Complete Mitogenome Sequencing, Annotation, and Phylogeny of Grateloupia turuturu, a Red Alga with Intronic cox1 Gene
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Isolation
2.2. Whole Genome Sequencing
2.3. Mitogenome Assembly and Annotation
2.4. Phylogenetic Analysis
2.5. Data Availability
3. Results and Discussion
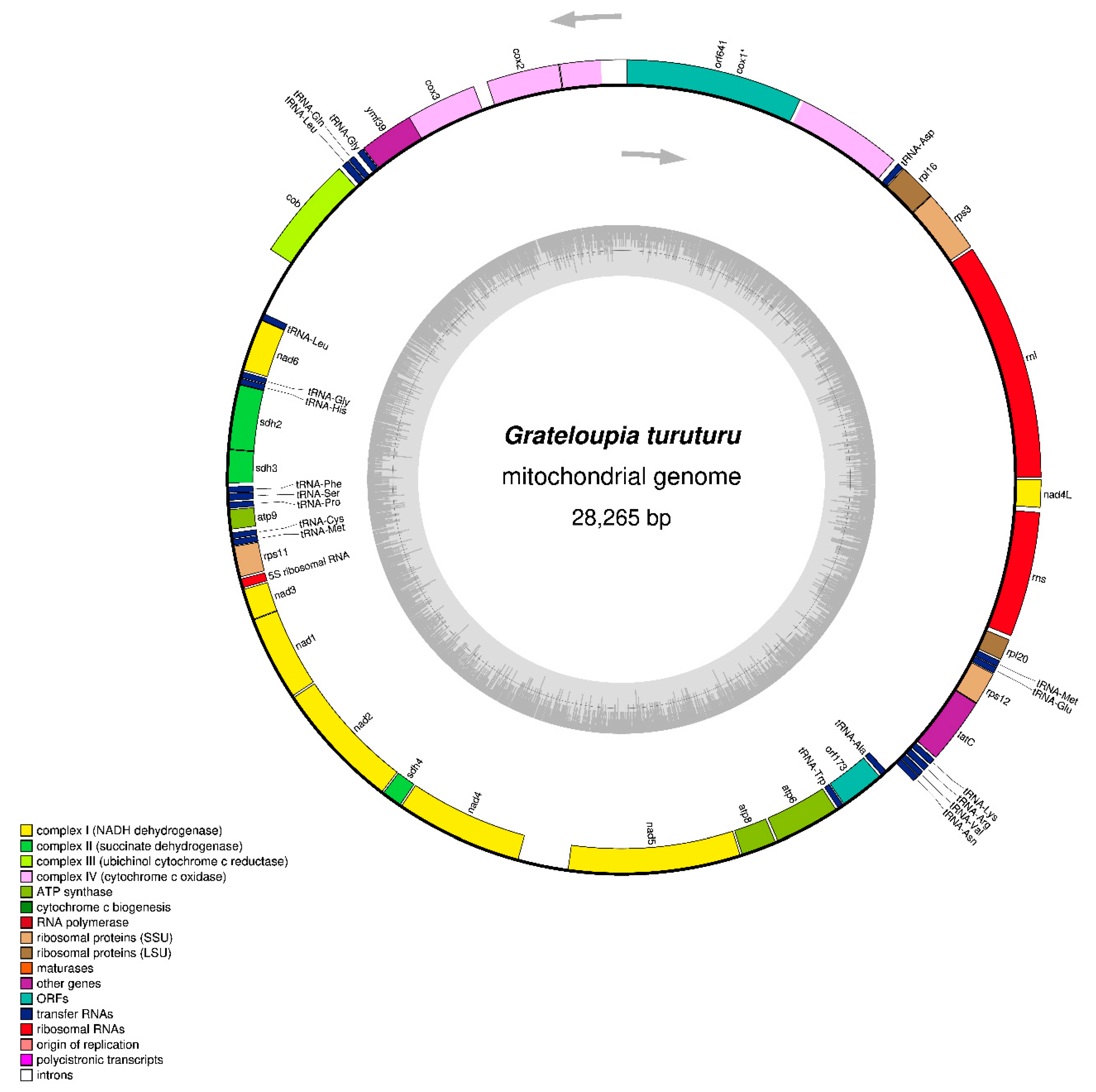
3.1. Genome Size and Organization
3.2. Protein-Coding Gene Features
3.3. Ribosomal RNA and Transfer RNA
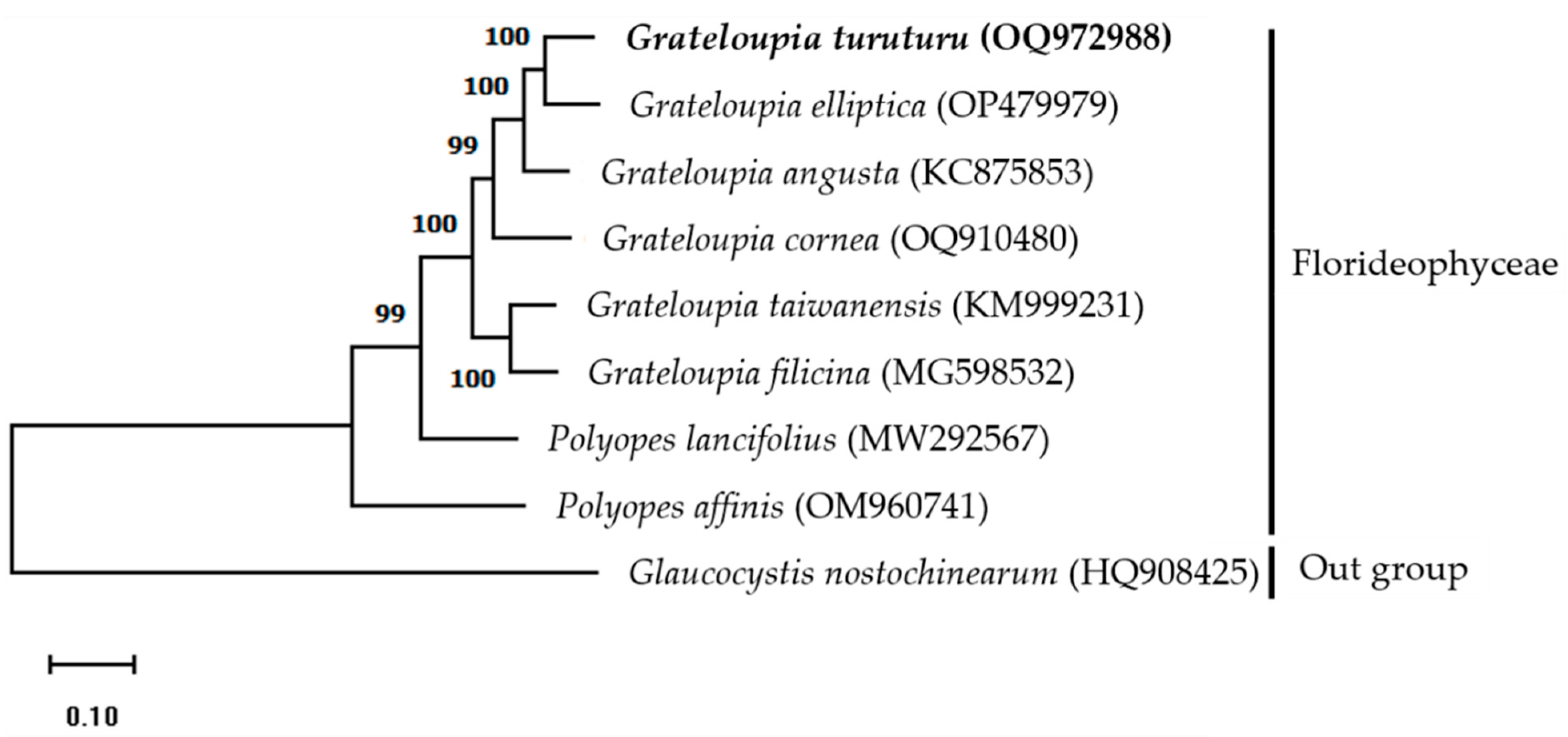
3.4. Phylogenetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurgel:, C.F.D.; Lopez-Bautista, J. Red algae. Encyclopedia of Life Sciences; John Willey & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.U.Y.; Fensome, R.A.; Fredericq, S.; et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Burger, G.; Saint-Louis, D.; Gray, M.W.; Lang, B.F. Complete sequence of the mitochondrial DNA of the red alga Porphyra purpurea: Cyanobacterial introns and shared ancestry of red and green algae. Plant Cell 1999, 11, 1675–1694. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2017. Available online: https://www.algaebase.org (accessed on 15 June 2023).
