Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes
Abstract
:1. Introduction
2. Inflammatory Mechanisms in Atherosclerosis and Coronary Artery Disease
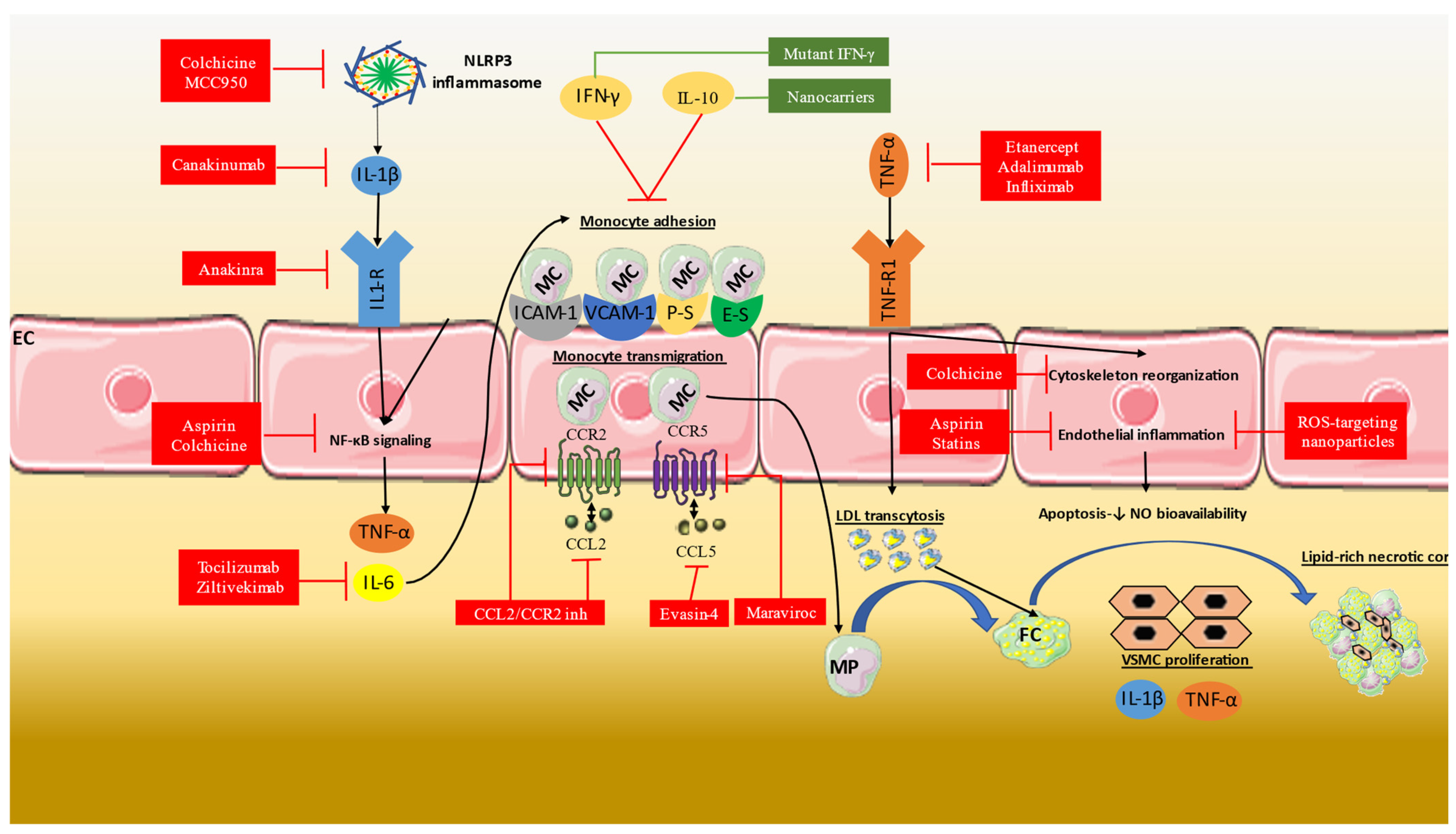
3. Anti-Inflammatory Therapies in CAD
3.1. Aspirin
3.2. Statins
3.3. Colchicine
3.4. IL-1b Inhibition
3.5. IL-6 Inhibition
3.6. Other Anti-Inflammatory Therapies
4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. History of Discovery: Inflammation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045. [Google Scholar] [CrossRef] [Green Version]
- Lagrand, W.K.; Visser, C.A.; Hermens, W.T.; Niessen, H.W.M.; Verheugt, F.W.A.; Wolbink, G.J.; Hack, C.E. C-Reactive Protein as a Cardiovascular Risk Factor: More than an Epiphenomenon? Circulation 1999, 100, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory Cytokines in Atherosclerosis: Current Therapeutic Approaches. Eur. Heart J. 2016, 37, 1723–1735. [Google Scholar] [CrossRef] [Green Version]
- Tsioufis, P.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. The Impact of Cytokines in Coronary Atherosclerotic Plaque: Current Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 15937. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Kampoli, A.M.; Tousoulis, D.; Antoniades, C.; Siasos, G.; Stefanadis, C. Biomarkers of Premature Atherosclerosis. Trends Mol. Med. 2009, 15, 323–332. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Androulakis, E.; Zacharia, E.; Papageorgiou, N.; Lioudaki, E.; Bertsias, D.; Charakida, M.; Siasos, G.; Tousoulis, D. High-Density Lipoprotein and Low-Density Lipoprotein Therapeutic Approaches in Acute Coronary Syndromes. Curr. Cardiol. Rev. 2017, 13, 168. [Google Scholar] [CrossRef]
- Barreto, J.; Karathanasis, S.K.; Remaley, A.; Sposito, A.C. Role of LOX-1 (Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1) as a Cardiovascular Risk Predictor: Mechanistic Insight and Potential Clinical Use. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 153–166. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 15937. [Google Scholar] [CrossRef]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef]
- Dimitroglou, Y.; Sakalidis, A.; Mavroudis, A.; Kalantzis, C.; Valatsou, A.; Andrikou, I.; Christofi, A.; Mantzouranis, E.; Kachrimanidis, I.; Bei, E.; et al. Lipoprotein-Associated Phospholipase A2 in Coronary Artery Disease. Curr. Top. Med. Chem. 2022, 22, 2344–2354. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and Atherosclerotic Cardiovascular Disease. Nat. Reviews. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Guo, X.; Ma, L. Inflammation in Coronary Artery Disease-Clinical Implications of Novel HDL-Cholesterol-Related Inflammatory Parameters as Predictors. Coron. Artery Dis. 2023, 34, 66–77. [Google Scholar] [CrossRef]
- Magnoni, M.; Andreini, D.; Pirillo, A.; Uboldi, P.; Latini, R.; Catapano, A.L.; Maggioni, A.P.; Norata, G.D. Predictive Value of HDL Function in Patients with Coronary Artery Disease: Relationship with Coronary Plaque Characteristics and Clinical Events. Ann. Med. 2022, 54, 1036–1046. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the Immune System Shapes Atherosclerosis: Roles of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2022, 22, 251. [Google Scholar] [CrossRef]
- Perrone, M.A.; Aimo, A.; Bernardini, S.; Clerico, A. Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers. Int. J. Mol. Sci. 2023, 24, 844. [Google Scholar] [CrossRef]
- von Vietinghoff, S.; Koltsova, E.K. Inflammation in Atherosclerosis: A Key Role for Cytokines. Cytokine 2019, 122, 154819. [Google Scholar] [CrossRef]
- Libby, P.; Ordovas, J.M.; Birinyi, L.K.; Auger, K.R.; Dinarello, C.A. Inducible Interleukin-1 Gene Expression in Human Vascular Smooth Muscle Cells. J. Clin. Investig. 1986, 78, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Ordovas, J.M.; Auger, K.R.; Robbins, A.H.; Birinyi, L.K.; Dinarello, C.A. Endotoxin and Tumor Necrosis Factor Induce Interleukin-1 Gene Expression in Adult Human Vascular Endothelial Cells. Am. J. Pathol. 1986, 124, 179. [Google Scholar]
- Frostegård, J.; Ulfgren, A.K.; Nyberg, P.; Hedin, U.; Swedenborg, J.; Andersson, U.; Hansson, G.K. Cytokine Expression in Advanced Human Atherosclerotic Plaques: Dominance of pro-Inflammatory (Th1) and Macrophage-Stimulating Cytokines. Atherosclerosis 1999, 145, 33–43. [Google Scholar] [CrossRef]
- Oikonomou, E.; Tsaplaris, P.; Anastasiou, A.; Xenou, M.; Lampsas, S.; Siasos, G.; Pantelidis, P.; Theofilis, P.; Tsatsaragkou, A.; Katsarou, O.; et al. Interleukin-1 in Coronary Artery Disease. Curr. Top. Med. Chem. 2022, 22, 2368–2389. [Google Scholar] [CrossRef]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and Regulation of the Inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An Update on the Regulatory Mechanisms of NLRP3 Inflammasome Activation. Cell. Mol. Immunol. 2021, 18, 1141. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 Inflammasome in Endothelial Dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Wang, L.; Qu, P.; Zhao, J.; Chang, Y. NLRP3 and Downstream Cytokine Expression Elevated in the Monocytes of Patients with Coronary Artery Disease. Arch. Med. Sci. AMS 2014, 10, 791. [Google Scholar] [CrossRef]
