Importance of Pharmacogenetics and Drug–Drug Interactions in a Kidney Transplanted Patient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
2.2. Case Presentation
2.3. TAC Pharmacokinetics
2.4. DNA Isolation and Genotyping
3. Results
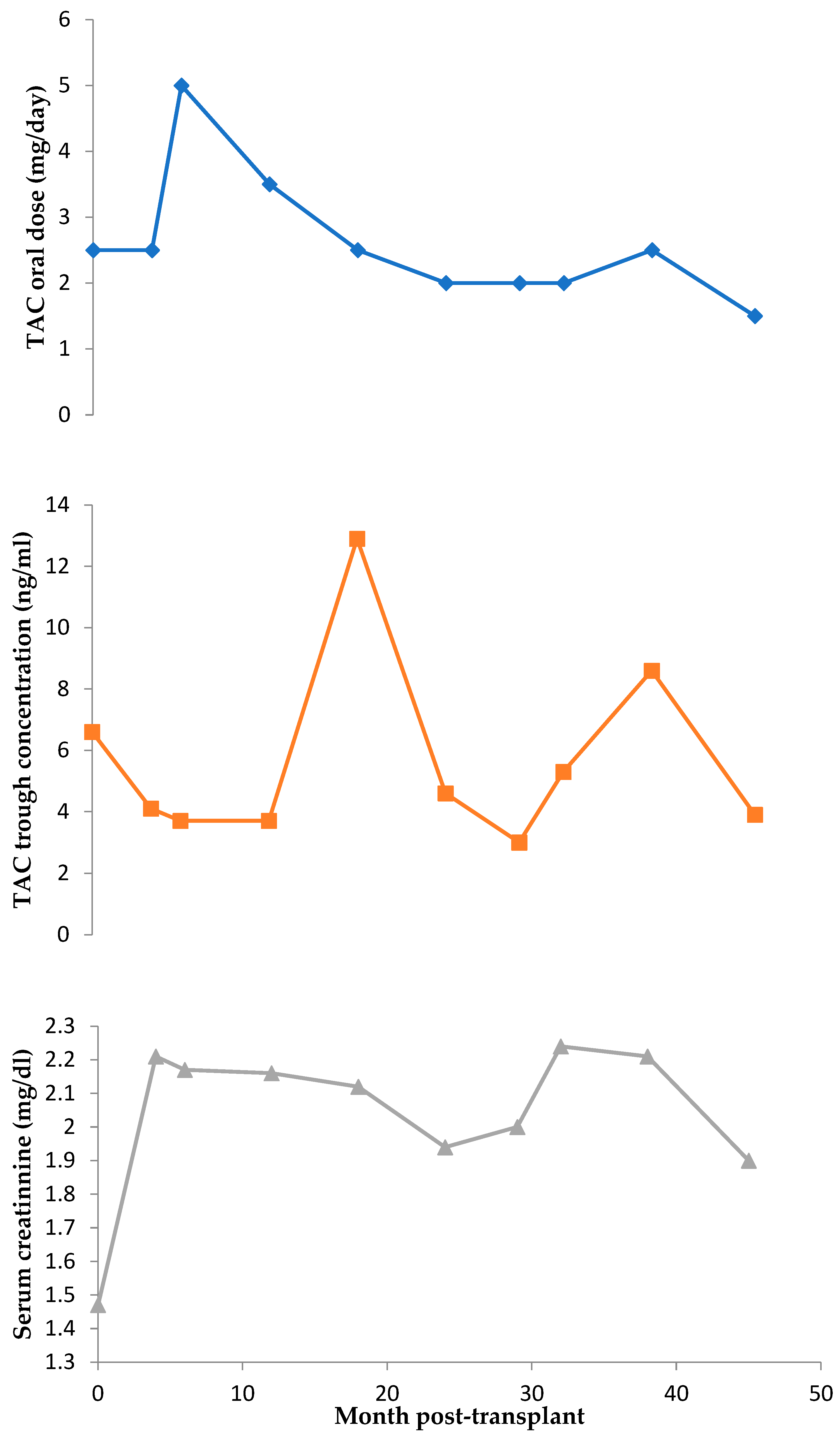
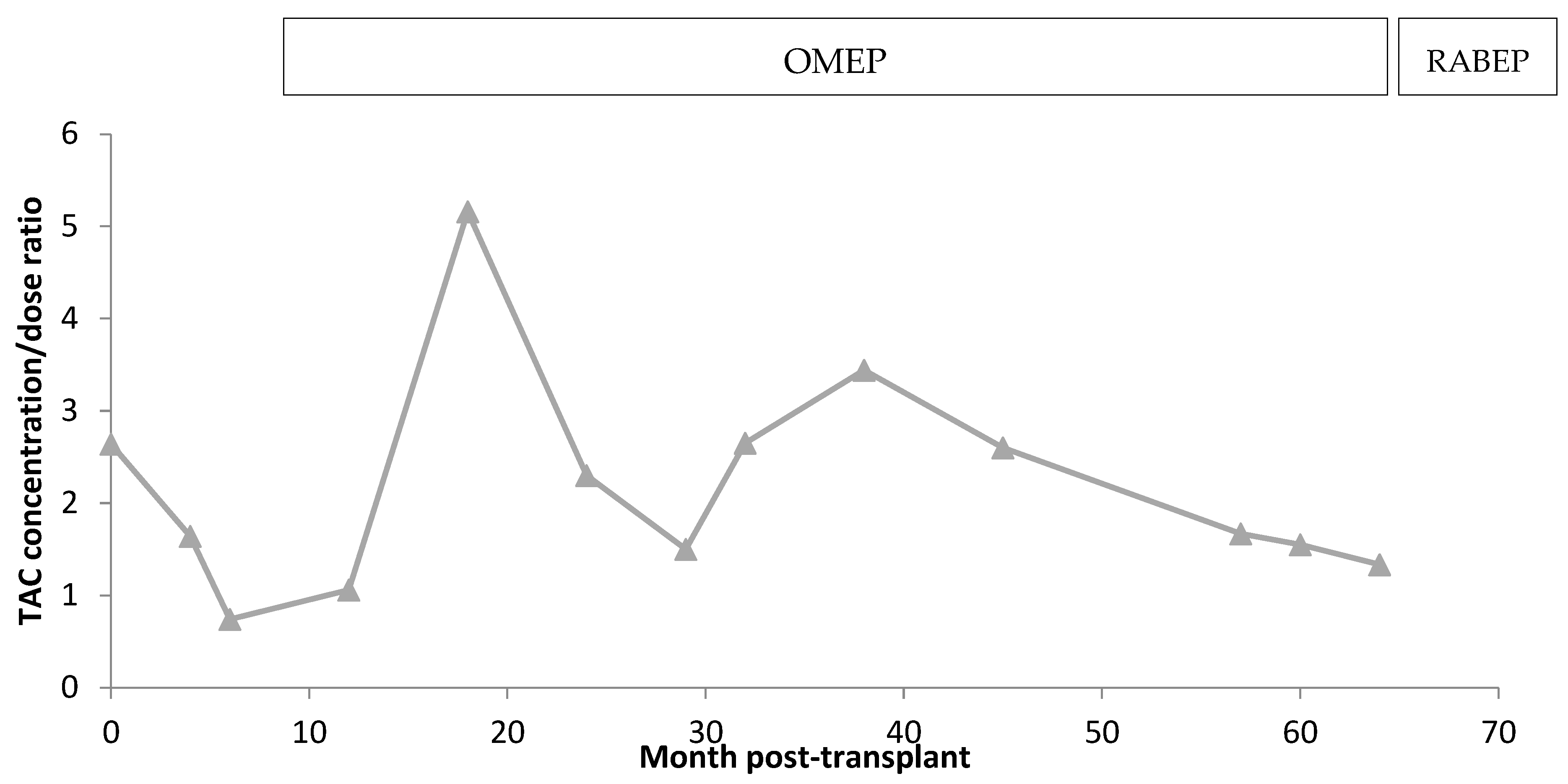
3.1. TAC Pharmacokinetics
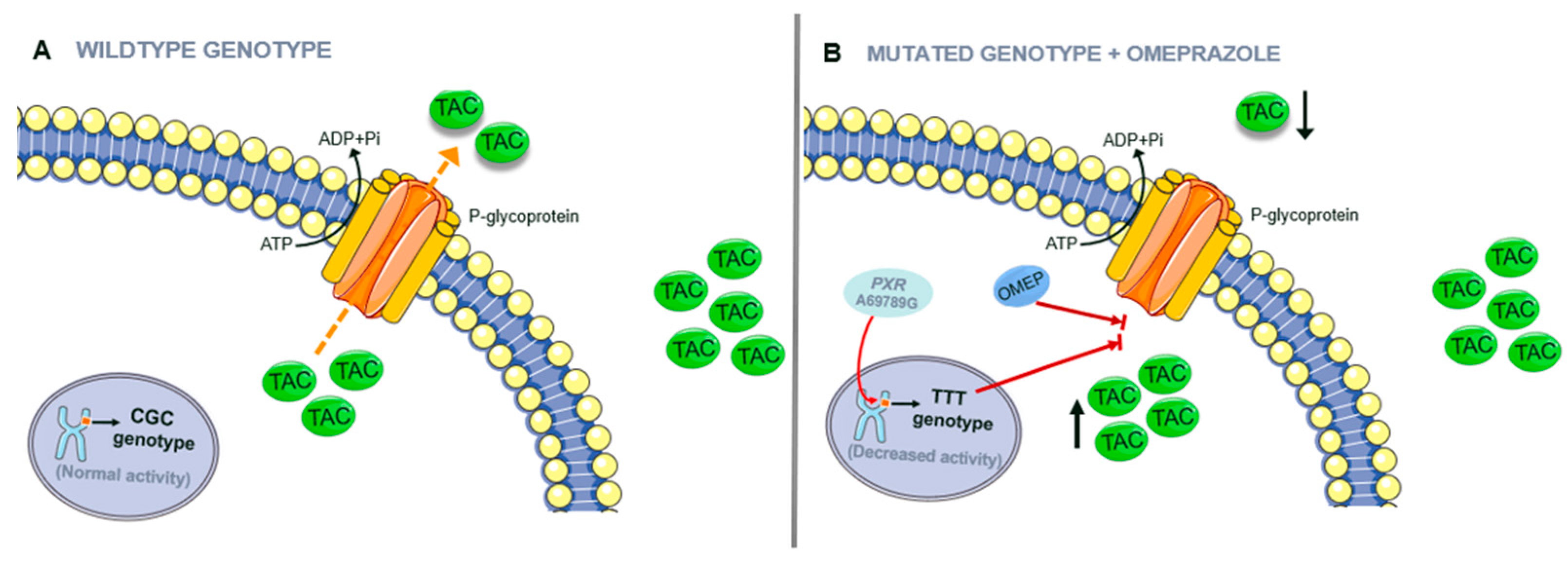
3.2. Genotype
4. Discussion
4.1. Clinical Variants Affecting Short-Term Transplantation Pharmacokinetics
4.2. Patient’s Pharmacogenetics and Drug–Drug Interactions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunet, M.; Pastor-Anglada, M. Insights into the Pharmacogenetics