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yang, E.C.; Boo, S.M.; Yoon, H.S. Complete mitochondrial genome of the marine red alga Grateloupia angusta (Halymeniales). Mitochondrial DNA 2014, 25, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kim, J.O.; Kim, K.; Kim, Y.R. The complete sequence of the mitochondrial DNA and phylogenetic analysis of the marine red alga Grateloupia elliptica (Rhodophyta: Halymeniales). Mitochondrial DNA Part B Resour. 2023, 8, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meinita, M.D.N.; Liu, T.; Chi, S.; Yin, H. Complete sequences of the mitochondrial DNA of the Grateloupia filicina (Rhodophyta). Mitochondrial DNA B Resour. 2018, 3, 76–77. [Google Scholar] [CrossRef] [PubMed]
- DePriest, M.S.; Bhattacharya, D.; Lopez-Bautista, J.M. The mitochondrial genome of Grateloupia taiwanensis (Halymeniaceae, Rhodophyta) and comparative mitochondrial genomics of red algae. Biol. Bull. 2014, 227, 191–200. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, J.O.; Kim, K.; Kim, Y.R.; Yoon, S. Complete mitochondrial genome and phylogenetic analysis of the marine red alga Polyopes affinis (Rhodophyta: Halymeniales). Mitochondrial DNA Part B Resour. 2022, 7, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, C.H.; Yang, E.C.; Yoon, H.S.; Kim, M.S. Complete mitochondrial genome of Polyopes lancifolius and comparison with related species in Halymeniales (Rhodophyta). Mitochondrial DNA Part B Resour. 2021, 6, 1365–1366. [Google Scholar] [CrossRef] [PubMed]
- Salomaki, E.D.; Lane, C.E. Red algal mitochondrial genomes are more complete than previously reported. Genome Biol. Evol. 2017, 9, 48–63. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.; Cho, C.H.; Noh, C.; Yang, J.H.; Park, S.I.; Lee, Y.M.; West, J.A.; Bhattacharya, D.; Jo, K.; Yoon, H.S. Origin of minicircular mitochondrial genomes in red algae. Nat. Commun. 2023, 14, 3363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Yoon, H.S.; Yi, G.; Shin, W.; Archibald, J.M. Comparative mitochondrial genomics of cryptophyte algae: Gene shuffling and dynamic mobile genetic elements. BMC Genom. 2018, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Cho, C.H.; Kim, E.J.; Bhattacharya, D.; Yoon, H.S. Group II intron and repeat-rich red algal mitochondrial genomes demonstrate the dynamic recent history of autocatalytic RNAs. BMC Biol. 2022, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yuan, C.; Tao, L.; Cai, Y.; Zhang, W. Life barcoded by DNA barcodes. Conserv. Genet. Resour. 2022, 14, 351–365. [Google Scholar] [CrossRef]
- Fang, J.; Xu, X.; Chen, Q.; Lin, A.; Lin, S.; Lei, W.; Zhong, C.; Huang, Y.; He, Y. The complete mitochondrial genome of Isochrysis galbana harbors a unique repeat structure and a specific trans-spliced cox1 gene. Front. Microbiol. 2022, 13, 966219. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Chikhi, R.; Medvedev, P. Informed and automated k-mer size selection for genome assembly. Bioinformatics 2014, 30, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Beck, N.; Lang, B. MFannot, Organelle Genome Annotation Webserver; Université de Montréal: Montréal, QC, USA, 2010; Available online: https://megasun.bch.umontreal.ca/apps/mfannot/ (accessed on 10 May 2023).
