Abstract
The trabecular meshwork (TM) route is the principal outflow egress of the aqueous humor. Actin cytoskeletal remodeling in the TM and extracellular matrix (ECM) deposition increase TM stiffness, outflow resistance, and elevate intraocular pressure (IOP). These alterations are strongly linked to transforming growth factor-β2 (TGFβ2), a known profibrotic cytokine that is markedly elevated in the aqueous humor of glaucomatous eyes. Sigma-1 receptor (S1R) has been shown to have neuroprotective effects in the retina, but data are lacking about its role in the TM. In this study, we identified the presence of S1R in mouse TM tissue and investigated the effect of an S1R agonist fluvoxamine (FLU) on TGFβ2-induced human TM cells regarding cell proliferation; ECM-related functions, including F-actin reorganization; and the accumulation of ECM elements. TGFβ2 increased the proliferation, cytoskeletal remodeling, and protein levels of fibronectin, collagen type IV, and connective tissue growth factor, and decreased the level of matrix metalloproteinase-2. Most importantly, FLU reversed all these effects of TGFβ2, suggesting that S1R agonists could be potential candidates for preserving TM function and thus maintaining normal IOP.
1. Introduction
The trabecular meshwork (TM) located in the iridocorneal angle of the eye is the main drainage pathway of aqueous humor (AH). Structural and functional impairment of the TM is associated with increased AH outflow resistance and intraocular pressure (IOP) elevation [1,2,3,4,5], which are the main risk factors of glaucoma [6,7]. Transforming growth factor-beta2 (TGFβ2) is the predominant isoform of TGFβ in the eye [8,9] and it is known to be strongly associated with the development of elevated IOP and primary open-angle glaucoma [10,11,12].
Numerous studies have shown that TGFβ2 increases the secretion and deposition of extracellular matrix (ECM) proteins [10,13,14,15] and connective tissue growth factor (CTGF), which is a downstream mediator of TGFβ2 [13,16,17,18] and a key inducer of ECM synthesis. There is increased deposition of these elements in the TM of glaucomatous eyes, among which fibronectin and collagen are the major components [10,19,20,21,22,23]. Research efforts have shed some light on the complex process of modulating ECM turnover in the TM, but treatment of TM dysfunction is still an unmet need.
The Sigma-1 receptor (S1R) is a multifunctional molecular chaperone, with a well-known protective effect in the central nervous system [24,25,26,27,28]. S1R has also been identified in the eye, including retinal tissue, the iris–ciliary body, lacrimal glands, the cornea, and the lens [29,30,31,32,33]. Studies have already reported its protective effect in the retina [34,35,36,37], but there are no data about S1R abundance and function in the TM except one investigation on pressure-induced cell death [29].
Here, we provide evidence about the presence of S1R and about the protective effect of S1R agonist fluvoxamine (FLU) against TGFβ2-induced ECM-related pathologies, including cell proliferation, morphological changes, F-actin enhancement, and rearrangement in the TM.
2. Materials and Methods
2.1. Materials
Unless stated otherwise, all standard plastic materials were supplied by Sarstedt (Numbrecht, Germany), all chemical agents were bought from Sigma-Aldrich/Merck (St. Luis, MO, USA), and each experiment was performed at least three times.
2.2. Human Trabecular Meshwork Cell Line
Nonglaucomatous transformed human trabecular meshwork cells (HTM5) were developed by Dr. Abbot Clark (University of North Texas—Health Science Center, Denton, TX, USA) [38] and provided by Dr. Xavier Gasull (University of Barcelona) for these experiments. HTM5 cells were grown in culture flasks in Dulbecco’s Modified Eagle Medium (DMEM, 31885-023, Gibco, Thermo Fischer, Waltham, MA, USA) complemented with 1% penicillin/streptomycin (Pen Strep, 15140-122, Gibco) and 10% fetal bovine serum (FBS, 10500-064, Gibco) in an atmosphere containing 5% CO2 and 95% air at 37 °C. The cells were passaged every 2–3 days using 0.25% Trypsin-EDTA buffer (25200-072, Gibco). For the characterization of TM cells, the induction of myocilin and alpha smooth muscle actin (α-SMA) was confirmed after 7 days of 100 nM dexamethasone (1177-87-3, Sigma-Aldrich) treatment. The expression of myocilin and α-SMA was investigated using immunolabeling and Western blotting.
2.3. Exposure to TGFβ2 and Fluvoxamine
HTM5 cells were seeded to subconfluence in serum-free DMEM for 24 h. For a dose curve, the cells were activated with various concentrations (1, 2.5, 5, 10, and 20 ng/mL) of TGFβ2 for a period of 24 h iewn serum-free media. To examine the viability of HTM5 cells in response to FLU, we treated the cells with different concentrations (5, 10, and 15 μM) of FLU for 24 h in serum-free media. For the rest of the experiments, cells were induced with 10 ng/mL TGFβ2 alone (302-B2, R&D Systems, Minneapolis, MN, USA) and treated with 10 µM FLU (F2802, Sigma-Aldrich) for 24 h in serum-free media. For each experiment, untreated cells were used as controls.
2.4. Proliferation and Cytotoxicity Assay
HTM5 cells were plated in 96-well plates (40,000 cells/100 µL/well, n = 6/treatment group) and treated as mentioned above (Section 2.3). To investigate cell proliferation, after 24 h of various treatments, cells were incubated with methyl-thiazolyldiphenyl-tetrazolium bromide (MTT, 0.5 mg/mL, M6494, Invitrogen) for 3 h at 37 °C. Then, MTT was discarded, and 100 µL of solubilizer (dimethyl sulfoxide (DMSO, D4540, Sigma-Aldrich) was mixed in a 1:1 ratio with ethanol) was added to each well to dissolve the water-insoluble formazan crystals. The absorbance of the formazan solution was recorded at 570 nm (SpectroStar Nano microplate reader, BMG Labtech, Ortenberg, Germany). To assess cell toxicity, the widely used lactate dehydrogenase (LDH) assay (C20300, Invitrogen, Carlsbad, CA, USA) was performed from the supernatant of the cells according to the manufacturer’s protocol and the samples were measured in the same way as in the case of MTT.
2.5. Immunocytochemistry
HTM5 cells were plated on gelatin-coated (0.1%, G1393, Sigma-Aldrich) 8-well chamber slides (µ-slide 8-well high Glass Bottom, 80807, Ibidi, Gräfelfing, Germany) (300 µL/well; 150,000 cells/well). After 24 h of treatments (Section 2.3), the cells were washed with phosphate-buffered saline (PBS), followed by fixation with 4% paraformaldehyde (30525-89-4, Sigma-Aldrich) at room temperature for 15 min. Then, the cells were washed, permeabilized with 0.1% TritonX100 (9036-19-5, Sigma-Aldrich) for 10 min, washed again, and blocked with 5% bovine serum albumin (BSA, A2153, Sigma-Aldrich) in PBS for 1 h. Next, repeated washes with PBS were followed by incubation with Alexa 546-phalloidin (1:40, A22283, Invitrogen) for 1 h; then, the cells were washed and the nuclei were counterstained with Hoechst 33342, (1 µg/mL, Invitrogen, Darmstadt, Germany) for 10 min. Finally, after rinsing with PBS, coverslips were mounted onto slides using ProLong antifade (P36980, Invitrogen). Fluorescent images were acquired with a Nikon Eclipse Ti2 inverted microscope (Nikon Instruments, Melville, NY, USA) equipped with 10×, 20×, 40×, and 60× oil immersion objective (Plan Apo lambda, N.A. 1.4) plus a 1.5× intermediate magnification and a cooled sCMOS camera (Zyla 4.2, Andor Technology, Belfast, UK). Image analysis for F-actin quantification was performed on images captured with 10× objective using NIS-Elements (Nis Elements 5.21.03 software version, Nikon Instruments) and data are presented as integrated density. To visualize the F-actin filaments, 60× objective plus 1.5× intermediate magnification was used and z-stack images were captured. Deconvolution was performed on the cropped 512 × 512-pixel cropped images using Huygens Essential 21.10 (Scientific Volume Imaging, Hilversum, The Netherlands) [39].