of Tacrolimus Pharmacokinetics and Pharmacodynamics. Pharmaceutics 2022, 14, 1755. [Google Scholar] [CrossRef]
- Steven, H.Y.W. Therapeutic Drug Monitoring for Immunosuppressants. Clin. Chim. Acta 2001, 23, 2745–2747. [Google Scholar] [CrossRef]
- Sikma, M.A.; Van Maarseveen, E.M.; Hunault, C.C.; Moreno, J.M.; Van de Graaf, E.A.; Kirkels, J.H.; Verhaar, M.C.; Grutters, J.C.; Kesecioglu, J.; De Lange, D.W.; et al. Unbound Plasma, Total Plasma, and Whole-Blood Tacrolimus Pharmacokinetics Early After Thoracic Organ Transplantation. Clin. Pharmacokinet. 2020, 59, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Bodnar-Broniarczyk, M.; Warzyszyńska, K.; Czerwińska, K.; Marszałek, D.; Dziewa, N.; Kosieradzki, M.; Pawiński, T. Development and Validation of the New Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Unbound Tacrolimus in the Plasma Ultrafiltrate of Transplant Recipients. Pharmaceutics 2022, 14, 632. [Google Scholar] [CrossRef]
- Frohlich, E. Understanding and Preventing Adverse Effects of Tacrolimus Metabolization in Transplant Patients. Curr. Drug Metab. 2019, 20, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Technical Sheet Tacforirus (EMA). Available online: https://www.ema.europa.eu/en/documents/product-information/tacforius-epar-product-information_en.pdf (accessed on 8 June 2023).
