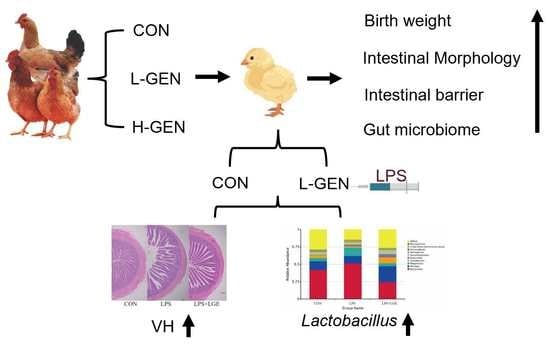
Supplementing Genistein for Breeder Hens Alters the Growth Performance and Intestinal Health of Offspring
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sample Collection
2.3. Serum Biochemical Assays
2.4. Histological Measurements
2.5. Total RNA Extraction and mRNA Quantification
2.6. Sequencing of the 16S Ribosomal RNA (rRNA) Gene
2.7. Data Analysis of the 16S rRNA Gene
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Indices
3.3. Intestinal Morphology
3.4. Intestinal Gene Expression
3.5. Description of the 16S rRNA Gene Sequencing Data
3.6. Structure of the Meconium and Ileum Microbiota
3.7. Predicted Functional Alterations of Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Shi, X.; Chen, Y.; Liu, J.; Wu, Y.; Xu, Y. Multi-Omics Revealed the Protective Effects of Rhamnolipids in Lipopolysaccharide Challenged Broilers. Front. Immunol. 2022, 13, 824664. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Nam, H.; Goo, D.; Wickramasuriya, S.S.; Zimmerman, N.; Smith, A.H.; Rehberger, T.G.; Lillehoj, H.S. Gut Microbiota-Derived Indole-3-Carboxylate Influences Mucosal Integrity and Immunity Through the Activation of the Aryl Hydrocarbon Receptors and Nutrient Transporters in Broiler Chickens Challenged With Eimeria maxima. Front. Immunol. 2022, 13, 867754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qi, L.; Wei, Q.; Shi, F. Maternal stevioside supplementation improves intestinal immune function of chicken offspring potentially via modulating gut microbiota and down-regulating the promoter methylation level of suppressor of cytokine signaling 1 (SOCS1). Anim. Nutr. 2022, 10, 329–346. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Dai, D.; Qi, G.; Wang, J.; Zhang, H.; Qiu, K.; Han, Y.; Wu, Y.; Wu, S. Dietary organic acids ameliorate high stocking density stress-induced intestinal inflammation through the restoration of intestinal microbiota in broilers. J. Anim. Sci. Biotechnol. 2022, 13, 124. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef]
- Huang, Z.; Jin, S.; Lv, Z. Dietary genistein supplementation alters mRNA expression profile and alternative splicing signature in the thymus of chicks with lipopolysaccharide challenge. Poult. Sci. 2022, 101, 101561. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, H.; Zhang, B.; Ning, C.; Xing, K.; Guo, Y. Dietary genistein supplementation in laying broiler breeder hens alters the development and metabolism of offspring embryos as revealed by hepatic transcriptome analysis. FASEB J. 2018, 32, 4214–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D.R.; Mourvaki, E.; Clerici, C.; Mattioli, S.; Brecchia, G.; Castellini, C. Dietary Isoflavone Aglycons from Soy Germ Pasta Improves Reproductive Performance of Aging Hens and Lowers Cholesterol Levels of Egg Yolk. Metabolites 2022, 12, 1112. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, H.; Zhang, B.; Xing, K.; Guo, Y. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018, 8, 5161. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Fan, H.; Song, B.; Li, G.; Liu, D.; Guo, Y. Supplementing Genistein for Breeder Hens Alters the Fatty Acid Metabolism and Growth Performance of Offsprings by Epigenetic Modification. Oxid. Med. Cell. Longev. 2019, 2019, 9214209. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; La, T.M.; Lee, H.J.; Choi, I.S.; Song, C.S.; Park, S.Y.; Lee, J.B.; Lee, S.W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019, 9, 6838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Dai, R.; Yang, L.; He, C.; Xu, K.; Liu, S.; Zhao, W.; Xiao, L.; Luo, L.; Zhang, Y.; et al. Inheritance and Establishment of Gut Microbiota in Chickens. Front. Microbiol. 2017, 8, 1967. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, K.Y.; Bai, S.P.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Xuan, Y.; Su, Z.W.; Ding, X.M. The impacts of egg storage time and maternal dietary vitamin E on the growth performance and antioxidant capacity of progeny chicks. Poult. Sci. 2021, 100, 101142. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, L.; Zhang, Q.; Yu, M.; Xiao, X. Genistein improves glucose metabolism and promotes adipose tissue browning through modulating gut microbiota in mice. Food Funct. 2022, 13, 11715–11732. [Google Scholar] [CrossRef]