- Satoh, M.; Tabuchi, T.; Itoh, T.; Nakamura, M. NLRP3 Inflammasome Activation in Coronary Artery Disease: Results from Prospective and Randomized Study of Treatment with Atorvastatin or Rosuvastatin. Clin. Sci. 2014, 126, 233–241. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, D.; Yang, Q.; You, J.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Interactions between PCSK9 and NLRP3 Inflammasome Signaling in Atherosclerosis. Front. Immunol. 2023, 14, 1126823. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ye, D.; Wang, Z.; Pan, H.; Lu, X.; Wang, M.; Xu, Y.; Yu, J.; Zhang, J.; Zhao, M.; et al. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front Cardiovasc. Med. 2022, 9, 818890. [Google Scholar] [CrossRef]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A Link between Inflammation and Thrombosis in Atherosclerotic Cardiovascular Diseases: Clinical and Therapeutic Implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Schuett, H.; Oestreich, R.; Waetzig, G.H.; Annema, W.; Luchtefeld, M.; Hillmer, A.; Bavendiek, U.; Von Felden, J.; Divchev, D.; Kempf, T.; et al. Transsignaling of Interleukin-6 Crucially Contributes to Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Huang, X.Z.; Li, X.N.; Feng, M.; Li, L.; Cai, X.J.; Zhang, C.; Liu, X.L.; Zhang, M.X.; Zhang, Y.; et al. Interleukin 6 Destabilizes Atherosclerotic Plaques by Downregulating Prolyl-4-Hydroxylase A1 via a Mitogen-Activated Protein Kinase and c-Jun Pathway. Arch. Biochem. Biophys. 2012, 528, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.A.; Sakkinen, P.; Conze, D.; Hardin, N.; Tracy, R. Interleukin-6 Exacerbates Early Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2364–2367. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Guo, Y.; Chung, C.; Peasey, A.; Pfister, R.; et al. The Interleukin-6 Receptor as a Target for Prevention of Coronary Heart Disease: A Mendelian Randomisation Analysis. Lancet 2012, 379, 1214–1224. [Google Scholar] [CrossRef] [Green Version]
- Schieffer, B.; Selle, T.; Hilfiker, A.; Hilfiker-Kleiner, D.; Grote, K.; Tietge, U.J.F.; Trautwein, C.; Luchtefeld, M.; Schmittkamp, C.; Heeneman, S.; et al. Impact of Interleukin-6 on Plaque Development and Morphology in Experimental Atherosclerosis. Circulation 2004, 110, 3493–3500. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The Role of Interleukin-10 Family Members in Cardiovascular Diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef]
- Kuan, R.; Agrawal, D.K.; Thankam, F.G. Treg Cells in Atherosclerosis. Mol. Biol. Rep. 2021, 48, 4897–4910. [Google Scholar] [CrossRef]
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze, V.; Emmanuel, F.; Bureau, M.F.; Soubrier, F.; Esposito, B.; Duez, H.; Fievet, C.; et al. Protective Role of Interleukin-10 in Atherosclerosis. Circ. Res. 1999, 85, e17–e24. [Google Scholar] [CrossRef]
- Wæhre, T.; Halvorsen, B.; Damås, J.K.; Yndestad, A.; Brosstad, F.; Gullestad, L.; Kjekshus, J.; Frøland, S.S.; Aukrust, P. Inflammatory Imbalance between IL-10 and TNFalpha in Unstable Angina Potential Plaque Stabilizing Effects of IL-10. Eur. J. Clin. Investig. 2002, 32, 803–810. [Google Scholar] [CrossRef]
- Goldwater, D.; Karlamangla, A.; Merkin, S.S.; Watson, K.; Seeman, T. Interleukin-10 as a Predictor of Major Adverse Cardiovascular Events in a Racially and Ethnically Diverse Population: Multi-Ethnic Study of Atherosclerosis. Ann. Epidemiol. 2019, 30, 9–14.e1. [Google Scholar] [CrossRef]
- Gencer, S.; Evans, B.R.; Van Der Vorst, E.P.C.; Döring, Y.; Weber, C. Inflammatory Chemokines in Atherosclerosis. Cells 2021, 10, 226. [Google Scholar] [CrossRef]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of Monocyte Chemoattractant Protein-1 Reduces Atherosclerosis in Low Density Lipoprotein Receptor-Deficient Mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef]
- Boring, L.; Gosling, J.; Cleary, M.; Charo, I.F. Decreased Lesion Formation in CCR2−/− Mice Reveals a Role for Chemokines in the Initiation of Atherosclerosis. Nature 1998, 394, 894–897. [Google Scholar] [CrossRef]