- Sendra, L.; Olivera, G.G.; López-Andújar, R.; Serrano, C.; Rojas, L.E.; Montalva, E.M.; Herrero, M.J.; Aliño, S.F. Pharmacogene Variants Associated with Liver Transplant in a Twelve-Year Clinical Follow-Up. Pharmaceutics 2022, 14, 354. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Li, J.; Deng, R.; Fu, Q.; Chen, J.; Huang, M.; Chen, X.; Wang, C. Dynamic effects of CYP3A5 polymorphism on dose requirement and trough concentration of tacrolimus in renal transplant recipients. J. Clin. Pharm. Ther. 2017, 42, 93–97. [Google Scholar] [CrossRef]
- Buendía, J.A.; Halac, E.; Bosaleh, A.; Garcia de Davila, M.T.; Imvertasa, O.; Bramuglia, G. Frequency of CYP3A5 Genetic Polymorphisms and Tacrolimus Pharmacokinetics in Pediatric Liver Transplantation. Pharmaceutics 2020, 12, 898. [Google Scholar] [CrossRef]
- Shi, W.L.; Tang, H.L.; Zhai, S.D. Effects of the CYP3A4*1B Genetic Polymorphism on the Pharmacokinetics of Tacrolimus in Adult Renal Transplant Recipients: A Meta-Analysis. PLoS ONE 2015, 10, e0127995. [Google Scholar] [CrossRef] [Green Version]
- Lloberas, N.; Elens, L.; Llaudó, I.; Padullés, A.; van Gelder, T.; Hesselink, D.A.; Colom, H.; Andreu, F.; Torras, J.; Bestard, O.; et al. The combination of CYP3A4*22 and CYP3A5*3 single-nucleotide polymorphisms determines tacrolimus dose requirement after kidney transplantation. Pharmacogenet. Genom. 2017, 27, 313–322. [Google Scholar] [CrossRef]
- Van Gelder, T. Drug Interactions with Tacrolimus. Drug Saf. 2002, 25, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Pauli-Magnus, C.; Rekersbrink, S.; Klotz, U.; Fromm, M.F. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 364, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Masuda, S.; Ogura, Y.; Oike, F.; Takada, Y.; Katsura, T.; Uemoto, S.; Inui, K. Interaction between Tacrolimus and Lansoprazole, but not Rabeprazole in Living-Donor Liver Transplant Patients with Defects of CYP2C19 and CYP3A5. Drug Metab. Pharmacokinet. 2008, 23, 134–138. [Google Scholar] [CrossRef]
- Zhao, W.; Fakhoury, M.; Maisin, A.; Baodouin, V.; Storme, T.; Deschenes, G.; Jacqz-Agrain, E. Pharmacogenetic determinant of the drug Interaction between tacrolimus and omeprazole. Ther. Drug Monit. 2012, 34, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Taburet, A.M.; Furlan, V.; Debray, D.; Loriot, M.A. Interaction between Tacrolimus and Omeprazole in a Pediatric Liver Transplant Recipient. Transplantation 2006, 82, 1382. [Google Scholar] [CrossRef]
- Maguire, M.; Franz, T.; Hains, D.S. A clinically significant interaction between tacrolimus and multiple proton pump inhibitors in a kidney transplant recipient. Ped. Transp. 2012, 16, E217–E220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yano, I.; Fukuhara, Y.; Katsura, T.; Takahashi, T.; Ito, N.; Yamamoto, S.; Ogawa, O.; Inui, K. Distinct Effects of Omeprazole and Rabeprazole on the Tacrolimus Blood Concentration in a Kidney Transplant Recipient. Drug Metab. Pharmacokinet. 2007, 22, 441–444. [Google Scholar] [CrossRef]
- Grinyo, J.M.; Ekberg, H.; Mamelok, R.D.; Oppenheimer, F.; Sanchez-Plumed, J.; Gentil, M.A.; Hernandez, D.; Kuypers, D.R.; Brunet, M. The pharmacokinetics of myco-phenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine, low-dose tacrolimus or low-dose sirolimus: The Symphony pharmacokinetic substudy. Nephrol. Dial. Transplant. 2009, 24, 2269–2276. [Google Scholar] [CrossRef] [Green Version]
- Ekberg, H.; Tedesco-Silva, H.; Demirbas, A.; Vitko, S.; Nashan, B.; Gürkan, A.; Margreiter, R.; Hugo, C.; Grinyó, J.M.; Frei, U.; et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 2007, 357, 2562–2575. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.F.; Holt, D.W.; Chang, R.; MacPhee, I. Black renal transplant recipients have poorer long-term graft survival than CYP3A5 expressers from other ethnic groups. Nephrol. Dial. Transplant. 2010, 25, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Cheong, H.S.; Kim, L.H.; Kin, J.O.; Seo, D.O.; Kim, Y.H.; Chung, M.W.; Han, S.Y.; Shin, H.D. Screening of Genetic Polymorphisms of CYP3A4 and CYP3A5 Genes. Korean J. Physiol. Pharmacol. 2013, 17, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levran, O.; Peles, E.; Hamon, S.; Randesi, M.; Adelson, M.; Kreek, M.J. CYP2B6 SNPs are associated with methadone dose required for effective treatment of opioid addiction. Addict. Biol. 2013, 18, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Wai-lin, W.; Jing, J.; Shu-sen, Z.; Li-hua, W.; Ting-bo, L.; Song-feng, Y.; Sheng, Y. Tacrolimus Dose Requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl. 2006, 12, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Scapoli, L.; Cura, F.; Rodia, M.T.; Ugolini, G.; Montroni, I.; Solmi, R. Colorectal cancer susceptibility: Apparent gender-related modulation by ABCB1 gene polymorphisms. J. Biomed. Sci. 2014, 21, 89. [Google Scholar] [CrossRef] [Green Version]