2.6. Western Blot Analysis
HTM5 cells were seeded at 100,000 cells/mL in 12-well plates (1 mL/well) using DMEM supplemented with 10% FBS. After 24 h, the nutritious media were replaced with serum-free DMEM for an additional 24 h, which was then followed by treatments (Section 2.3) for another 24 h. At the end of treatment, whole-cell lysates were collected. The cells were rinsed with ice-cold PBS and lysed in a lysis buffer containing 1.7 mg/mL aprotinin, 1 M Tris base, 5 mg/mL leupeptin, 0.5 M EGTA, 0.25 M NaF, 1% TritonX100, 0.5 M PMSF, and 0.5 M Na3VO4. Then, the cells were vortexed for 1 min, sonicated 2 times for 3 min, and vortexed for 30 s, followed by 1 min cooling on ice after each step. The lysates were then cleared using centrifugation at a speed of 13,000 rpm at 4 °C for 10 min. The supernatant containing proteins was transferred to a clean Eppendorf tube and the protein concentration was measured using a Lowry assay with a Protein DC kit (5000113-115, Bio-Rad Laboratories, Inc., Hercules, CA, USA). Then, 4× Laemmli sample buffer (161-0747, Bio-Rad) supplemented with 2-mercaptoethanol (M3148, Sigma-Aldrich) was added to the samples, which were all denatured by heating at 95 °C for 5 min. In the case of collagen type IV, we used 4X Laemmli buffer without a reducing agent and the denaturing step was omitted according to the manufacturer’s protocol. Total protein (10 µg) was loaded to polyacrylamide gradient gels (4–20%) (4561096, Bio-Rad) and transferred to nitrocellulose membranes (1704158, Bio-Rad). The membranes were stained in Ponceau S (0.1%, P3504, Sigma-Aldrich) for 3 min to correct for any differences in total protein loading. After destaining Ponceau S with distilled water, the membranes were blocked by 5% nonfat dry milk or 5% BSA in Tris-buffered saline at room temperature for 1 h and then incubated with primary antibodies (S1R, 1:1000, Santa Cruz sc137075; fibronectin, 1:5000, Abcam ab2413; collagen type IV, 1:500, Abcam ab6586; CTGF, 1:200, Santa Cruz sc14939; MMP2, 1:2000, Abcam ab92536) at 4 °C overnight. The membranes were washed and then probed for secondary antibodies (1:3000, Cell Signaling, Danvers, MA, USA). The signals were detected using a ChemiDocTM MP Imaging System (Bio-Rad) and analyzed using Image Lab software (version 6.1.0, Bio-Rad).
2.7. In Vivo Experiments
2.7.1. TGFβ2 Injection
Four-month-old male C57BL/6J mice were purchased from Animalab (Budapest, Hungary) and were kept in a 12 h light/12 h dark cycle at constant temperature (22 ± 2 °C) with ad libitum access to food and water. The animal studies were carried out in accordance with the Animal Experiment Regulations of the Animal Welfare Committee of Semmelweis University (PE/EA/916-7/2020), Budapest, Hungary.
3.5 µL of TGFβ2 (5 ng/µL) or PBS was injected intracamerally twice a week using a Hamilton glass microsyringe (5 mL volume, Hamilton Company, Reno, NV, USA). For the injection, the mice were anesthetized with 90 mg/10 mg/bwkg of ketamine/xylazine. In addition, 0.4% oxybuprocainhydrochloride (Novesine, OmniVision GmbH, Puchheim, Germany) was used as topical anesthesia. After 5 injections, the eyes were enucleated and processed for further investigations.
2.7.2. Fluorescent Immunohistochemistry for Mouse Anterior Segments
After enucleation, the eyes were fixed in 4% paraformaldehyde overnight at 4 °C and then kept in PBS with 0.01% sodium azide at 4 °C. First, the eyes were dissected and only the anterior segments (ASs) were used for the staining. The ASs were washed with PBS 4 times and then permeabilized with 0.5% TritonX100 for 1 h at room temperature, washed again, and blocked with 5% goat serum (31873, Invitrogen), 1% BSA, and 0.1% TritonX100 in PBS for 1 h at room temperature. Next, the ASs were incubated with the primary antibody (S1R, 1:50, Invitrogen, 42-3300) at 4 °C overnight. Then, repeated washes with PBS were followed by incubation with the secondary antibody (goat anti-rabbit Star Red, 1:500, Abberior, Star Red-1002) combined with Hoechst (5 µg/mL) for 1 h. After being washed with PBS, the ASs were stained for F-actin using Phalloidin Star Orange (Abberior, 1:20, Star Orange-0100) and for staining the nuclei, Hoechst was used (5 µg/mL). Both were applied for 1 h at room temperature. Finally, after rinsing with PBS, the samples were kept at 4 °C until further use. Fluorescent images were acquired with an Abberior Expert Line confocal microscope (Abberior, Instruments, Göttingen, Germany).
2.8. Statistical Analysis
The data were analyzed statistically using Prism software (version 8.0, GraphPad, Boston, MA, USA). Normal (Gaussian) distribution was checked using a Shapiro–Wilk normality test. Multiple comparisons and interactions were evaluated with a one-way ANOVA followed by a Holm–Sidak post hoc test. For nonparametric data, the Kruskal–Wallis ANOVA on ranks followed by Dunn’s correction was used. All data are presented as mean ± SEM and p < 0.05 was considered significant.
3. Results
3.1. S1R Is Present in Mouse TM and in HTM5 Cells, TGFβ2 Elevates F-Actin Level in Mouse Anterior Segments and Does Not Affect the Level of S1R
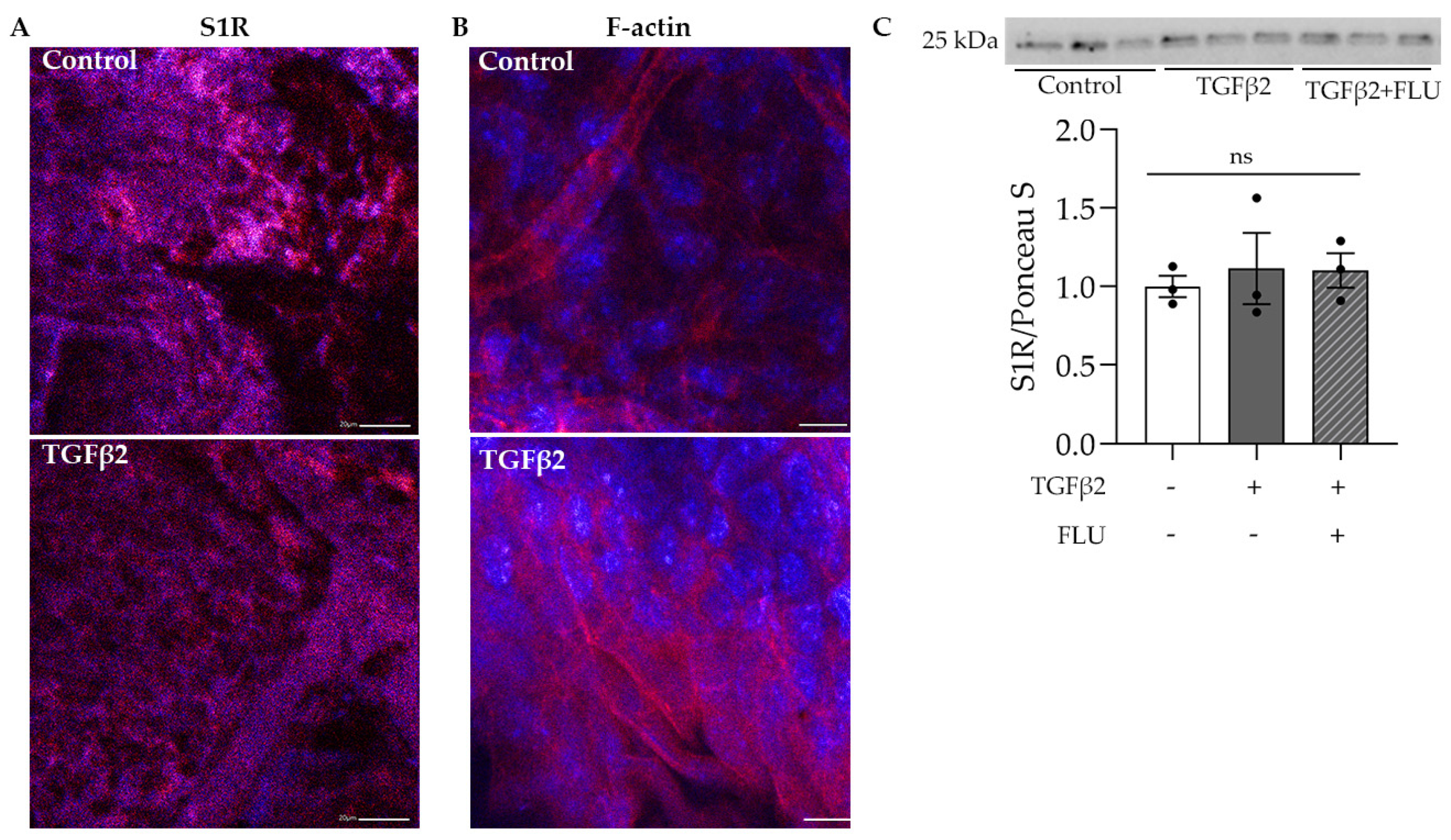
The protective role of S1R in the retina has been widely investigated [34,35,36,37]; however, much less is known about its function in the anterior segment, especially in the TM tissue. In the first step, we visualized S1R in the mouse anterior segments. Confocal immunofluorescence microscope images confirmed the abundant presence of S1R in the TM region, which was not altered by the profibrotic TGFβ2 (Figure 1A). Additionally, we demonstrated the presence of S1R in HTM5 cells. Moreover, Western blot analysis revealed that TGFβ2 and FLU treatment did not change the level of S1R protein (Figure 1C).