- Makarewicz-wujec, M.; Henzel, J.; Kępka, C.; Kruk, M.; Wardziak, Ł.; Trochimiuk, P.; Parzonko, A.; Dzielińska, Z.; Demkow, M.; Kozłowska-wojciechowska, M. Usefulness of MCP-1 Chemokine in the Monitoring of Patients with Coronary Artery Disease Subjected to Intensive Dietary Intervention: A Pilot Study. Nutrients 2021, 13, 3047. [Google Scholar] [CrossRef]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-Derived Chemokines in Inflammation and Atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Weber, K.S.C.; Huo, Y.; Proudfoot, A.E.I.; Nelson, P.J.; Ley, K.; Weber, C. RANTES Deposition by Platelets Triggers Monocyte Arrest on Inflamed and Atherosclerotic Endothelium. Circulation 2001, 103, 1772–1777. [Google Scholar] [CrossRef]
- Virani, S.S.; Nambi, V.; Hoogeveen, R.; Wasserman, B.A.; Coresh, J.; Gonzalez, F.; Chambless, L.E.; Mosley, T.H.; Boerwinkle, E.; Ballantyne, C.M. Relationship between Circulating Levels of RANTES (Regulated on Activation, Normal T-Cell Expressed, and Secreted) and Carotid Plaque Characteristics: The Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur. Heart J. 2011, 32, 459–468. [Google Scholar] [CrossRef]
- Lees, J.R. Interferon Gamma in Autoimmunity: A Complicated Player on a Complex Stage. Cytokine 2015, 74, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Elyasi, A.; Voloshyna, I.; Ahmed, S.; Kasselman, L.J.; Behbodikhah, J.; De Leon, J.; Reiss, A.B. The Role of Interferon-γ in Cardiovascular Disease: An Update. Inflamm. Res. 2020, 69, 975–988. [Google Scholar] [CrossRef]
- Gupta, S.; Pablo, A.M.; Jiang, X.C.; Wang, N.; Tall, A.R.; Schindler, C. IFN-Gamma Potentiates Atherosclerosis in ApoE Knock-out Mice. J. Clin. Investig. 1997, 99, 2752–2761. [Google Scholar] [CrossRef]
- Buono, C.; Come, C.E.; Stavrakis, G.; Maguire, G.F.; Connelly, P.W.; Lichtman, A.H. Influence of Interferon-Gamma on the Extent and Phenotype of Diet-Induced Atherosclerosis in the LDLR-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.Y.H.; Oldham, W.M.; He, H.; Wang, R.; Mulhern, R.; Handy, D.E.; Loscalzo, J. Interferon-γ Impairs Human Coronary Artery Endothelial Glucose Metabolism by Tryptophan Catabolism and Activates Fatty Acid Oxidation. Circulation 2021, 144, 1612–1628. [Google Scholar] [CrossRef]
- Sbrana, S.; Campolo, J.; Clemente, A.; Bastiani, L.; Cecchettini, A.; Ceccherini, E.; Caselli, C.; Neglia, D.; Parodi, O.; Chiappino, D.; et al. Blood Monocyte Phenotype Fingerprint of Stable Coronary Artery Disease: A Cross-Sectional Substudy of SMARTool Clinical Trial. BioMed Res. Int. 2020, 2020, 8748934. [Google Scholar] [CrossRef]
- Turner, N.A.; Mughal, R.S.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Porter, K.E. Mechanism of TNFα-Induced IL-1α, IL-1β and IL-6 Expression in Human Cardiac Fibroblasts: Effects of Statins and Thiazolidinediones. Cardiovasc. Res. 2007, 76, 81–90. [Google Scholar] [CrossRef]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of Tumor Necrosis Factor-Alpha Gene Diminishes the Development of Atherosclerosis in ApoE-Deficient Mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef]
- Huang, R.; Zhao, S.R.; Li, Y.; Liu, F.; Gong, Y.; Xing, J.; Xu, Z.S. Association of Tumor Necrosis Factor-α Gene Polymorphisms and Coronary Artery Disease Susceptibility: A Systematic Review and Meta-Analysis. BMC Med. Genet. 2020, 21, 29. [Google Scholar] [CrossRef] [Green Version]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Gregory Hundley, W.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef]
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities Between Peripheral Artery Disease and Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1982–1989. [Google Scholar] [CrossRef]
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. New Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Kronish, I.M.; Rieckmann, N.; Shimbo, D.; Burg, M.; Davidson, K.W. Aspirin Adherence, Aspirin Dosage, and C-Reactive Protein in the First 3 Months after Acute Coronary Syndrome. Am. J. Cardiol. 2010, 106, 1090–1094. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [Green Version]
- Sahebkar, A.; Kotani, K.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Ray, K.K.; Blaha, M.J.; Rysz, J.; Toth, P.P.; et al. Statin Therapy Reduces Plasma Endothelin-1 Concentrations: A Meta-Analysis of 15 Randomized Controlled Trials. Atherosclerosis 2015, 241, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Arabi, S.M.; Chambari, M.; Malek-Ahmadi, M.; Bahrami, L.S.; Hadi, V.; Rizzo, M.; Sahebkar, A. The Effect of Statin Therapy in Combination with Ezetimibe on Circulating C-Reactive Protein Levels: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Inflammopharmacology 2022, 30, 1597–1615. [Google Scholar] [CrossRef]