- Balan, S.; Bharathan, S.P.; Vellichiramal, N.N.; Sathyan, S.; Joseph, V.; Radhakhrishnan, K.; Banerjee, M. Genetic Association Analysis of ATP Binding Cassette Protein Family Reveals a Novel Association of ABCB1 Genetic Variants with Epilepsy Risk, but Not with Drug-Resistance. PLoS ONE 2014, 9, e89253. [Google Scholar] [CrossRef]
- Reyes-Hernández, O.D.; Vega, L.; Jiménez-Ríos, M.A.; Martinez-Cervera, P.F.; Lungo-García, J.A.; Hernandez-Candela, L.; Ostrosky-Wegman, P.; Orozco, L.; Elizondo, G. The PXR rs7643645 polymorphism is associated with the risk of higher prostate-specific antigen levels in prostate cancer patients. PLoS ONE 2014, 9, e99974. [Google Scholar] [CrossRef] [Green Version]
- Nieuwoudt, E. Effect of Genetic Variants in Genes Encoding Two Nuclear Receptors (PXR and CAR) on Efavirenz Levels and Treatment Outcome in South African HIV-Infected Females. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Tabari, R.G.; Marjani, A.; Ataby, O.A.; Mansourian, A.R.; Samai, N.M. Genetic Polymorphism of Cytochrome p450 (2C19) Enzyme in Iranian Turkman Ethnic Group. Oman Med. J. 2013, 28, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kattel, K.; Evande, R.; Tan, C.; Mondal, G.; Grem, J.L.; Mahato, R.I. Impact of CYP2C19 polymorphism on the pharmacokinetics of nelfinavir in patients with pancreatic cancer. Br. J. Clin. Pharmacol. 2015, 80, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apellániz-Ruiz, M.; Inglada-Pérez, L.; Naranjo, M.E.; Sánchez, L.; Mancikova, V.; Currás-Freixes, M.; de Cubas, A.A.; Comino-Méndez, I.; Triki, S.; Rebai, A.; et al. High frequency and founder effect of the CYP3A4*20 loss-of-function allele in the Spanish population classifies CYP3A4 as a polymorphic enzyme. Pharmacogenomics 2015, 15, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, H.; Yoon, H.Y.; Yee, J.; Gwak, H.S. Association of P450 Oxidoreductase Gene Polymorphism with Tacrolimus Pharmacokinetics in Renal Transplant Recipients: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 261. [Google Scholar] [CrossRef]
- Hodges, L.M.; Markova, S.M.; Chinn, L.W.; Gow, J.M.; Kroetz, D.L.; Klein, T.E.; Altman, R.B. Very important pharmacogene summary. Pharmacogenet. Genom. 2011, 21, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xiang, X.; Huang, W.W.; Sandford, A.; Wu, S.; Zhang, M.M.; Wang, M.; Chen, G.; He, J. Association of PXR and CAR Polymorphisms and Antituberculosis Drug-Induced Hepatotoxicity. Sci. Rep. 2019, 9, 2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamba, J.; Lamba, V.; Strom, S.; Venkataramanan, R.; Schuetz, E. Novel Single Nucleotide Polymorphisms in the Pro-moter and Intron 1 of Human Pregnane X Receptor/NR1I2 and Their Association with CYP3A4 Expression. Drug Metab. Dispos. 2007, 36, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Denisenko, N.; Sychev, D.; Sizova, Z.; Smirnov, V.V.; Ryzhikova, K.A.; Sozaeva, Z.A.; Grishina, E.A. Urine metabolic ratio of omeprazole in relation to CYP2C19 polymorphisms in Russian peptic ulcer patients. Pharmacogenom. Pers. Med. 2017, 10, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Andrews, L.M.; Hesselink, D.A.; Schaik, R.H.N.; Gelder, T.; Fijter, J.W.; Lloberas, N.; Elens, L.; Moes, D.J.A.R.; Winter, B.C.M. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br. J. Clin. Pharmacol. 2019, 85, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Staatz, C.E.; Tett, S.E. Clinical Pharmacokinetics and Pharmacodynamics of Tacrolimus in Solid Organ Transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar] [CrossRef] [PubMed]
- Schijvens, A.M.; van Hesteren, F.H.S.; Cornelissen, E.A.M.; Bootsma-Robroeks, C.M.H.H.T.; Brüggemann, J.M.; Burger, D.M.; Wildt, S.N.; Schreuder, M.F.; Ter Heine, R. The potential impact of hematocrit correction on evaluation of tacrolimus target exposure in pediatric kidney transplant patients. Pediatr. Nephrol. 2019, 34, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Størset, E.; Holford, N.; Midtvedt, K.; Bremer, S.; Bergan, S.; Asberg, A. Importance of hematocrit for a tacrolimus target concentration strategy. Eur. J. Clin. Pharmacol. 2014, 70, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Larkins, N.; Matsell, D.G. Tacrolimus therapeutic drug monitoring and pediatric renal transplant graft outcomes. Pediatr. Transplant. 2014, 18, 803–809. [Google Scholar] [CrossRef]
- Higgins, K. Update on the Use of Tacrolimus in Pediatrics. Univ. Va. Child. Hosp. 2018, 24, 12. Available online: https://med.virginia.edu/pediatrics/opportunities/pharmacotherapy-newsletter/ (accessed on 1 June 2023).