- Gish, W.; States, D.J. Identification of protein coding regions by database similarity search. Nat. Genet. 1993, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Search and Contextual Analysis of Transfer RNA Genes. Nucl. Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Lang, B.F.; Laforest, M.J.; Burger, G. Mitochondrial introns: A critical view. Trends Genet. 2007, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P. The Sequence Manipulation Suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinf. 2003, 1, 2–3. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.C.; Kim, K.M.; Kim, S.Y.; Lee, J.; Boo, G.H.; Lee, J.H.; Nelson, W.A.; Yi, G.; Schmidt, W.E.; Fredericq, S.; et al. Highly conserved mitochondrial genomes among multicellular red algae of the Florideophyceae. Genome Biol. Evol. 2015, 7, 2394–2406. [Google Scholar] [CrossRef] [PubMed]
- Burger, G.; Lang, B.F.; Braun, H.P.; Marx, S. The enigmatic mitochondrial ORF ymf39 codes for ATP synthase chain b. Nucleic Acids Res. 2003, 31, 2353–2360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burger, G.; Nedelcu, A.M. Mitochondrial Genomes of Algae. In Genomics of Chloroplasts and Mitochondria (Advances in Photosynthesis and Respiration); Bock, R., Knoop, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 127–157. [Google Scholar] [CrossRef]
| Algae | G. turuturu | G. angusta | G. cornea | G. elliptica | G. filicina | G. taiwanensis | P. affinis | P. lancifolius |
|---|---|---|---|---|---|---|---|---|
| GenBank no. | OQ972988 | KC875853 | OQ910480 | OP479979 | MG598532 | KM999231 | OM960741 | MW292567 |
| Size (bp) | 28,265 | 27,943 | 30,595 | 28,503 | 29,274 | 28,906 | 25,988 | 26,132 |
| Nucleotide composition | ||||||||
| A (%) | 36.1 | 36.7 | 35.3 | 36.2 | 35.6 | 36.0 | 37.9 | 36.1 |
| T (%) | 32.7 | 33.1 | 31.6 | 32.6 | 32.4 | 32.6 | 34.6 | 32.9 |
| G (%) | 16.1 | 15.4 | 16.8 | 15.9 | 16.4 | 16.2 | 14.3 | 15.8 |
| C (%) | 15.1 | 14.7 | 16.3 | 15.3 | 15.5 | 15.3 | 13.3 | 15.2 |
| AT (%) | 68.8 | 69.8 | 66.9 | 68.8 | 68.0 | 68.6 | 72.5 | 69.0 |
| GC (%) | 31.2 | 30.1 | 33.1 | 31.2 | 31.9 | 31.5 | 27.6 | 31.0 |
| AT-Skew | 0.049 | 0.052 | 0.055 | 0.052 | 0.047 | 0.050 | 0.046 | 0.046 |
| GC-Skew | 0.032 | 0.023 | 0.015 | 0.019 | 0.028 | 0.029 | 0.036 | 0.019 |
| Group of genes (numbers) | ||||||||
| rRNA | 3 | 2 | 3 | 3 | 2 | 2 | 3 | 2 |
| tRNA | 20 | 18 | 23 | 20 | 24 | 24 | 23 | 23 |
| PCGs a | 26 | 26 | 25 | 26 | 26 | 26 | 25 | 25 |
| Other features | ||||||||
| Intronic ORF | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Intronic cox1 | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Intronic tRNA | No | Yes | No | No | Yes | Yes | No | Yes |
| Unique genes | orf641, orf173 | Gang5, Gang35 | orf632, orf173 | orf634 | cox1-intronic ORF, orf174 | cox1-intronic ORF, orf172 | orf164 | orf165 |
| Reference | In this study | [6] | - | [7] | [8] | [9] | [10] | [11] |