- Mostafa Arabi, S.; Sadat Bahrami, L.; MalekAhmadi, M.; Chambari, M.; Milkarizi, N.; Orekhov, A.N.; Sahebkar, A. The Effect of Combination Therapy with Statins and Ezetimibe on Proinflammatory Cytokines: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. Immunopharmacol. 2022, 113, 109477. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients with Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 Inhibition with Ziltivekimab in Patients at High Atherosclerotic Risk (RESCUE): A Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; García Rodríguez, L.A.; Landolfi, R.; Baigent, C. Low-Dose Aspirin for the Prevention of Atherothrombosis. N. Engl. J. Med. 2005, 353, 2373–2383. [Google Scholar] [CrossRef] [Green Version]
- Hohlfeld, T.; Schrör, K. Antiinflammatory Effects of Aspirin in ACS: Relevant to Its Cardiocoronary Actions? Thromb. Haemost. 2015, 114, 469–477. [Google Scholar] [CrossRef]
- Capra, V.; Bäck, M.; Angiolillo, D.J.; Cattaneo, M.; Sakariassen, K.S. Impact of Vascular Thromboxane Prostanoid Receptor Activation on Hemostasis, Thrombosis, Oxidative Stress, and Inflammation. J. Thromb. Haemost. 2014, 12, 126–137. [Google Scholar] [CrossRef]
- Wilson, S.J.; Cavanagh, C.C.; Lesher, A.M.; Frey, A.J.; Russell, S.E.; Smyth, E.M. Activation-Dependent Stabilization of the Human Thromboxane Receptor: Role of Reactive Oxygen Species. J. Lipid Res. 2009, 50, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Zucker, T.P.; Bönisch, D.; Muck, S.; Weber, A.A.; Bretschneider, E.; Glusa, E.; Schrör, K. Thrombin-Induced Mitogenesis in Coronary Artery Smooth Muscle Cells Is Potentiated by Thromboxane A2 and Involves Upregulation of Thromboxane Receptor MRNA. Circulation 1998, 97, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Praticò, D. Prostanoid and Isoprostanoid Pathways in Atherogenesis. Atherosclerosis 2008, 201, 8–16. [Google Scholar] [CrossRef]
- Gabrielsen, A.; Qiu, H.; Bäck, M.; Hamberg, M.; Hemdahl, A.L.; Agardh, H.; Folkersen, L.; Swedenborg, J.; Hedin, U.; Paulsson-Berne, G.; et al. Thromboxane Synthase Expression and Thromboxane A2 Production in the Atherosclerotic Lesion. J. Mol. Med. 2010, 88, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Tellier, C.; Michiels, C.; Ellertsen, I.; Dogné, J.M.; Bäck, M. Effects of the Dual TP Receptor Antagonist and Thromboxane Synthase Inhibitor EV-077 on Human Endothelial and Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2013, 441, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayatte, A.J.; Du, Y.; Oliver-Krasinski, J.; Lavielle, G.; Verbeuren, T.J.; Cohen, R.A. The Thromboxane Receptor Antagonist S18886 but Not Aspirin Inhibits Atherogenesis in Apo E-Deficient Mice: Evidence That Eicosanoids Other than Thromboxane Contribute to Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1724–1728. [Google Scholar] [CrossRef] [Green Version]
- Eikelboom, J.W.; Hankey, G.J.; Thom, J.; Bhatt, D.L.; Steg, P.G.; Montalescot, G.; Johnston, S.C.; Steinhubl, S.R.; Mak, K.H.; Easton, J.D.; et al. Incomplete Inhibition of Thromboxane Biosynthesis by Acetylsalicylic Acid: Determinants and Effect on Cardiovascular Risk. Circulation 2008, 118, 1705–1712. [Google Scholar] [CrossRef] [Green Version]
- Solheim, S.; Pettersen, A.A.; Arnesen, H.; Seljeflot, I. No Difference in the Effects of Clopidogrel and Aspirin on Inflammatory Markers in Patients with Coronary Heart Disease-PubMed. Thromb. Haemost. 2006, 96, 660–664. [Google Scholar] [PubMed]
- Woodward, M.; Lowe, G.D.O.; Francis, L.M.A.; Rumley, A.; Cobbe, S.M.; Bain, R.; Dean, J.; Fulcher, R.; Gershlick, A.; Haywood, G.; et al. A Randomized Comparison of the Effects of Aspirin and Clopidogrel on Thrombotic Risk Factors and C-Reactive Protein Following Myocardial Infarction: The CADET Trial. J. Thromb. Haemost. 2004, 2, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lekakis, J.; Vamvakou, G.; Andreotti, F.; Nihoyannopoulos, P. Cigarette Smoking Is Associated with Increased Circulating Proinflammatory and Procoagulant Markers in Patients with Chronic Coronary Artery Disease: Effects of Aspirin Treatment. Am. Heart J. 2005, 149, 832–839. [Google Scholar] [CrossRef]
- Taubert, D.; Berkels, R.; Grosser, N.; Schröder, H.; Gründemann, D.; Schömig, E. Aspirin Induces Nitric Oxide Release from Vascular Endothelium: A Novel Mechanism of Action. Br. J. Pharmacol. 2004, 143, 159–165. [Google Scholar] [CrossRef] [Green Version]
- O’Kane, P.D.; Queen, L.R.; Ji, Y.; Reebye, V.; Stratton, P.; Jackson, G.; Ferro, A. Aspirin Modifies Nitric Oxide Synthase Activity in Platelets: Effects of Acute versus Chronic Aspirin Treatment. Cardiovasc. Res. 2003, 59, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Morris, T.; Stables, M.; Hobbs, A.; de Souza, P.; Colville-Nash, P.; Warner, T.; Newson, J.; Bellingan, G.; Gilroy, D.W. Effects of Low-Dose Aspirin on Acute Inflammatory Responses in Humans. J. Immunol. 