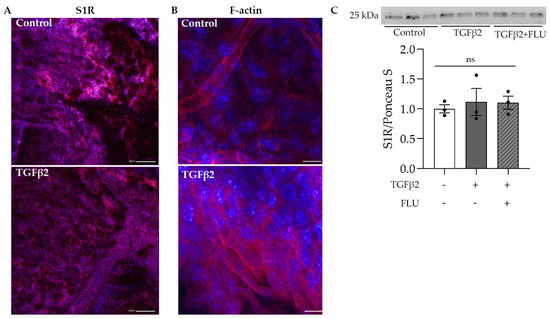
Figure 1.
Confocal microscope images of the mouse anterior segments and Western blot of HTM5 cells. (A) Sigma-1 receptor (S1R) expression in the trabecular meshwork region of C57BL/6J mice in control and TGFβ2-injected mice (S1R: red, nuclei: blue, 20× objective, scale bar = 20 µm). (B) F-actin level in the anterior segment of TGFβ2-injected mice compared to control mice (F-actin: red, nuclei: blue, 40× objective, scale bar = 10 µm). (C) Representative Western blot of S1R (25 kDa) protein in HTM5 cells. Cells were treated with 10 ng/mL TGFβ2 alone or in combination with 10 µM FLU for 24 h (data: mean ± SEM; n = 3/group; ns: nonsignificant; ANOVA followed by Holm–Šidak multiple comparison test). The uncropped blots are shown in File S1.
The normal function and structure of this spongy TM tissue are crucial in maintaining IOP and changes in its ECM have been associated with POAG [1,2,3,4,5]. In addition, TGFβ2, which is the main isoform of TGFβ, has been found in increased amounts in the aqueous humor of POAG patients [11,40] and proved to be involved in the ECM production and degradation of TM [10,13,14,15]. To verify the effect of TGFβ2 in the TM, C57BL/6J mice were injected with TGFβ2, and the anterior segments were stained for F-actin, an essential component of the ECM. Fluorescent confocal microscopy images revealed a massive increase in the F-actin level upon TGFβ2 induction in the TM region (Figure 1B), a result that indicated the usage of TGFβ2 as an ECM-altering agent in our further investigations.
Secondly, we aimed to elucidate the role of S1R in ECM-related changes in the TM; the direct effect of the specific S1R agonist FLU on TGFβ2-induced human trabecular meshwork cell culture (HTM5) was investigated.
3.2. FLU Prevents TGFβ2-Induced Cell Proliferation and Morphological Changes and Is Not Toxic to the Cells
Before performing experiments, to confirm the characteristics of HTM5 cells, dexamethasone treatment was applied as previously described [41], and the induction of myocilin and αSMA was detected with immunocytochemistry (Supplementary Materials).
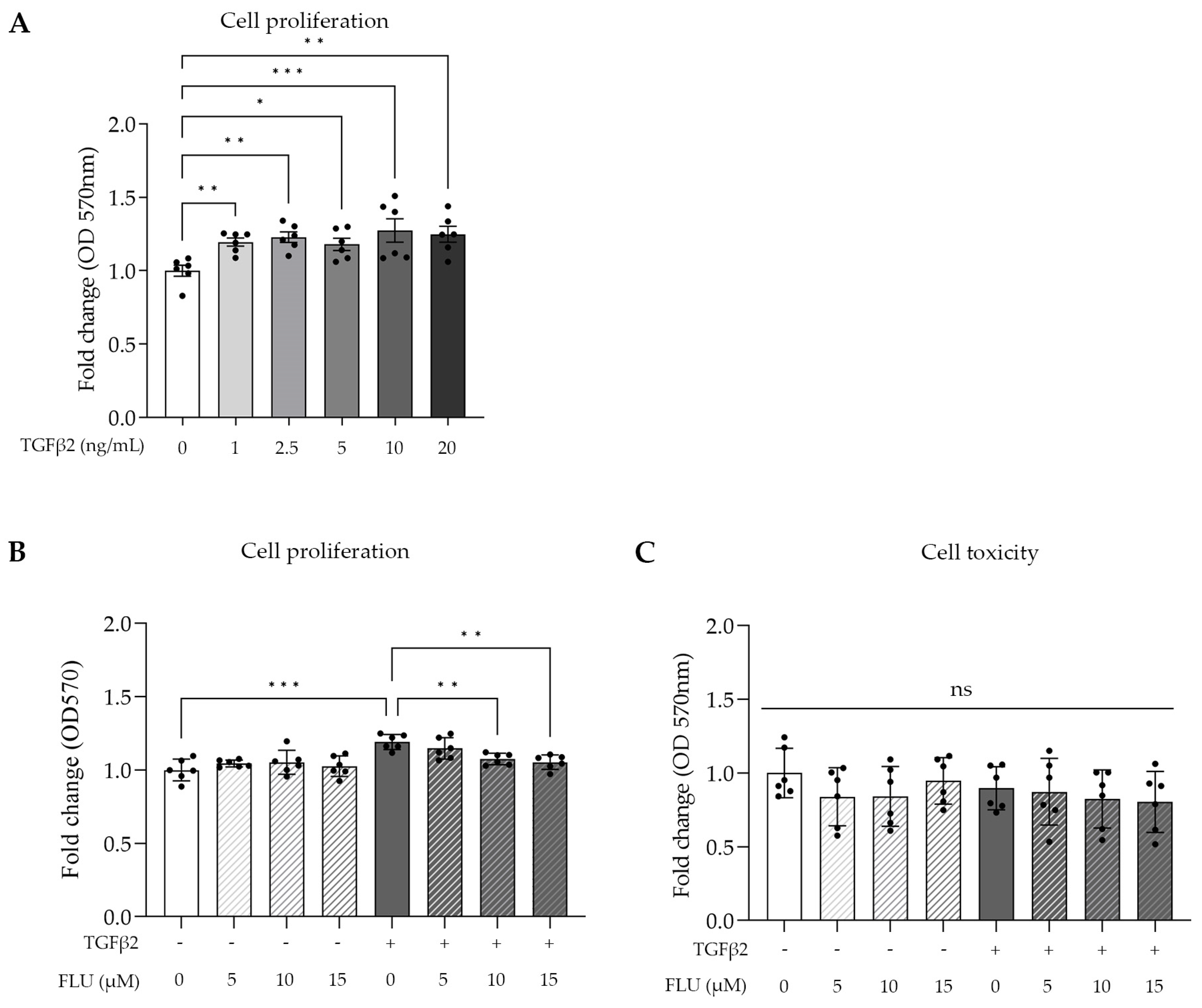
TGFβ2 is involved in cell proliferation; therefore, we determined its lowest concentration needed for a maximum effect on TM cell proliferation, using an MTT assay, which is a common colorimetric method to measure proliferation and cell viability in vitro. The HTM5 cells were activated with TGFβ2 in a range of 1–20 ng/mL for 24 h. Dose-dependent cell growth was measured with a peak at 10 ng/mL (Figure 2A).