| Group | Group of Genes | Gene Name | Three Letter Code | Location | Size (bp) | No. of Amino Acid | Strand | Start Codon | Stop Codon | Anti-Codon | Intergenic Nucleotides a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | |||||||||||
| rRNA | Large subunit of a ribosome | rnl | - | 20 | 2615 | 2596 | - | H | - | - | - | 23 |
| Small subunit of a ribosome | rrn5 | - | 15281 | 15388 | 108 | - | L | - | - | - | 15 | |
| rns | - | 26545 | 27911 | 1367 | - | H | - | - | - | 48 | ||
| tRNA | Transfer RNA genes | trnD | Asp | 3760 | 3831 | 72 | - | H | - | - | GTC | 51 |
| trnG | Gly | 10054 | 10128 | 75 | - | H | - | - | TCC | 49 | ||
| trnQ | Gln | 10178 | 10249 | 70 | - | H | - | - | TTG | 7 | ||
| trnL | Leu | 10257 | 10341 | 85 | - | H | - | - | TAA | 40 | ||
| trnL | Leu | 12178 | 12259 | 82 | - | L | - | - | TAG | −1 | ||
| trnG | Gly | 12886 | 12957 | 72 | - | L | - | - | GCC | 5 | ||
| trnH | His | 12963 | 13037 | 75 | - | L | - | - | GTG | −1 | ||
| trnF | Phe | 14214 | 14286 | 73 | - | L | - | - | GAA | 4 | ||
| trnS | Ser | 14291 | 14375 | 85 | - | L | - | - | TGA | 15 | ||
| trnP | Pro | 14391 | 14464 | 74 | - | L | - | - | TGG | 11 | ||
| trnC | Cys | 14744 | 14814 | 71 | - | L | - | - | GCA | 7 | ||
| trnM | Met | 14822 | 14895 | 74 | - | L | - | - | CAT | 4 | ||
| trnW | Trp | 23819 | 23891 | 73 | - | L | - | - | TCA | 7 | ||
| trnA | Ala | 24444 | 24518 | 75 | - | L | - | - | TGC | 140 | ||
| trnN | Asn | 24659 | 24731 | 73 | - | H | - | - | GTT | 2 | ||
| trnV | Val | 24734 | 24805 | 72 | - | H | - | - | TAC | 13 | ||
| trnR | Arg | 24819 | 24893 | 75 | - | H | - | - | ACG | 17 | ||
| trnK | Lys | 24911 | 24983 | 73 | - | H | - | - | TTT | 21 | ||
| trnE | Glu | 26106 | 26178 | 73 | - | H | - | - | TTC | 3 | ||
| trnM | Met | 26182 | 26254 | 73 | - | H | - | - | CAT | 15 | ||
| CDS | NADH dehydrogenase subunits (complex 1) | nad6 | - | 12259 | 12867 | 609 | 202 | L | ATG | TAA | - | 18 |
| nad3 | - | 15404 | 15769 | 366 | 121 | L | ATG | TAA | - | 2 | ||
| nad1 | - | 15772 | 16752 | 981 | 326 | L | ATG | TAA | - | 18 | ||
| nad2 | - | 16771 | 18258 | 1488 | 495 | L | ATG | TAA | - | 14 | ||
| nad4 | - | 18529 | 20004 | 1476 | 491 | L | ATG | TAA | - | 477 | ||
| nad5 | - | 20582 | 22579 | 1998 | 665 | L | ATG | TAA | - | 18 | ||
| nad4L | - | 27960 | 28265 | 306 | 101 | H | ATG | TAA | - | 19 | ||
| Succinate dehydrogenase (complex 2) | sdh2 | - | 13037 | 13789 | 753 | 250 | L | ATG | TAG | - | 1 | |
| sdh3 | - | 13791 | 14174 | 384 | 127 | L | ATG | TAA | - | 39 | ||
| sdh4 | - | 18273 | 18512 | 240 | 79 | L | ATG | TAA | - | 16 | ||
| Apocytochrome b (complex 3) | cob | - | 10382 | 11527 | 1146 | 381 | H | ATG | TAA | - | 650 | |
| Cytochrome c oxidase (complex 4) | cox1 b | - | 3883 | 5041 | 1599 | 532 | H | ATG | - | - | - | |
| 7299 | 7738 | H | - | TAA | - | 3 | ||||||
| cox2 | - | 7742 | 8539 | 798 | 265 | H | ATG | TAG | - | 144 | ||
| cox3 | - | 8684 | 9502 | 819 | 272 | H | ATG | TAA | - | −51 | ||
| ATP synthase (complex 5) | ymf39 | - | 9450 | 10049 | 600 | 199 | H | ATG | TAA | - | 4 | |