2009, 183, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; Bermudez, E.A.; Ridker, P.M.; Hurwitz, S.; Serhan, C.N. Aspirin Triggers Antiinflammatory 15-Epi-Lipoxin A4 and Inhibits Thromboxane in a Randomized Human Trial. Proc. Natl. Acad. Sci. USA 2004, 101, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Brancaleone, V.; Gobbetti, T.; Cenac, N.; Le Faouder, P.; Colom, B.; Flower, R.J.; Vergnolle, N.; Nourshargh, S.; Perretti, M. A Vasculo-Protective Circuit Centered on Lipoxin A4 and Aspirin-Triggered 15-Epi-Lipoxin A4 Operative in Murine Microcirculation. Blood 2013, 122, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Santilli, F.; Paloscia, L.; Liani, R.; Nicola, M.D.; Marco, M.D.; Lattanzio, S.; Barba, S.L.; Pascale, S.; Mascellanti, M.; Davì, G. Circulating Myeloid-Related Protein-8/14 Is Related to Thromboxane-Dependent Platelet Activation in Patients with Acute Coronary Syndrome, with and without Ongoing Low-Dose Aspirin Treatment. J. Am. Heart Assoc. 2014, 3, e000903. [Google Scholar] [CrossRef]
- Magen, E.; Viskoper, J.R.; Mishal, J.; Priluk, R.; London, D.; Yosefy, C. Effects of Low-Dose Aspirin on Blood Pressure and Endothelial Function of Treated Hypertensive Hypercholesterolaemic Subjects. J. Hum. Hypertens. 2005, 19, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Istvan, E.S.; Deisenhofer, J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, N.; Zacharia, E.; Briasoulis, A.; Androulakis, E.; Tousoulis, D. Statins and Myocardial Infarction: Type, Dose, and Administration Time: Does It Matter? Trends Cardiovasc. Med. 2016, 26, 433–441. [Google Scholar] [CrossRef]
- Tousoulis, D.; Psarros, C.; Demosthenous, M.; Patel, R.; Antoniades, C.; Stefanadis, C. Innate and Adaptive Inflammation as a Therapeutic Target in Vascular Disease: The Emerging Role of Statins. J. Am. Coll. Cardiol. 2014, 63, 2491–2502. [Google Scholar] [CrossRef] [Green Version]
- Vogiatzi, G.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Briasoulis, A.; Androulakis, E.; Latsios, G.; Papaioannou, S.; Tsioufis, K.; Tousoulis, D. Statins and Inflammation in Cardiovascular Disease. Curr. Pharm. Des. 2017, 23, 7027–7039. [Google Scholar] [CrossRef]
- Zivkovic, S.; Maric, G.; Cvetinovic, N.; Lepojevic-Stefanovic, D.; Bozic Cvijan, B. Anti-Inflammatory Effects of Lipid-Lowering Drugs and Supplements-A Narrative Review. Nutrients 2023, 15, 1517. [Google Scholar] [CrossRef]
- Drakopoulou, M.; Toutouzas, K.; Michelongona, A.; Tousoulis, D. Statins and Vulnerable Plaque. Curr. Pharm. Des. 2017, 23, 7069–7085. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. Inflammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Vasiliadou, C.; Kourtellaris, P.; Koniari, K.; Marinou, K.; Charakida, M.; Ntarladimas, I.; Siasos, G.; Stefanadis, C. Effects of Atorvastatin and Vitamin C on Forearm Hyperaemic Blood Flow, Asymmentrical Dimethylarginine Levels and the Inflammatory Process in Patients with Type 2 Diabetes Mellitus. Heart 2007, 93, 244. [Google Scholar] [CrossRef]
- Stumpf, C.; Petzi, S.; Seybold, K.; Wasmeier, G.; Arnold, M.; Raaz, D.; Yilmaz, A.; Daniel, W.G.; Garlichs, C.D. Atorvastatin Enhances Interleukin-10 Levels and Improves Cardiac Function in Rats after Acute Myocardial Infarction. Clin. Sci. 2009, 116, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Kapelouzou, A.; Giaglis, S.; Peroulis, M.; Katsimpoulas, M.; Moustardas, P.; Aravanis, C.V.; Kostakis, A.; Karayannakos, P.E.; Cokkinos, D.V. Overexpression of Toll-Like Receptors 2, 3, 4, and 8 Is Correlated to the Vascular Atherosclerotic Process in the Hyperlipidemic Rabbit Model: The Effect of Statin Treatment. J. Vasc. Res. 2017, 54, 156–169. [Google Scholar] [CrossRef]
- Almeida, S.O.; Budoff, M. Effect of Statins on Atherosclerotic Plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of Atorvastatin Therapy on Fibrous Cap Thickness in Coronary Atherosclerotic Plaque as Assessed by Optical Coherence Tomography: The EASY-FIT Study. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef]
- Almquist, T.; Jacobson, S.H.; Mobarrez, F.; Näsman, P.; Hjemdahl, P. Lipid-Lowering Treatment and Inflammatory Mediators in Diabetes and Chronic Kidney Disease. Eur. J. Clin. Investig. 2014, 44, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, N.; Briasoulis, A.; Lazaros, G.; Imazio, M.; Tousoulis, D. Colchicine for Prevention and Treatment of Cardiac Diseases: A Meta-Analysis. Cardiovasc. Ther. 2017, 35, 10–18. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Beerkens, F.J.; Shah, B.; Giannopoulos, G.; Vrachatis, D.A.; Giotaki, S.G.; Siasos, G.; Nicolas, J.; Arnott, C.; Patel, S.; et al. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation 2022, 145, 61–78. [Google Scholar] [CrossRef]
- Angelidis, C.; Kotsialou, Z.; Kossyvakis, C.; Vrettou, A.-R.; Zacharoulis, A.; Kolokathis, F.; Kekeris, V.; Giannopoulos, G. Colchicine Pharmacokinetics and Mechanism of Action. Curr. Pharm. Des. 2018, 24, 659–663. [Google Scholar] [CrossRef]