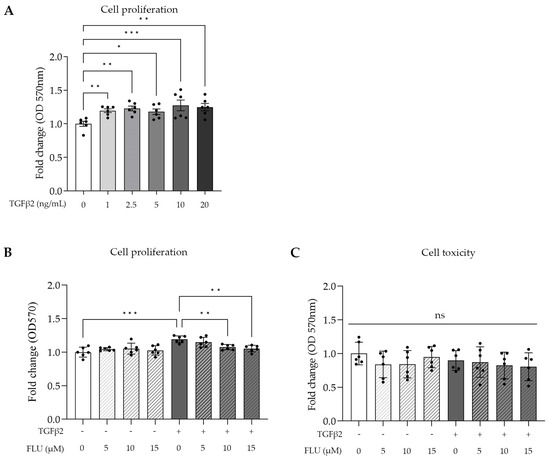
Figure 2.
Cell proliferation and cell toxicity of human trabecular meshwork (HTM5) cells after TGFβ2 stimuli and fluvoxamine (FLU) treatment. (A) Cell proliferation after treatment with different concentrations of TGFβ2 (0, 1, 2.5, 5, 10, and 20 ng/mL; 24 h). (B) Cell proliferation treatment with 10 ng/mL TGFβ2 combined with 5, 10, or 15 µM FLU (24 h). (C) Cellular toxicity determined with LDH assay after 24 h induction with 10 ng/mL TGFβ2 and/or treatment with FLU (5, 10, and 15 µM). (Data: mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, ns: nonsignificant; n = 6/group; one-way ANOVA.)
Based on this, 10 ng/mL TGFβ2 was used in further experiments. To assess the impact of FLU on cell proliferation and cellular toxicity, an MTT assay and a commonly used lactate dehydrogenase assay (LDH) were performed, respectively. The cells were treated with three different concentrations of FLU (5, 10, and 15 µM; 24 h), which were applied to the cells alone or in combination with TGFβ2. The MTT results revealed that FLU prevented TGFβ2-induced cell proliferation in a dose-dependent manner. However, when FLU was applied alone, it did not affect the proliferation (Figure 2B). Based on these findings, for our subsequent experiments, we selected the minimum effective dose of FLU required for proliferation inhibition, which in this case was determined to be 10 μM.
The cytotoxicity measurements confirmed our expectations, as none of the treatments exhibited cytotoxicity. This included FLU alone at concentrations of 5, 10, or 15 µM; TGFβ2 at a concentration of 10 ng/mL; and the combination of TGFβ2 with FLU at concentrations of 5, 10, or 15 µM (Figure 2C). The cells remained unaffected by these treatments, indicating their nontoxic nature.
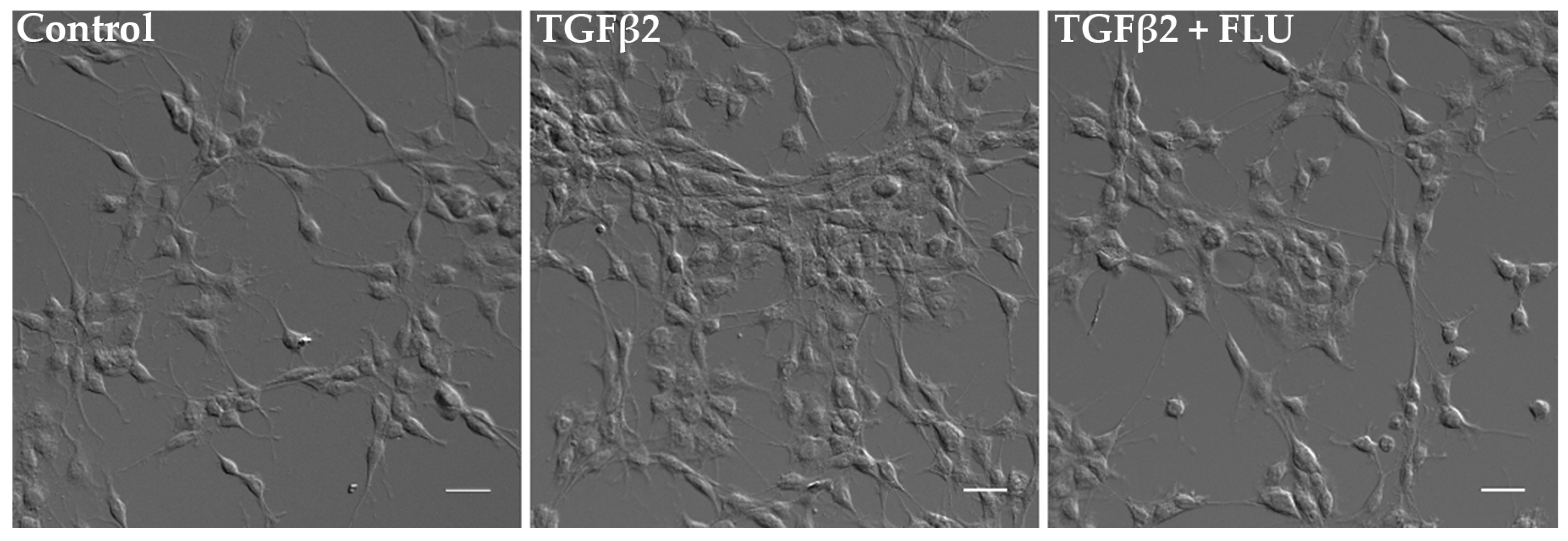
In parallel, the cells’ morphological changes were visualized. Consistent with the MTT results, differential interference contrast (DIC) images confirmed a noticeable increase in cell proliferation upon TGFβ2 induction. However, the presence of FLU ameliorated this effect, as observed in the images (Figure 3).
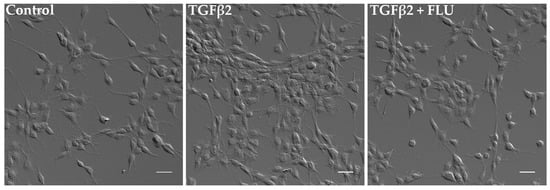
Figure 3.
Morphology of human trabecular meshwork (HTM5) cells. Cells were treated with 10 ng/mL TGFβ2 alone or in combination with 10 µM FLU for 24 h (20× objective; scale bar = 20 µm).