- Lv, Z.; Dai, H.; Wei, Q.; Jin, S.; Wang, J.; Wei, X.; Yuan, Y.; Yu, D.; Shi, F. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult. Sci. 2020, 99, 3411–3427. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.; Yan, S.; Song, B.; Liu, Y.; Gao, M.; Tang, D.; Guo, Y. Soya saponin improves egg-laying performance and immune function of laying hens. J. Anim. Sci. Biotechnol. 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G. Muscle development in the embryo and hatchling. Poult. Sci. 2007, 86, 1050–1054. [Google Scholar] [CrossRef]
- Li, F.; Ning, H.; Duan, X.; Chen, Z.; Xu, L. Effect of dietary l-arginine of broiler breeder hens on embryonic development, apparent metabolism, and immunity of offspring. Domest. Anim. Endocrinol. 2021, 74, 106537. [Google Scholar] [CrossRef] [PubMed]
- Gawlinska, K.; Gawlinski, D.; Filip, M.; Przegalinski, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.S.; Chang, W.H.; Liu, G.H.; Zhang, S.; Zheng, A.J.; Li, Y.; Xie, Q.; Liu, Z.Y.; Cai, H.Y. Effects of flavones of sea buckthorn fruits on growth performance, carcass quality, fat deposition and lipometabolism for broilers. Poult. Sci. 2015, 94, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Yang, Q.; Stewart, S.; Whitmore, M.A.; Zhang, G. Biogeography, succession, and origin of the chicken intestinal mycobiome. Microbiome 2022, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Wijtten, P.J.A.; Langhout, D.J.; Verstegen, M.W.A. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: A review. Acta Agric. Scand. Sect. A Anim. Sci. 2012, 62, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Peebles, E.D.; Morgan, T.W.; Harkess, R.L.; Zhai, W. Protein source and nutrient density in the diets of male broilers from 8 to 21 d of age: Effects on small intestine morphology. Poult. Sci. 2015, 94, 61–67. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; D’Antongiovanni, V.; Antonioli, L.; Bernardini, N.; Derkinderen, P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol. Hepatol. 2023, 8, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Huang, J.; Zhao, L.; Pan, X.; Liao, C.; Jiang, Q.; Lei, J.; Guo, F.; Cui, J.; Guo, Y.; et al. Dietary genistein increases microbiota-derived short chain fatty acid levels, modulates homeostasis of the aging gut, and extends healthspan and lifespan. Pharmacol. Res. 2023, 188, 106676. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Sayoc-Becerra, A.; Santos, A.N.; Shawki, A.; Canale, V.; Krishnan, M.; Niechcial, A.; Obialo, N.; Scharl, M.; Li, J.; et al. PTPN2 Regulates Interactions Between Macrophages and Intestinal Epithelial Cells to Promote Intestinal Barrier Function. Gastroenterology 2020, 159, 1763–1777.e1714. [Google Scholar] [CrossRef]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X.; Ma, X.; Gao, K.; Hu, Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015, 28, 288–294. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Zhao, T.; Quan, X.; Han, Y.; Cheng, Y.; Chen, Y.; Shen, X.; Zheng, Y.; Zhao, Y. Comparative study of extracellular vesicles derived from mesenchymal stem cells and brain endothelial cells attenuating blood-brain barrier permeability via regulating Caveolin-1-dependent ZO-1 and Claudin-5 endocytosis in acute ischemic stroke. J. Nanobiotechnol. 2023, 21, 70. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, F.T.; Ding, J.; Zhou, H.; Xu, K.; He, C.; Han, C.; Zheng, Y.; Luo, H.; Yang, K.; Gu, C.; et al. Dynamic distribution of gut microbiota during embryonic development in chicken. Poult. Sci. 2020, 99, 5079–5090. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell. Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Shi, L.; Ge, Y.; Leng, D.; Zeng, B.; Wang, T.; Jie, H.; Li, D. Dynamic Changes in the Gut Microbial Community and Function during Broiler Growth. Microbiol. Spectr. 2022, 10, e0100522. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Ali, A.; Khatoon, A.; Almohaimeed, H.M.; Al-Sarraj, F.; Albiheyri, R.; Alotibi, I.; Abidin, Z.U. Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period. Toxins 2022, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xi, Y.; Xia, Y.; Wu, T.; Zhao, D.; Zhang, Z.; Ding, B. Dietary Lactobacillus fermentum and Bacillus coagulans Supplementation Modulates Intestinal Immunity and Microbiota of Broiler Chickens Challenged by Clostridium perfringens. Front. Vet. Sci. 2021, 8, 680742. [Google Scholar] [CrossRef] [PubMed]






| Items 1 | Breeder Hens | 1 to 21 d |
|---|---|---|
| Ingredient (%) | ||
| Corn (CP 7.8%) | 54.99 | 53.28 |
| Soybean meal (CP 43%) | 34.15 | 38.57 |
| Soybean oil | 0.50 | 3.70 |
| Limestone | 7.23 | 1.05 |
| Dicalcium phosphate | 2.09 | 1.98 |
| DL-Methionine | 0.17 | 0.17 |
| NaCl | 0.35 | 0.35 |
| 1 Trace element premix | 0.30 | 0.50 |
| 2 Vitamin premix | 0.10 | 0.10 |
| Choline chloride (50%) | 0.12 | 0.30 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| Metabolizable energy (MJ /Kg) | 11.84 | 12.35 |
| Crude protein (%) | 16.10 | 21.57 |
| Available phosphorus (%) | 0.47 | 1.15 |
| Calcium (%) | 3.48 | 1.05 |
| Methionine (%) | 0.34 | 0.49 |
| Lysine (%) | 0.81 | 1.05 |
| Gene | Primer Sequence (5′→3′) | Amplicon Size (bp) | GenBank Accession Number |
|---|---|---|---|
| E-cadherin | F: AGCCCCAGTGCTTCTCTCTA | 197 | NM_131820.1 |
| R: CCTCCCGATCAGCAACTCTC | |||
| Claudin-1 | F: ACCCACAGCCTAAGTGCTTC | 200 | NM_001013611.2 |
| R: AGGTCTCATAAGGCCCCACT | |||
| Claudin-2 | F: AGGGGCTATGGATGGAGTGT | 189 | NM_001277622.1 |
| R: AGCCCTGATTGAAGACGGTG | |||
| Claudin-3 | F: AGGGGTTCTCAGCTCTCACT | 332 | NM_204202.1 |
| R: GTTTCTCCGCCAGACTCTCC | |||
| Claudin-5 | F: TTGCAGGTCGCCAGAGATAC | 269 | NM_204201.1 |
| R: AGGCAAGTGCATGTTACCGA | |||
| ZO-1 | F: ACTGTGACCCCAAAACCTGG | 294 | XM_015278981.2 |
| R: CTCCCTGCTTGTGGCATGTA | |||
| ZO-2 | F: GGCTCCCAAAATGAGATGCG | 123 | NM_204918.1 |
| R: TTGGGCGTGACGTATAGCTG | |||
| ZO-3 | F: CACAAAGGGTTACGCAAGGC | 198 | XM_015299760.2 |
| R: AGAGCTCCAGGAGGGTCTTC | |||
| Occludin | F: ACCAGAATGGTACCCTGAGC | 141 | XM_025144247.1 |
| R: ATTACACAGCTTCAGCCTTACA | |||
| GAPDH | F: TCAGCACCATCTGACAATGC | 148 | NC_006088.5 |
| R: AGTAGGCAGCATCCCATCTG |
| Indexes | CON | LGE | HGE | p-Value |
|---|---|---|---|---|
| UA (μmol/L) | 995 ± 147 a | 621 ± 56.8 b | 673 ± 94.1 b | 0.035 |
| CREA (μmol/L) | 21.6 ± 1.30 a | 17.2 ± 0.86 b | 14.7 ± 1.62 b | 0.006 |
| GLU (mmol/L) | 9.60 ± 0.17 | 8.93 ± 0.42 | 8.27 ± 0.46 | 0.134 |
| TCHO (mmol/L) | 9.47 ± 0.05 | 9.31 ± 0.19 | 9.28 ± 0.15 | 0.623 |
| TG (mmol/L) | 1.59 ± 0.21 | 1.74 ± 0.21 | 1.56 ± 0.12 | 0.770 |
| TP (g/L) | 26.6 ± 0.92 b | 29.7 ± 0.71 a | 31.9 ± 2.41 a | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Wang, J.; Lv, Z. Supplementing Genistein for Breeder Hens Alters the Growth Performance and Intestinal Health of Offspring. Life 2023, 13, 1468. https://doi.org/10.3390/life13071468
Gao M, Wang J, Lv Z. Supplementing Genistein for Breeder Hens Alters the Growth Performance and Intestinal Health of Offspring. Life. 2023; 13(7):1468. https://doi.org/10.3390/life13071468
Chicago/Turabian StyleGao, Mingkun, Jiao Wang, and Zengpeng Lv. 2023. "Supplementing Genistein for Breeder Hens Alters the Growth Performance and Intestinal Health of Offspring" Life 13, no. 7: 1468. https://doi.org/10.3390/life13071468
APA StyleGao, M., Wang, J., & Lv, Z. (2023). Supplementing Genistein for Breeder Hens Alters the Growth Performance and Intestinal Health of Offspring. Life, 13(7), 1468. https://doi.org/10.3390/life13071468