- Birdwell, K.A.; Decker, B.; Barbino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and Tacrolimus dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elens, L.; Hesselink, D.A.; Bouamar, R.; Budde, K.; de Fijter, J.W.; De Meyer, M.; Mourad, M.; Kuypers, D.R.; Haufroid, V.; van Gelder, T.; et al. Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine A in renal transplant patients. Ther. Drug Monit. 2014, 36, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kimchi-Sarfaty, C.; Marple, A.H.; Shinar, S.; Kimchi, A.M.; Scavo, D.; Roma, M.I.; Kim, I.W.; Jones, A.; Arora, M.; Gribar, J.; et al. Ethnicity-related polymorphisms and haplotypes in the human ABCB1 gene. Pharmacogenomics 2007, 8, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, B.; Tulsyan, S.; Mittal, R. The effect of ABCB1 polymorphisms on the outcome of breast cancer treatment. Pharmacogenom. Pers. Med. 2016, 9, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Johne, A. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin. Pharmacol. Ther. 2002, 72, 84–94. [Google Scholar] [CrossRef]
- Zawadzka, I.; Jeleń, A.; Pietrzak, J.; Åebrowska-Nawrocka, M.; Michalska, K.; Szmajda-Krygier, D.; Mirowski, M.; Åochowski, M.; Kozak, J.; Balcerczak, E. The impact of ABCB1 gene polymorphism and its expression on non-small-cell lung cancer development, progression and therapy–Preliminary report. Sci. Rep. 2002, 10, 6188. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paulo, G.N.; Darpía, I.; Luvomirov, R.; Borobia, A.M.; Alonso-Sánchez, N.L.; Espinosa, L.; Carcas-Sansuán, A.J. Weight of ABCB1 and POR genes on oral tacrolimus exposure in CYP3A5 nonexpressor pediatric patients with stable kidney transplant. Pharmacogenom. J. 2018, 18, 180–186. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef]
- García-García, J.A. ¿Qué debemos conocer de los inhibidores de bomba protones, para su uso en las unidades de dolor? Rev. Soc. Esp. Dolor 2007, 14, 501–510. [Google Scholar]
- Hosohata, K.; Masuda, S.; Katsura, T.; Takada, Y.; Kaido, T.; Ogura, Y.; Oike, F.; Egawa, H.; Uemoto, S.; Inui, K. Impact of intestinal CYP2C19 genotypes on the interaction between tacrolimus and omeprazole, but not lansoprazole, in adult living-donor liver transplant patients. Drug Metab. Dispos. 2009, 4, 821–826. [Google Scholar] [CrossRef]
- Pascual, J.; Marcén, R.; Orea, O.E.; Navarro, M.; Alarcón, M.C.; Ocaña, J.; Villafruela, J.J.; Burgos, F.J.; Ortuño, J. Inter-action Between Omeprazole and Tacrolimus in Renal Allograft Recipients: A Clinical-Analytical Study. Transplant. Proc. 2005, 37, 3752–3753. [Google Scholar] [CrossRef] [PubMed]
- Katsakiori, P.; Papapetrou, E.; Goumenos, D.; Nikiforidis, G.; Flordellis, C. Investigation of clinical interaction between omeprazole and tacrolimus in CYP3A5 non-expressors, renal transplant recipients. Ther. Clin. Risk Manag. 2010, 6, 265. [Google Scholar] [CrossRef] [Green Version]
- Degraeve, A.; Moudio, S.; Haufroid, V.; Chaib Eddour, D.; Mourad, M.; Bindels, L.B.; Elens, L. Predictors of tacrolimus pharmacokinetic variability: Current evidences and future perspectives. Expert Opin. Drug Metab. Toxicol. 2020, 16, 769–782. [Google Scholar] [CrossRef]