3.3. FLU Attenuates TGFβ2-Induced Cytoskeletal Remodeling
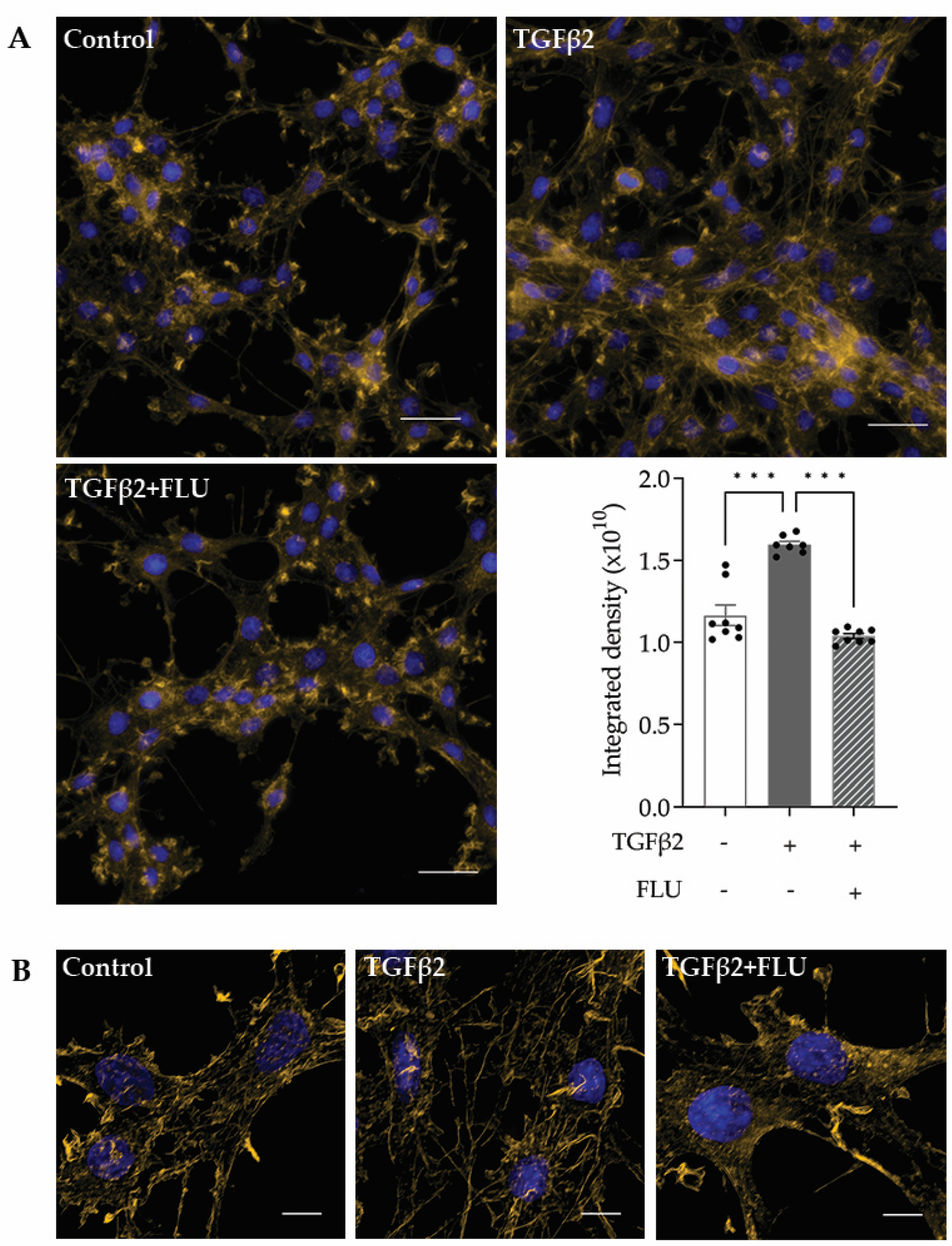
F-actin, or filamentous actin, plays a critical role in many cellular processes, including cell movement and the maintenance of cell shape [42,43]. In the TM, the F-actin network maintains the ECM surrounding the trabecular meshwork cells, and that plays a role in modulating the AH outflow facility. Therefore, investigating the effect of FLU on the actin cytoskeleton is rational. The representative fluorescent images of phalloidin-stained HTM5 cells showed a marked induction of F-actin level upon TGFβ2 induction, with increased actin clump and stress fiber formation, compared to the diffuse actin network of thin actin filaments seen in the control cells (Figure 4A).
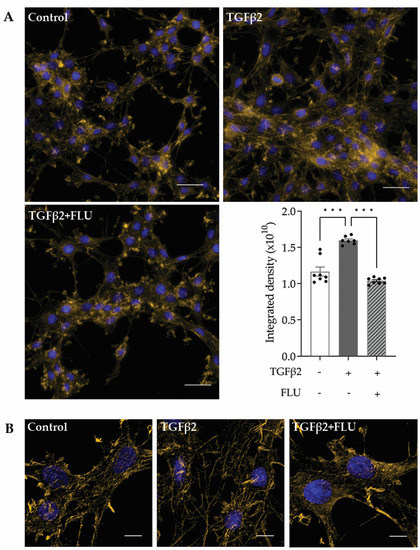
Figure 4.
F-actin reorganization in HTM5 cells. Representative images of cytoskeletal rearrangement after 10 ng/mL TGFβ2 induction for 24 h with or without 10 µM FLU with (A) smaller and (B) higher magnification. F-actin was visualized with phalloidin-Alexa Fluor 546 (F-actin: yellow, nuclei: blue; (A) 60× objective along with a 1.5× intermediate magnification, scale bar = 20 µm. Integrated density values represent quantification of fluorescence (data: mean ± SEM. *** p < 0.001; n = 6–8/group; one-way ANOVA). (B) 512 × 512-pixel cropped deconvolved images (60× objective along with a 1.5× intermediate magnification, scale bar = 5 µm).
The S1R agonist FLU reduced F-actin enhancement; furthermore, fewer stress fibers and less clump accumulation were observed. To clearly visualize the actin stress fibers, image deconvolution was performed on the cropped images using Huygens Deconvolution software, Huygens Essential 21.10 (Figure 4B). This postprocessing step enables the resolution of finer details in the image. In addition to visualization, a quantification of the fluorescent signal was performed, and it is presented as integrated density, which is a commonly used metric in image evaluation (Figure 4A, lower panel).
All these data confirmed the protective effect of FLU on TGFβ2-induced F-actin network reorganization in HTM5 cells.
3.4. FLU Ameliorates the TGFβ2-Induced Production of ECM Compounds
There is an increased deposition of ECM elements in the TM of glaucomatous eyes, among which fibronectin and collagen are the main components [10,11,19,20,21,22,23]. In addition to this, CTGF stimulates the accumulation and remodeling of the ECM [16,18,44].
