 2.65 |
| Cimetidine 100 mg/kg | 2.37 ± 0.16 | 0.87 ± 0.05 * | 5.06 ± 0.79 * | 13.30 ± 2.35 * |
| ELJ extract 150 mg/kg | 3.43 ± 0.39 | 1.18 ± 0.17 | 1.87 ± 0.10 | 27.02 ± 4.83 |
| ELJ extract 300 mg/kg | 3.05 ± 0.43 | 1.07 ± 0.13 | 2.31 ± 0.28 | 27.42 ± 3.25 |
| ELJ extract 600 mg/kg | 3.38 ± 0.24 | 1.26 ± 0.15 | 2.03 ± 0.42 | 34.76 ± 5.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhawa, S.; Arpornchayanon, W.; Jaijoy, K.; Chansakaow, S.; Soonthornchareonnon, N.; Sireeratawong, S. Anti-Inflammatory, Antinociceptive, Antipyretic, and Gastroprotective Effects of Eurycoma longifolia Jack Ethanolic Extract. Life 2023, 13, 1465. https://doi.org/10.3390/life13071465
Subhawa S, Arpornchayanon W, Jaijoy K, Chansakaow S, Soonthornchareonnon N, Sireeratawong S. Anti-Inflammatory, Antinociceptive, Antipyretic, and Gastroprotective Effects of Eurycoma longifolia Jack Ethanolic Extract. Life. 2023; 13(7):1465. https://doi.org/10.3390/life13071465
Chicago/Turabian StyleSubhawa, Subhawat, Warangkana Arpornchayanon, Kanjana Jaijoy, Sunee Chansakaow, Noppamas Soonthornchareonnon, and Seewaboon Sireeratawong. 2023. "Anti-Inflammatory, Antinociceptive, Antipyretic, and Gastroprotective Effects of Eurycoma longifolia Jack Ethanolic Extract" Life 13, no. 7: 1465. https://doi.org/10.3390/life13071465
APA StyleSubhawa, S., Arpornchayanon, W., Jaijoy, K., Chansakaow, S., Soonthornchareonnon, N., & Sireeratawong, S. (2023). Anti-Inflammatory, Antinociceptive, Antipyretic, and Gastroprotective Effects of Eurycoma longifolia Jack Ethanolic Extract. Life, 13(7), 1465. https://doi.org/10.3390/life13071465