- Robertson, S.; Martínez, G.J.; Payet, C.A.; Barraclough, J.Y.; Celermajer, D.S.; Bursill, C.; Patel, S. Colchicine Therapy in Acute Coronary Syndrome Patients Acts on Caspase-1 to Suppress NLRP3 Inflammasome Monocyte Activation. Clin. Sci. 2016, 130, 1237–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvis, M.J.M.; Fiolet, A.T.L.; Opstal, T.S.J.; Dekker, M.; Suquilanda, D.; Zivkovic, M.; Duyvendak, M.; The, S.H.K.; Timmers, L.; Bax, W.A.; et al. Colchicine Reduces Extracellular Vesicle NLRP3 Inflammasome Protein Levels in Chronic Coronary Disease: A LoDoCo2 Biomarker Substudy. Atherosclerosis 2021, 334, 93–100. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Angelidis, C.; Alexopoulos, N.; Filippatos, G.; Papoutsidakis, N.; Sianos, G.; Goudevenos, J.; Alexopoulos, D.; Pyrgakis, V.; et al. Anti-Inflammatory Treatment with Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation 2015, 132, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Mewton, N.; Roubille, F.; Bresson, D.; Prieur, C.; Bouleti, C.; Bochaton, T.; Ivanes, F.; Dubreuil, O.; Biere, L.; Hayek, A.; et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Pillinger, M.; Zhong, H.; Cronstein, B.; Xia, Y.; Lorin, J.D.; Smilowitz, N.R.; Feit, F.; Ratnapala, N.; Keller, N.M.; et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020, 13, e008717. [Google Scholar] [CrossRef]
- Cole, J.; Htun, N.; Lew, R.; Freilich, M.; Quinn, S.; Layland, J. COlchicine to Prevent PeriprocEdural Myocardial Injury in Percutaneous Coronary Intervention (COPE-PCI): A Descriptive Cytokine Pilot Sub-Study. Cardiovasc. Revascularization Med. Incl. Mol. Interv. 2022, 39, 84–89. [Google Scholar] [CrossRef]
- Tong, D.C.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; Stub, D.; et al. Colchicine in Patients with Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation 2020, 142, 1890–1900. [Google Scholar] [CrossRef]
- Akrami, M.; Izadpanah, P.; Bazrafshan, M.; Hatamipour, U.; Nouraein, N.; Drissi, H.B.; Manafi, A. Effects of Colchicine on Major Adverse Cardiac Events in next 6-Month Period after Acute Coronary Syndrome Occurrence; a Randomized Placebo-Control Trial. BMC Cardiovasc. Disord. 2021, 21, 583. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimo, A.; Pascual Figal, D.A.; Bayes-Genis, A.; Emdin, M.; Georgiopoulos, G. Effect of Low-Dose Colchicine in Acute and Chronic Coronary Syndromes: A Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2021, 51, e13464. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Tardif, J.C.; Bouabdallaoui, N.; Khairy, P.; Dubé, M.P.; Blondeau, L.; Guertin, M.C. Colchicine for Secondary Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. J. Cardiol. 2021, 37, 776–785. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Koné-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018, 378, 1908–1919. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, R.P.; Birks, J.S.; Mani, V.; Biasiolli, L.; Robson, M.D.; L’Allier, P.L.; Gingras, M.A.; Alie, N.; McLaughlin, M.A.; Basson, C.T.; et al. Arterial Effects of Canakinumab in Patients with Atherosclerosis and Type 2 Diabetes or Glucose Intolerance. J. Am. Coll. Cardiol. 2016, 68, 1769. [Google Scholar] [CrossRef] [Green Version]
- Everett, B.M.; MacFadyen, J.G.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial. J. Am. Coll. Cardiol. 2020, 76, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Baylis, R.A.; Gomez, D.; Mallat, Z.; Pasterkamp, G.; Owens, G.K. The CANTOS Trial: One Important Step for Clinical Cardiology but a Giant Leap for Vascular Biology. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiller, C.; Reindl, M.; Holzknecht, M.; Lechner, I.; Schwaiger, J.; Brenner, C.; Mayr, A.; Klug, G.; Bauer, A.; Metzler, B.; et al. Association of Plasma Interleukin-6 with Infarct Size, Reperfusion Injury, and Adverse Remodelling after ST-Elevation Myocardial Infarction. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Koshino, A.; Schechter, M.; Sen, T.; Vart, P.; Neuen, B.L.; Neal, B.; Arnott, C.; Perkovic, V.; Ridker, P.M.; Tuttle, K.R.; et al. Interleukin-6 and Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: New Insights From CANVAS. Diabetes Care 2022, 45, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Batra, G.; Ghukasyan Lakic, T.; Lindbäck, J.; Held, C.; White, H.D.; Stewart, R.A.H.; Koenig, W.; Cannon, C.P.; Budaj, A.; Hagström, E.; et al. Interleukin 6 and Cardiovascular Outcomes in Patients with Chronic Kidney Disease and Chronic Coronary Syndrome. JAMA Cardiol. 2021, 6, 1440–1445. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory Cytokines and Risk of Coronary Heart Disease: New Prospective Study and Updated Meta-Analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [Green Version]