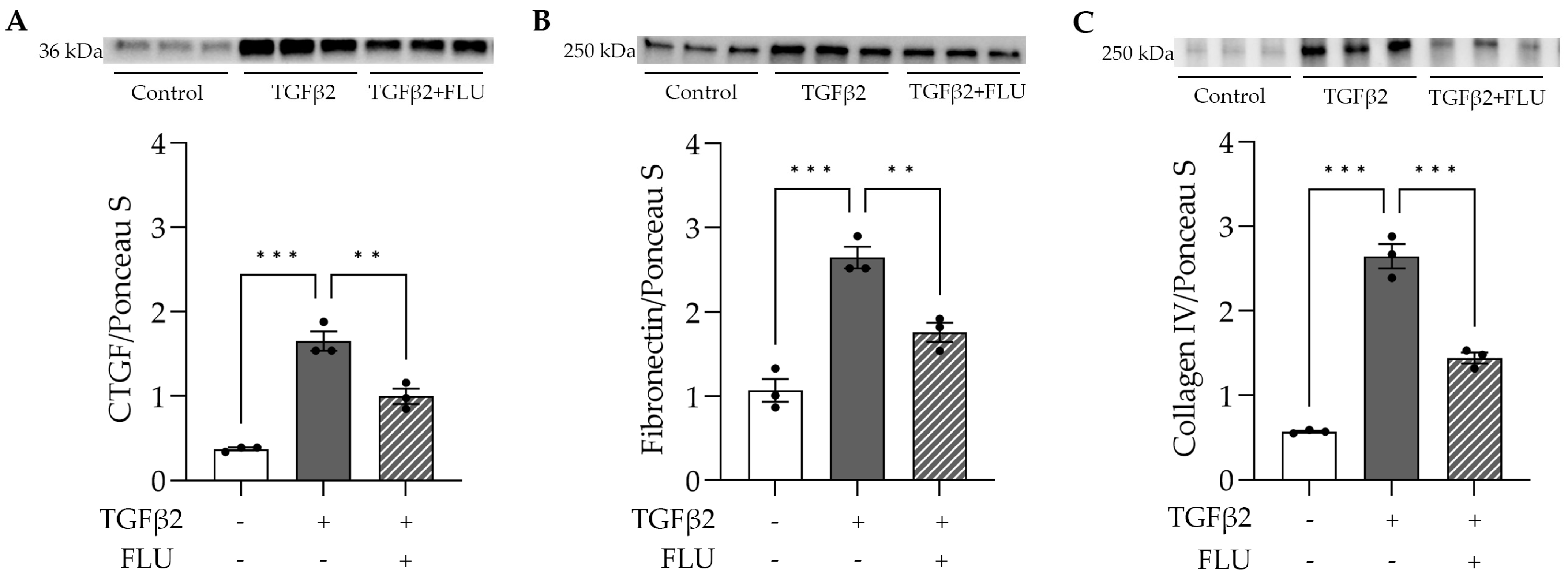
To assess the effect of FLU on the ECM’s key players, HTM5 cells were treated with TGFβ2 and FLU, and CTGF, fibronectin, and collagen type IV were analyzed with a Western blot. In line with the literature, in the HTM5 cells, TGFβ2 markedly increased the levels of CTGF, fibronectin, and collagen IV, while FLU suppressed the elevation of all proteins (Figure 5). To the best of our knowledge, these results are the first evidence for the impact of FLU on these ECM components in TM cells.
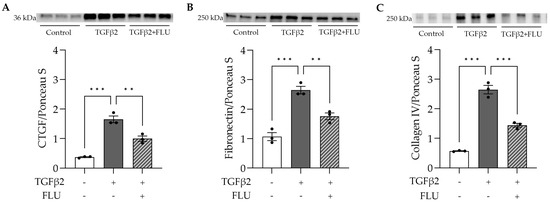
Figure 5.
ECM-related protein levels in HTM5 cells after induction with 10 ng/mL TGFβ2 for 24 h alone or in combination with 10 µM FLU. (A) Representative Western blot images of CTGF (36 kDa), (B) fibronectin (250 kDa), and (C) collagen IV (250 kDa) (all data: mean ± SEM. ** p < 0.01, *** p < 0.001; n = 3/group; one-way ANOVA). The uncropped blots are shown in File S1.
3.5. FLU Increases the Level of the ECM-Degrading Enzyme MMP2
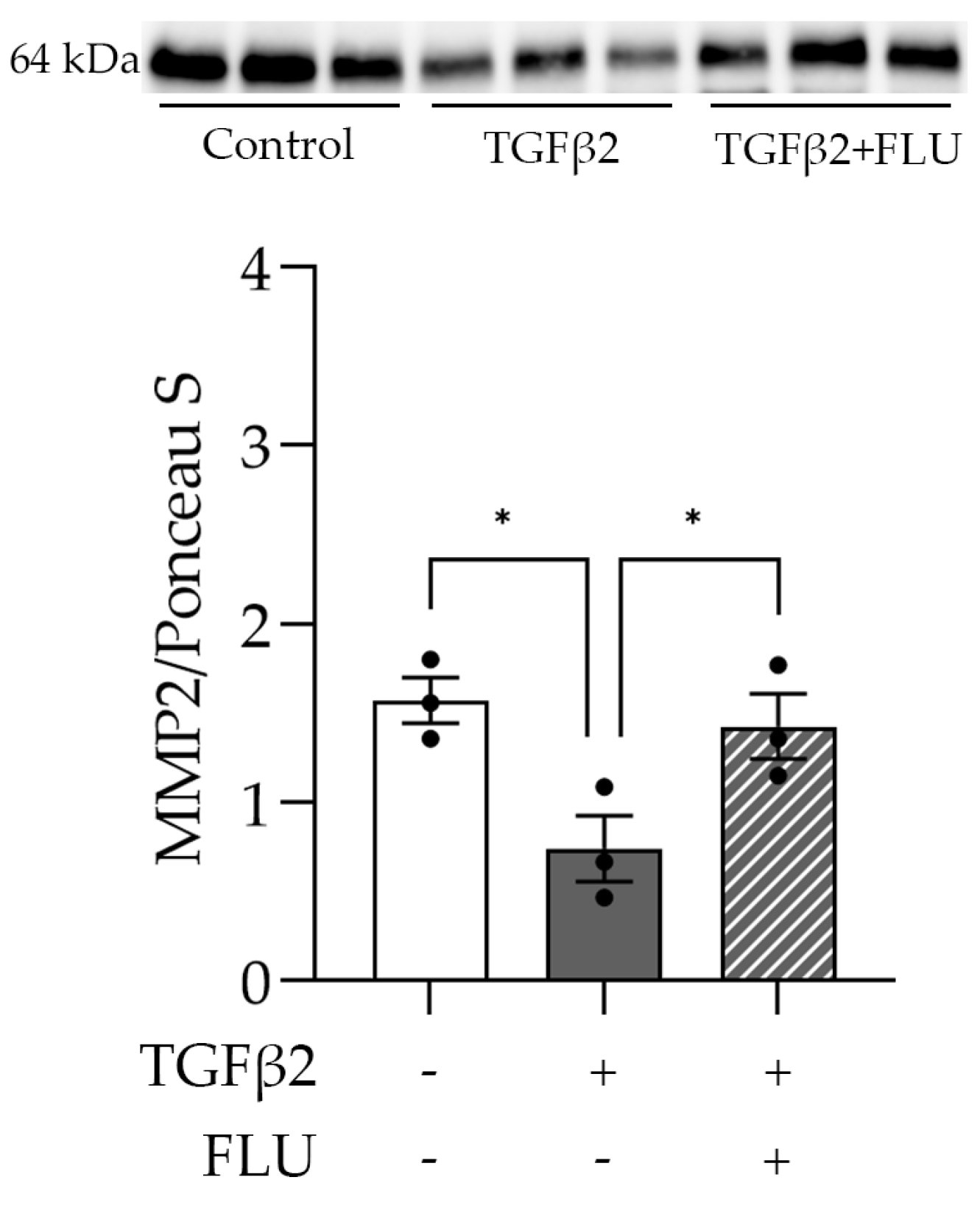
One may speculate that FLU attenuates ECM accumulation by facilitating its degradation. To verify this, we examined the level of the active form of the proteolytic enzyme MMP2 that plays a role in the breakdown of ECM components. As the Western blot analysis revealed, TGFβ2 reduced the MMP2 level (Figure 6). More importantly, the TGFβ2-induced suppression of MMP2 was reversed by FLU (Figure 6), and this may facilitate ECM degradation. This is in line with our F-actin and ECM element measurements and confirms the protective effect of FLU against TGFβ2-induced alterations in HTM5 cells.
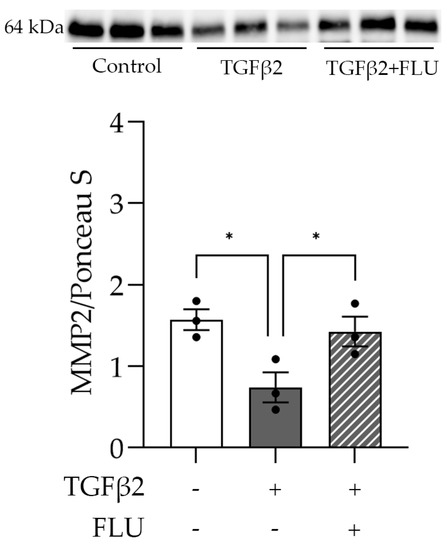
Figure 6.