- Capron, A.; Mourad, M.; De Meyer, M.; De Pauw, L.; Eddour, D.C.; Latinne, D.; Elens, L.; Haufroid, V.; Wallemacq, P. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics 2010, 11, 703–714. [Google Scholar] [CrossRef]
- Sallustio, B.C. Monitoring Intra-cellular Tacrolimus Concentrations in Solid Organ Transplantation: Use of Peripheral Blood Mononuclear Cells and Graft Biopsy Tissue. Front. Pharmacol. 2021, 12, 733285. [Google Scholar] [CrossRef]
- Tron, C.; Woillard, J.B.; Houssel-Debry, P.; David, V.; Jezequel, C.; Rayar, M.; Balakirouchenane, D.; Blanchet, B.; Debord, J.; Petitcollin, A.; et al. Pharmacogenetic-Whole blood and intracellular pharmacokinetic-Pharmacodynamic (PG-PK2-PD) relationship of tacrolimus in liver transplant recipients. PLoS ONE 2020, 15, e0230195. [Google Scholar] [CrossRef] [PubMed]
- De Nicolòf, A.; Pinon, M.; Palermiti, A.; Nonnato, A.; Manca, A.; Mula, J.; Catalano, S.; Tandoi, F.; Romagnoli, R.; D’Avolio, A.; et al. Monitoring Tacrolimus Concentrations in Whole Blood and Peripheral Blood Mononuclear Cells: Inter- and Intra-Patient Variability in a Cohort of Pediatric Patients. Front. Pharmacol. 2021, 12, 750433. [Google Scholar] [CrossRef] [PubMed]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar] [CrossRef]
- Isoda, K.; Takeuchi, T.; Kotani, T.; Hirano-Kuwata, S.; Shoda, T.; Hata, K.; Yoshida, S.; Makino, S.; Hanafusa, T. The Proton Pump Inhibitor Lansoprazole, but not Rabeprazole, the Increased Blood Concentrations of Calcineurin Inhibitors in Japanese Patients with Connective Tissue Diseases. Intern. Med. 2014, 53, 1413–1418. [Google Scholar] [CrossRef] [Green Version]
- Sallustio, B.C.; Noll, B.D.; Hu, R.; Barratt, D.T.; Tuke, J.; Coller, J.K.; Russ, G.R.; Somogyi, A.A. Tacrolimus Dose, Blood Concentrations and Acute Nephrotoxicity, but Not CYP3A5/ABCB1 Genetics, Are Associated with Allograft Tacrolimus Concentrations in Renal Transplant Recipients. Br. J. Clin. Pharmacol. 2021, 87, 3901–3909. [Google Scholar] [CrossRef]
- Yokogawa, K.; Takahashi, M.; Tamai, I.; Konishi, H.; Nomura, M.; Moritani, S.; Miyamoto, K.; Tsuji, A. P-glycoprotein-dependent disposition kinetics of tacrolimus: Studies in mdr1a knockout mice. Pharm. Res. 1999, 16, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Vitek, C.R.; Giri, J.; Caraballo, P.J.; Curry, T.B.; Nicholson, W.T. Pharmacogenomics education and perceptions: Is there a gap between internal medicine resident and attending physicians? Pharmacogenomics 2021, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]

| Gene | Variant | Analysis Technique | Primers (Sequencing or PCR-RFLP) | Enzyme | References |
|---|---|---|---|---|---|
| CYP3A5 (NM_000777.5) | c.6986A > G | Sequencing and rhAmp | F: 5′-ACTGCCCTTGCAGCATTTAG-3′ R: 5′-CCAGGAAGCCAGACTTTGAT-3′ | [22] | |
| CYP3A4 (NM_001202855.3) | g.-290A > G | Sequencing and rhAmp | F: 5′-CAGAAGGGATGACATGCAGA-3′ F: 5′-GGAAGAGGCTTCTCCACCTT-3′ | [23] | |
| CYP3A4 (NM_001202855.3) | c.15389G > T | TaqMan | ID Assay C__59013445_10 | ||
| CYP3A4 (NM_001202855.3) | c.1461_1462 insA | Sequencing | F: 5′-GAAGGAGTGTCTCACTCA-3′ R: 5′-GAGGTCTCTGGTGTTCTCAG-3′ | [24] | |