- Ferencik, M.; Mayrhofer, T.; Lu, M.T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Voora, D.; et al. Coronary Atherosclerosis, Cardiac Troponin, and Interleukin-6 in Patients with Chest Pain: The PROMISE Trial Results. JACC Cardiovasc. Imaging 2022, 15, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Eggers, K.M.; Lindhagen, L.; Agewall, S.; Brolin, E.B.; Collste, O.; Daniel, M.; Ekenbäck, C.; Frick, M.; Henareh, L.; et al. Increased Inflammatory Activity in Patients 3 Months after Myocardial Infarction with Nonobstructive Coronary Arteries. Clin. Chem. 2019, 65, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Eggers, K.M.; Lakic, T.G.; Lindbäck, J.; Budaj, A.; Cornel, J.H.; Giannitsis, E.; Katus, H.A.; Siegbahn, A.; Storey, R.F.; et al. Biomarker Concentrations and Their Temporal Changes in Patients with Myocardial Infarction and Nonobstructive Compared with Obstructive Coronary Arteries: Results From the PLATO Trial. J. Am. Heart Assoc. 2023, 12, e027466. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Chiang, C.Y.; Chan, J.S.; Lee, C.Y.; Leu, H.B.; Huang, P.H.; Chen, J.S.; Lin, S.J.; Chen, J.W. Tocilizumab, a Humanized Monoclonal Antibody Against the Interleukin-6 Receptor, Inhibits High Glucose-Induced Vascular Smooth Muscle Cell Migration Through Mitogen-Activated Protein Kinase Signaling Pathways. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2018, 38, 510–516. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab Improves Oxidative Stress and Endothelial Glycocalyx: A Mechanism That May Explain the Effects of Biological Treatment on COVID-19. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 145, 111694. [Google Scholar] [CrossRef]
- Greco, D.; Gualtierotti, R.; Agosti, P.; Adorni, M.P.; Ingegnoli, F.; Rota, M.; Bernini, F.; Meroni, P.L.; Ronda, N. Anti-Atherogenic Modification of Serum Lipoprotein Function in Patients with Rheumatoid Arthritis after Tocilizumab Treatment, a Pilot Study. J. Clin. Med. 2020, 9, 2157. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Nielsen, C.; Nybo, M.; Just, S.A.; Vinholt, P.J. The in Vitro Effect of Antirheumatic Drugs on Platelet Function. Platelets 2020, 31, 248–257. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Devalaraja, M.; Fishbane, S.; Chonchol, M.; Mathur, V.S.; Smith, M.T.; Lo, L.; Herzog, K.; Kakkar, R.; Davidson, M.H. Ziltivekimab for Treatment of Anemia of Inflammation in Patients on Hemodialysis: Results from a Phase 1/2 Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Soc. Nephrol. JASN 2021, 32, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Adamstein, N.H.; Cornel, J.H.; Davidson, M.; Libby, P.; De Remigis, A.; Jensen, C.; Ekström, K.; Ridker, P.M. Association of Interleukin 6 Inhibition with Ziltivekimab and the Neutrophil-Lymphocyte Ratio: A Secondary Analysis of the RESCUE Clinical Trial. JAMA Cardiol. 2023, 8, 177–181. [Google Scholar] [CrossRef]
- ZEUS—A Research Study to Look at How Ziltivekimab Works Compared to Placebo in People with Cardiovascular Disease, Chronic Kidney Disease and Inflammation-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT05021835 (accessed on 15 April 2023).
- Tocci, G.; Goletti, D.; Marino, V.; Matucci, A.; Mariamilano, G.; Cantini, F.; Scarpa, R. Cardiovascular Outcomes and Tumour Necrosis Factor Antagonists in Chronic Inflammatory Rheumatic Disease: A Focus on Rheumatoid Arthritis. Expert Opin. Drug Saf. 2016, 15, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ogrič, M.; Poljšak, K.M.; Lakota, K.; Žigon, P.; Praprotnik, S.; Semrl, S.S.; Čučnik, S. Neutralizing Effects of Anti-Infliximab Antibodies on Synergistically-Stimulated Human Coronary Artery Endothelial Cells. Atherosclerosis 2019, 291, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barbati, C.; Colasanti, T.; Vomero, M.; Ceccarelli, F.; Celia, A.I.; Perricone, C.; Spinelli, F.R.; Conti, F.; Valesini, G.; Alessandri, C. Up-Regulation of Autophagy by Etanercept Treatment Results in TNF-Induced Apoptosis Reduction in EA.Hy926 Endothelial Cell Line. Clin. Exp. Rheumatol. 2021, 39, 606–611. [Google Scholar] [CrossRef]
- Avgerinou, G.; Tousoulis, D.; Siasos, G.; Oikonomou, E.; Maniatis, K.; Papageorgiou, N.; Paraskevopoulos, T.; Miliou, A.; Koumaki, D.; Latsios, G.; et al. Anti-Tumor Necrosis Factor α Treatment with Adalimumab Improves Significantly Endothelial Function and Decreases Inflammatory Process in Patients with Chronic Psoriasis. Int. J. Cardiol. 2011, 151, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Gravos, A.; Georgiopoulos, G.; Terentes-Printzios, D.; Ioakeimidis, N.; Vassilopoulos, D.; Stamatelopoulos, K.; Tousoulis, D. The Effect of TNF-a Antagonists on Aortic Stiffness and Wave Reflections: A Meta-Analysis. Clin. Rheumatol. 2018, 37, 515–526. [Google Scholar] [CrossRef]