Level of matrix metalloproteinase-2 (MMP2) in HTM5 cells after induction with 10 ng/mL TGFβ2 for 24 h alone or in combination with 10 µM FLU. Western blot of ECM-degrading MMP2 enzyme (64 kDa) (Data: mean ± SEM. * p < 0.05; n = 3/group; one-way ANOVA). The uncropped blots are shown in File S1.
4. Discussion
S1R is a ubiquitously expressed chaperone that has been studied rigorously in the brain, with a large body of evidence supporting its neuroprotective effect in various central nervous system diseases [24,25,26,27,28,45]. S1R is located in various ocular tissues; however, most papers have reported on its retinal effects [34,35,36,37,46,47,48,49]. There is only one study, which investigated the role of S1R in the pathologies of TM, showing that S1R agonist (+)-pentazocine protects human TM cells from pressure-induced apoptosis [29]. Our study is the first that deeply investigates S1R abundance and function in the anterior segment of the eye (more precisely in the TM). Additionally, we confirmed the presence of S1R in HTM5 cells. Moreover, we demonstrated that none of the treatments altered the protein level of S1R.
TGFβ2 is the predominant isoform of TGFβ in the eye and is one of the two factors (along with dexamethasone) that is known to cause glaucomatous alterations in the TM. Although numerous investigations have been performed to examine TGFβ2’s effect on ECM remodeling and TM function [10,11,12,13,14,15], the detailed mechanism leading to increased AH outflow resistance and elevated IOP is still not clear. Novel compounds that could ameliorate TM dysfunctions are of immense interest.
Here, we provide evidence that the S1R agonist FLU prevents ECM-related alterations of HTM5 cells caused by TGFβ2. In line with the literature, we also detected F-actin enhancement and the formation of stress fibers and actin clumps upon TGFβ2 induction both in vivo (Figure 1B) and in vitro (Figure 4). Similarly to other studies [14,15,19,20], we confirmed that TGFβ2 significantly increased cell proliferation (Figure 2), caused morphological changes (Figure 3), and induced F-actin level and rearrangement (Figure 4) and the accumulation of ECM elements (Figure 5) in HTM5 cells. More importantly, we demonstrated the protective effect of FLU by mitigating various changes, including the downregulation of F-actin, CTGF, fibronectin, and collagen IV proteins (Figure 4 and Figure 5), which are key factors in maintaining normal outflow resistance in the TM. Additionally, our study reveals that none of the treatments induced cellular toxicity, affirming the safety of allying FLU.
Multiple possibilities have to be considered beyond this protective effect against TGFβ2-induced changes. First of all, FLU markedly decreased the level of F-actin, the main component of the cell’s cytoskeleton, and inhibited actin clump and stress fiber formation. Thus, cellular processes such as cell division, cell movement, and the maintenance of cell shape, in all of which F-actin plays a critical role [42], are directly targeted by FLU.
Secondly, the effect of FLU on CTGF is also a possibility. CTGF is involved in a variety of biological processes, including cell adhesion and migration. It is well known to stimulate the deposition and remodeling of the ECM [16,17,44], while its inhibition could attenuate fibrotic processes [17,44]. We postulate that the increased fibronectin and collagen IV level could be a consequence of CTGF activation. Therefore, its inhibition by FLU can lead to the decreased level of these fibrotic proteins, and through this, FLU could ameliorate tissue stiffness. However, further research is needed to fully understand their potential interactions.
Targeting ECM degradation is another manner in which FLU might be protective. The accumulation of the ECM could be the result of the reduction in ECM-degrading enzyme activity (MMPs and some other proteolytic enzymes). The inhibition of MMP2 expression or activity in human TM cell cultures through TGFβ2 treatment has been reported [15,50]. The overexpression of ECM elements (fibronectin and collagen IV) and reduction in the MMP2 trigger fibrotic processes and, therefore, may increase AH outflow resistance. We found that FLU prevented the TGFβ2-induced MMP2 decrease (Figure 6). Therefore, we propose that it could maintain the ECM-degrading function of the enzyme. It is worth noting that further experiments investigating the enzyme activity using gel zymography would provide further details about the mechanism of FLU’s effect on ECM decomposition.
In conclusion, our research describes the presence of S1R in TM cells. We reported a novel effect of the S1R agonist FLU, which protects TM cells from ECM remodeling, accumulation, and dysfunction caused by the TGFβ2/CTGF pathway. Therefore, we postulate that S1R activation is a valuable approach to preserve the TM’s structure and function. Investigating the effect of FLU in vivo is a feasible and rational continuation that will further validate our in vitro results.
5. Patents
Patent number: US20190209575A1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13071581/s1, Figure S1: Immunocytochemistry and Western blot of HTM5 cells after treatment with 100 nM dexamethasone (Dex) for 7 days. File S1: Full pictures of the Western blots.
Author Contributions
Conceptualization, I.K., A.F. and J.H.; funding acquisition, A.F.; investigation, M.N.T., T.M. and J.H.; methodology, M.N.T., T.M., X.G., I.K. and J.H.; project administration, A.J.S. and A.F.; resources, A.J.S., X.G. and A.F.; supervision, A.F. and J.H.; visualization, B.B. and G.T.; writing—original draft, M.N.T.; writing—review and editing, A.F. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by OTKA-K135398 (National Research, Development and Innovation Office), LP2021-3/2021 (Hungarian Academy of Sciences), and TKP2021-EGA-24 grants. Project no. TKP2021-EGA-24 was implemented with support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Semmelweis University (protocol code PE/EA/916-7/2020, approval date: 7 October 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Maria Bernath for excellent technical assistance. We also acknowledge the TKP2021-EGA-23 grant (Gyorgy Torok).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic Modulus Determination of Normal and Glaucomatous Human Trabecular Meshwork. Investig. Opthalmol. Vis. Sci. 2011, 52, 2147. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.M. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 1963, 69, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef]
- Knepper, P.A.; Goossens, W.; Palmberg, P.F. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2414–2425. [Google Scholar]
- Rohen, J.W. Why is Intraocular Pressure Elevated in Chronic Simple Glaucoma?: Anatomical Considerations. Ophthalmology 1983, 90, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.V.; Quigley, H.A. Risk factors and open-angle glaucoma: Classification and application. J. Glaucoma 2007, 16, 406–418. [Google Scholar] [CrossRef]
- Nouri-Mahdavi, K.; Hoffman, D.; Coleman, A.L.; Liu, G.; Li, G.; Gaasterland, D.; Caprioli, J. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004, 111, 1627–1635. [Google Scholar] [CrossRef]
- Hirsch, L.; Nazari, H.; Sreekumar, P.G.; Kannan, R.; Dustin, L.; Zhu, D.; Barron, E.; Hinton, D.R. TGF-β2 secretion from RPE decreases with polarization and becomes apically oriented. Cytokine 2015, 71, 394–396. [Google Scholar] [CrossRef]