| POR (NM_000941.3) | c.1508C > T | Sequencing and rhAmp | F: 5′-CATCTGTGCGGTGGTTGT-3′ R: 5′-TGAAGGGCAGGCGGA-3′ | ||
| ABCB1 (NM_000927.3) | c.3435C > T | PCR-RFLP and rhAmp | F: 5′-GATCTGTGAACTCTTGTTTT-3′ R: 5′-GAAGAGAGACTTACATTAGGC-3′ | MboI | [25] |
| ABCB1 (NM_000927.3) | c.1236C > T | PCR-RFLP and rhAmp | F:5′-TTGAATGAAGAGTTTCTGATGTTTT-3′ R: 5′CTCTGCATCAGCTGGACTGT-3′ | BsuRI | [26] |
| ABCB1 (NM_000927.3) | c.2677G > T | PCR-RFLP and TaqMan | F: 5′-TGCAGGCTATAGGTTCCAGG-3′ R: 5′-TTTAGTTTGACTCACCTTCCCG-3′ | BanI | [27] |
| PXR (NM_022002.2) | c.69789A > G | PCR-RFLP and rhAmp | F: 5′-CACCATGCTTAGCTACAGCTCTATT-3′ R: 5′-GGCAAGATCACAACATGGGAAGA-3′ | BstDSI | [28] |
| PXR (NM_022002.2) | c.63396C > T | PCR-RFLP | F: 5′-TGCTAGCAGTGCATAAGGGCTCAG-3′ R: 5′-TCCTGACCTTAGGTGATCCATGCC-3′ | Hpy188I | [28] |
| CYP2C19 (NM_000769.4) | c.681G > A | PCR-RFLP and rhAmp | F: 5′-AATTACAACCAGAGCTTGGC-3′ R: 5′-TATCACTTTCCATAAAAGCAAG-3′ | SmaI | [29] |
| CYP2C19 (NM_000769.4) | c.636G > A | PCR-RFLP | F: 5′-AAATTGTTTCCAATCATTTAGCT-3′ R: 5′-ACTTCAGGGCTTGGTCAATA-3′ | BamHI | [30] |
| Gene | Variant | RefSNP (ID Number) | Patient Results | Refs. | |
|---|---|---|---|---|---|
| Genotype | Expected Phenotype | ||||
| CYP3A5 (NM_000777.5) | c.6986A > G | rs776746 | GG | Non-expresser | [8] |
| CYP3A4 (NM_001202855.3) | g.-290A > G | rs2740574 | AA | Wildtype. Normal activity | [10] |
| CYP3A4 (NM_001202855.3) | c.15389G > T | rs35599367 | CC | Wildtype. Normal activity | [11] |
| CYP3A4 (NM_001202855.3) | c.1461_1462 insA | rs67666821 | No insertion | Wildtype. Normal activity | [31] |
| POR (NM_000941.3) | c.1508C > T | rs1057868 | CC | Wildtype. Normal activity | [32] |
| ABCB1 (NM_000927.3) | c.3435C > T | rs1045642 | TT | TTT haplotype. P-gp decreased activity and expression. | [33] |
| ABCB1 (NM_000927.3) | c.1236C > T | rs1128503 | TT | ||
| ABCB1 (NM_000927.3) | c.2677G > T | rs2032582 | TT | ||
| PXR (NM_022002.2) | c.69789A > G | rs7643645 | AG | Decreased activity of ABCB1 and CY3A | [34,35] |
| PXR (NM_022002.2) | c.63396C > T | rs2472677 | CC | Wildtype. Normal activity | |
| CYP2C19 (NM_000769.4) | c.681G > A | rs4244285 | GG | Wildtype. Normal activity | [36] |
| CYP2C19 (NM_000769.4) | c.636G > A | rs4986893 | GG | Wildtype. Normal activity | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha, J.; Sangüesa, E.; Saez-Benito, A.M.; Aznar, I.; Berenguer, N.; Saez-Benito, L.; Ribate, M.P.; García, C.B. Importance of Pharmacogenetics and Drug–Drug Interactions in a Kidney Transplanted Patient. Life 2023, 13, 1627. https://doi.org/10.3390/life13081627
Concha J, Sangüesa E, Saez-Benito AM, Aznar I, Berenguer N, Saez-Benito L, Ribate MP, García CB. Importance of Pharmacogenetics and Drug–Drug Interactions in a Kidney Transplanted Patient. Life. 2023; 13(8):1627. https://doi.org/10.3390/life13081627
Chicago/Turabian StyleConcha, Julia, Estela Sangüesa, Ana M. Saez-Benito, Ignacio Aznar, Nuria Berenguer, Loreto Saez-Benito, M. Pilar Ribate, and Cristina B. García. 2023. "Importance of Pharmacogenetics and Drug–Drug Interactions in a Kidney Transplanted Patient" Life 13, no. 8: 1627. https://doi.org/10.3390/life13081627