| atp9 | - | 14476 | 14706 | 231 | 76 | L | ATG | TAA | - | 37 | ||
| atp8 | - | 22598 | 23008 | 411 | 136 | L | ATG | TAG | - | 24 | ||
| atp6 | - | 23033 | 23794 | 762 | 253 | L | ATG | TAG | - | 24 | ||
| SSU ribosomal proteins | rps3 | - | 2639 | 3334 | 696 | 231 | H | ATG | TAA | - | 2 | |
| rps11 | - | 14900 | 15262 | 363 | 120 | L | ATG | TAG | - | 18 | ||
| rps12 | - | 25735 | 26100 | 366 | 121 | H | ATG | TAA | - | 5 | ||
| LSU ribosomal proteins | rpl16 | - | 3337 | 3753 | 417 | 138 | H | ATG | TAA | - | 6 | |
| rpl20 | - | 26270 | 26503 | 234 | 77 | H | ATG | TAA | - | 41 | ||
| Independent protein translocase | tatC | - | 25005 | 25733 | 729 | 242 | H | TTG | TAA | - | 1 | |
| Hypothetical proteins | orf641 | - | 5084 | 7009 | 1926 | 641 | H | ATG | TAA | - | 289 | |
| orf173 | - | 23899 | 24420 | 522 | 173 | L | ATG | TAA | - | 23 | ||
| Amino Acids | Codon | Number | % | Fraction | Amino Acids | Codon | Number | % | Fraction | Amino Acids | Codon | Number | % | Fraction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ala | GCG | 42 | 0.626 | 0.12 | Gly | GGT | 139 | 2.070 | 0.39 | Ser | AGT | 122 | 1.817 | 0.23 |
| GCA | 130 | 1.936 | 0.38 | GGC | 39 | 0.581 | 0.11 | AGC | 50 | 0.745 | 0.09 | |||
| GCT | 150 | 2.234 | 0.44 | His | CAT | 100 | 1.489 | 0.74 | TCG | 41 | 0.611 | 0.08 | ||
| GCC | 20 | 0.298 | 0.06 | CAC | 36 | 0.536 | 0.26 | TCA | 153 | 2.279 | 0.29 | |||
| Arg | AGG | 17 | 0.253 | 0.09 | Ile | ATA | 194 | 2.889 | 0.29 | TCT | 131 | 1.951 | 0.25 | |
| AGA | 57 | 0.849 | 0.30 | ATT | 390 | 5.809 | 0.59 | TCC | 37 | 0.551 | 0.07 | |||
| CGG | 8 | 0.119 | 0.04 | ATC | 75 | 1.117 | 0.11 | Thr | ACG | 40 | 0.596 | 0.11 | ||
| CGA | 33 | 0.492 | 0.17 | Leu | TTG | 122 | 1.817 | 0.12 | ACA | 111 | 1.653 | 0.31 | ||
| CGT | 52 | 0.775 | 0.27 | TTA | 544 | 8.102 | 0.55 | ACT | 176 | 2.621 | 0.49 | |||
| CGC | 26 | 0.387 | 0.13 | CTG | 35 | 0.521 | 0.04 | ACC | 35 | 0.521 | 0.10 | |||
| Asn | AAT | 214 | 3.187 | 0.69 | CTA | 117 | 1.743 | 0.12 | Trp | TGG | 28 | 0.417 | 0.20 | |
| AAC | 96 | 1.430 | 0.31 | CTT | 153 | 2.279 | 0.15 | TGA | 110 | 1.638 | 0.80 | |||
| Asp | GAT | 111 | 1.653 | 0.67 | CTC | 19 | 0.283 | 0.02 | Tyr | TAT | 169 | 2.517 | 0.60 | |
| GAC | 55 | 0.819 | 0.33 | Lys | AAG | 67 | 0.998 | 0.19 | TAC | 113 | 1.683 | 0.40 | ||
| Cys | TGT | 55 | 0.819 | 0.65 | AAA | 291 | 4.334 | 0.81 | Val | GTG | 38 | 0.566 | 0.09 | |
| TGC | 30 | 0.447 | 0.35 | Met | ATG | 166 | 2.472 | 1.00 | GTA | 140 | 2.085 | 0.34 | ||
| Gln | CAG | 35 | 0.521 | 0.18 | Phe | TTT | 507 | 7.551 | 0.84 | GTT | 199 | 2.964 | 0.48 | |
| CAA | 159 | 2.368 | 0.82 | TTC | 97 | 1.445 | 0.16 | GTC | 39 | 0.581 | 0.09 | |||
| Glu | GAG | 39 | 0.571 | 0.19 | Pro | CCG | 24 | 0.357 | 0.11 | * | TAA | - | - | - |
| GAA | 165 | 2.458 | 0.81 | CCA | 73 | 1.087 | 0.33 | TAG | - | - | - | |||
| Gly | GGG | 36 | 0.536 | 0.10 | CCT | 104 | 1.549 | 0.48 | ||||||
| GGA | 143 | 2.130 | 0.40 | CCC | 17 | 0.253 | 0.08 |