- Tanihara, H.; Yoshida, M.; Matsumoto, M.; Yoshimura, N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1993, 34, 413–419. [Google Scholar]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.-H.; Clark, A.F. TGFβ2-Induced Changes in Human Trabecular Meshwork: Implications for Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Patel, P.D.; Searby, C.; Sheffield, V.C.; Zode, G.S. Transforming growth factor β2 (TGFβ2) signaling plays a key role in glucocorticoid-induced ocular hypertension. J. Biol. Chem. 2018, 293, 9854–9868. [Google Scholar] [CrossRef]
- Gottanka, J.; Chan, D.; Eichhorn, M.; Lütjen-Drecoll, E.; Ethier, C.R. Effects of TGF-β2 in Perfused Human Eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Jain, A.; Zode, G.S.; Wordinger, R.J.; Clark, A.F. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Honjo, M.; Aihara, M. mTOR inhibitors potentially reduce TGF-β2-induced fibrogenic changes in trabecular meshwork cells. Sci. Rep. 2021, 11, 14111. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Moriniere, J.; Welge-Lussen, U. Vitamin E reduces TGF-beta2-induced changes in human trabecular meshwork cells. Curr. Eye Res. 2013, 38, 952–958. [Google Scholar] [CrossRef]
- Junglas, B.; Kuespert, S.; Seleem, A.A.; Struller, T.; Ullmann, S.; Bosl, M.; Bosserhoff, A.; Kostler, J.; Wagner, R.; Tamm, E.R.; et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 2012, 180, 2386–2403. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef]
- Junglas, B.; Yu, A.H.L.; Welge-Lüssen, U.; Tamm, E.R.; Fuchshofer, R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp. Eye Res. 2009, 88, 1065–1075. [Google Scholar] [CrossRef]
- Li, H.; Henty-Ridilla, J.L.; Bernstein, A.M.; Ganapathy, P.S.; Herberg, S. TGFβ2 Regulates Human Trabecular Meshwork Cell Contractility via ERK and ROCK Pathways with Distinct Signaling Crosstalk Dependent on the Culture Substrate. Curr. Eye Res. 2022, 47, 1165–1178. [Google Scholar] [CrossRef]
- Buffault, J.; Brignole-Baudouin, F.; Reboussin, É.; Kessal, K.; Labbé, A.; Mélik Parsadaniantz, S.; Baudouin, C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. J. Clin. Med. 2022, 11, 1001. [Google Scholar] [CrossRef]
- Keller, K.E.; Peters, D.M. Pathogenesis of glaucoma: Extracellular matrix dysfunction in the trabecular meshwork-A review. Clin. Exp. Ophthalmol. 2022, 50, 163–182. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Millar, J.C.; Clark, A.F.; Zode, G.S. Increased synthesis and deposition of extracellular matrix proteins leads to endoplasmic reticulum stress in the trabecular meshwork. Sci. Rep. 2017, 7, 65. [Google Scholar] [CrossRef]
- Overby, D.R.; Bertrand, J.; Tektas, O.Y.; Boussommier-Calleja, A.; Schicht, M.; Ethier, C.R.; Woodward, D.F.; Stamer, W.D.; Lutjen-Drecoll, E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4922–4933. [Google Scholar] [CrossRef]
- Albayrak, Y.; Hashimoto, K. Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders. Adv. Exp. Med. Biol. 2017, 964, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Wang, J.-Y.; Chen, H.-L.; Chang, H.-X.; Zeng, Z.-R.; Li, G.-X.; Ma, H.; Zhao, Y.-Q.; Li, Y.-F. Sigma-1 receptor agonist properties that mediate the fast-onset antidepressant effect of hypidone hydrochloride (YL-0919). Eur. J. Pharmacol. 2023, 946, 175647. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef]
- Hayashi, T.; Tsai, S.-Y.; Mori, T.; Fujimoto, M.; Su, T.-P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets 2011, 15, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Omi, T.; Tanimukai, H.; Kanayama, D.; Sakagami, Y.; Tagami, S.; Okochi, M.; Morihara, T.; Sato, M.; Yanagida, K.; Kitasyoji, A.; et al. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014, 5, e1332. [Google Scholar] [CrossRef]
- Meng, B.; Li, H.; Sun, X.; Qu, W.; Yang, B.; Cheng, F.; Shi, L.; Yuan, H. σ-1 receptor stimulation protects against pressure-induced damage through InsR-MAPK signaling in human trabecular meshwork cells. Mol. Med. Rep. 2017, 16, 617–624. [Google Scholar] [CrossRef]
- Ola, M.S.; Moore, P.; El-Sherbeny, A.; Roon, P.; Agarwal, N.; Sarthy, V.P.; Casellas, P.; Ganapathy, V.; Smith, S.B. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res. Mol. Brain Res. 2001, 95, 86–95. [Google Scholar] [CrossRef]
- Bucolo, C.; Campana, G.; Di Toro, R.; Cacciaguerra, S.; Spampinato, S. Sigma Recognition Sites in Rabbit Iris-Ciliary Body: Topical sigmal1-Site Agonists Lower Intraocular Pressure. J. Pharmacol. Exp. Ther. 1999, 289, 1362. [Google Scholar] [PubMed]
- Wang, L.; Duncan, G. Silencing of sigma-1 receptor induces cell death in human lens cells. Exp. Cell Res. 2006, 312, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Schoenwald, R.D.; Barfknecht, C.F.; Xia, E.; Newton, R.E. The presence of sigma-receptors in the lacrimal gland. J. Ocul. Pharmacol. 1993, 9, 125–139. [Google Scholar] [CrossRef]
- Zhao, J.; Gonsalvez, G.B.; Mysona, B.A.; Smith, S.B.; Bollinger, K.E. Sigma 1 Receptor Contributes to Astrocyte-Mediated Retinal Ganglion Cell Protection. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1. [Google Scholar] [CrossRef]
- Martin, P.M.; Ola, M.S.; Agarwal, N.; Ganapathy, V.; Smith, S.B. The sigma receptor ligand (+)−pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Mol. Brain Res. 2004, 123, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, F.; Meng, B.; Yang, B.; Song, W.; Yuan, H. Pregnenolone sulfate decreases intraocular pressure and changes expression of sigma receptor in a model of chronic ocular hypertension. Mol. Biol. Rep. 2012, 39, 6607–6614. [Google Scholar] [CrossRef]
- Zhao, J.; Mysona, B.A.; Qureshi, A.; Kim, L.; Fields, T.; Gonsalvez, G.B.; Smith, S.B.; Bollinger, K.E. (+)-Pentazocine Reduces NMDA-Induced Murine Retinal Ganglion Cell Death Through a σR1-Dependent Mechanism. Investig. Ophthalmol. Vis. Sci. 2016, 57, 453–461. [Google Scholar] [CrossRef]
- Pang, I.H.; Shade, D.L.; Clark, A.F.; Steely, H.T.; DeSantis, L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr. Eye Res. 1994, 13, 51–63. [Google Scholar] [CrossRef]
- Huygens Deconvolution: Restore Microscopy Images. Available online: https://svi.nl/Huygens-Deconvolution (accessed on 5 July 2023).
- Tripathi, R.C.; Li, J.; Chan, W.F.; Tripathi, B.J. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Structure and Organization of Actin Filaments. In The Cell: A Molecular Approach; Sinauer Associates: Sunderland MA, USA, 2000. [Google Scholar]
- Fuchshofer, R.; Kuespert, S.; Junglas, B.; Tamm, E.R. The Prostaglandin F2α Analog Fluprostenol Attenuates the Fibrotic Effects of Connective Tissue Growth Factor on Human Trabecular Meshwork Cells. J. Ocul. Pharmacol. Ther. 2014, 30, 237–245. [Google Scholar] [CrossRef]
- Aishwarya, R.; Abdullah, C.S.; Morshed, M.; Remex, N.S.; Bhuiyan, M.S. Sigmar1’s Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front. Physiol. 2021, 12, 705575. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, Y.; Qin, H.; Zhang, S.; Li, P.; Yao, K. Sigma-1 Receptor in Retina: Neuroprotective Effects and Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 7572. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S.; Winters, H.; Pannicke, T.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Sigma-1 receptor activation inhibits osmotic swelling of rat retinal glial (Müller) cells by transactivation of glutamatergic and purinergic receptors. Neurosci. Lett. 2016, 610, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Saul, A.; Roon, P.; Smith, S.B. Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, E3764–E3772. [Google Scholar] [CrossRef]
- Mueller, B.H.; Park, Y.; Ma, H.-Y.; Dibas, A.; Ellis, D.Z.; Clark, A.F.; Yorio, T. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp. Eye Res. 2014, 128, 156–169. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Welge-Lussen, U.; Lütjen-Drecoll, E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp. Eye Res. 2003, 77, 757–765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).