| Algae | G. turuturu (OQ972988) | G. angusta(KC875853) | G. cornea (OQ910480) | G. elliptica (OP479979) | G. filicina (MG598532) | G. taiwanensis (KM999231) | P. affinis (OM960741) | P. lancifolius (MW292567) |
|---|---|---|---|---|---|---|---|---|
| rrn5 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| rns | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| rnl | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnA (TGC) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnC (GCA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnD (GTC) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnE (TTC) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnF (GAA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnG (TCC) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnG (GCC) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnH (GTG) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnI (GAT) | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| trnK (TTT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnL (TAA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnL (TAG) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnM (CAT) | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| trnN (GTT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnP (TGG) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnQ (TTG) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnR (ACG) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnR (TCT) | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| trnS (GCT) | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| trnS (TGA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnV (TAC) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnW (TCA) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| trnY (GTA) | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Total tRNA | 20 | 18 | 23 | 20 | 24 | 24 | 23 | 23 |
| Ref. | In this study | [6] | - | [7] | [8] | [9] | [10] | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, M.P.; Kim, J.-O.; Kim, Y.-R.; Yoon, S.; Kim, K. Complete Mitogenome Sequencing, Annotation, and Phylogeny of Grateloupia turuturu, a Red Alga with Intronic cox1 Gene. Life 2023, 13, 1642. https://doi.org/10.3390/life13081642
Patil MP, Kim J-O, Kim Y-R, Yoon S, Kim K. Complete Mitogenome Sequencing, Annotation, and Phylogeny of Grateloupia turuturu, a Red Alga with Intronic cox1 Gene. Life. 2023; 13(8):1642. https://doi.org/10.3390/life13081642
Chicago/Turabian StylePatil, Maheshkumar Prakash, Jong-Oh Kim, Young-Ryun Kim, Seokjin Yoon, and Kyunghoi Kim. 2023. "Complete Mitogenome Sequencing, Annotation, and Phylogeny of Grateloupia turuturu, a Red Alga with Intronic cox1 Gene" Life 13, no. 8: 1642. https://doi.org/10.3390/life13081642
APA StylePatil, M. P., Kim, J.-O., Kim, Y.-R., Yoon, S., & Kim, K. (2023). Complete Mitogenome Sequencing, Annotation, and Phylogeny of Grateloupia turuturu, a Red Alga with Intronic cox1 Gene. Life, 13(8), 1642. https://doi.org/10.3390/life13081642