- Barnabe, C.; Martin, B.J.; Ghali, W.A. Systematic Review and Meta-Analysis: Anti-Tumor Necrosis Factor α Therapy and Cardiovascular Events in Rheumatoid Arthritis. Arthritis Care Res. 2011, 63, 522–529. [Google Scholar] [CrossRef]
- Armstrong, A.; Brezinski, E.; Follansbee, M.; Armstrong, E. Effects of Biologic Agents and Other Disease-Modifying Antirheumatic Drugs on Cardiovascular Outcomes in Psoriasis and Psoriatic Arthritis: A Systematic Review. Curr. Pharm. Des. 2014, 20, 500–512. [Google Scholar] [CrossRef]
- Vallejo, S.; Palacios, E.; Romacho, T.; Villalobos, L.; Peiró, C.; Sánchez-Ferrer, C.F. The Interleukin-1 Receptor Antagonist Anakinra Improves Endothelial Dysfunction in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Diabetol. 2014, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, I.; Tzortzis, S.; Andreadou, I.; Paraskevaidis, I.; Katseli, C.; Katsimbri, P.; Pavlidis, G.; Parissis, J.; Kremastinos, D.; Anastasiou-Nana, M.; et al. Increased Benefit of Interleukin-1 Inhibition on Vascular Function, Myocardial Deformation, and Twisting in Patients with Coronary Artery Disease and Coexisting Rheumatoid Arthritis. Circ. Cardiovasc. Imaging 2014, 7, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Del Buono, M.G.; Damonte, J.I.; Trankle, C.R.; Kadariya, D.; Carbone, S.; Thomas, G.; Turlington, J.; Markley, R.; Canada, J.M.; Biondi-Zoccai, G.G.; et al. Effect of Interleukin-1 Blockade with Anakinra on Leukocyte Count in Patients with ST-Segment Elevation Acute Myocardial Infarction. Sci. Rep. 2022, 12, 2157. [Google Scholar] [CrossRef]
- Abbate, A.; Kontos, M.C.; Abouzaki, N.A.; Melchior, R.D.; Thomas, C.; Van Tassell, B.W.; Oddi, C.; Carbone, S.; Trankle, C.R.; Roberts, C.S.; et al. Comparative Safety of Interleukin-1 Blockade with Anakinra in Patients with ST-Segment Elevation Acute Myocardial Infarction (from the VCU-ART and VCU-ART2 Pilot Studies). Am. J. Cardiol. 2015, 115, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Zinellu, A.; Sotgia, S.; Carru, C.; Piga, M.; Erre, G.L. Protective Effects of Methotrexate against Proatherosclerotic Cytokines: A Review of the Evidence. Mediat. Inflamm 2017, 2017, 9632846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, M.; Kai, H.; Yasukawa, H.; Yamamoto, T.; Kawai, Y.; Kato, S.; Kusaba, K.; Kai, M.; Egashira, K.; Kataoka, Y.; et al. Inhibition of Progression and Stabilization of Plaques by Postnatal Interferon-Gamma Function Blocking in ApoE-Knockout Mice. Circ. Res. 2007, 101, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M.; Kawashima, S.; Yamashita, T.; Ozaki, M.; Sakoda, T.; Inoue, N.; Hirata, K.I.; Morishita, R.; Kaneda, Y.; Yokoyama, M. Intramuscular Gene Transfer of Interleukin-10 CDNA Reduces Atherosclerosis in Apolipoprotein E-Knockout Mice. Atherosclerosis 2004, 172, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.S.; Chan Kwon, I.; Tae, G. Targeted Delivery of Anti-Inflammatory Cytokine by Nanocarrier Reduces Atherosclerosis in Apo E−/− Mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, S.; Shamsi, S. MCC950 in the Treatment of NLRP3-Mediated Inflammatory Diseases: Latest Evidence and Therapeutic Outcomes. Int. Immunopharmacol. 2022, 106, 108595. [Google Scholar] [CrossRef]
- Van Der Heijden, T.; Kritikou, E.; Venema, W.; Van Duijn, J.; Van Santbrink, P.J.; Slütter, B.; Foks, A.C.; Bot, I.; Kuiper, J. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1457–1461. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Shi, H.; Chang, S.; Gao, Y.; Li, X.; Lv, C.; Yang, H.; Xiang, H.; Yang, J.; Xu, L.; et al. The Selective NLRP3-Inflammasome Inhibitor MCC950 Reduces Myocardial Fibrosis and Improves Cardiac Remodeling in a Mouse Model of Myocardial Infarction. Int. Immunopharmacol. 2019, 74, 105575. [Google Scholar] [CrossRef]
- Živković, L.; Asare, Y.; Bernhagen, J.; Dichgans, M.; Georgakis, M.K. Pharmacological Targeting of the CCL2/CCR2 Axis for Atheroprotection: A Meta-Analysis of Preclinical Studies. Arterioscler. Thromb. Vasc. Biol. 2022, 42, E131–E144. [Google Scholar] [CrossRef]
- Veillard, N.R.; Kwak, B.; Pelli, G.; Mulhaupt, F.; James, R.W.; Proudfoot, A.E.I.; Mach, F. Antagonism of RANTES Receptors Reduces Atherosclerotic Plaque Formation in Mice. Circ. Res. 2004, 94, 253–261. [Google Scholar] [CrossRef]
- Cipriani, S.; Francisci, D.; Mencarelli, A.; Renga, B.; Schiaroli, E.; D’Amore, C.; Baldelli, F.; Fiorucci, S. Efficacy of the CCR5 Antagonist Maraviroc in Reducing Early, Ritonavir-Induced Atherogenesis and Advanced Plaque Progression in Mice. Circulation 2013, 127, 2114–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Sha, X.; Song, X.; Zhang, X.; Xing, M.; Liu, S.; Xu, K.; Li, J. Targeted Therapy of Atherosclerosis Vulnerable Plaque by ROS-Scavenging Nanoparticles and MR/Fluorescence Dual-Modality Imaging Tracing. Int. J. Nanomed. 2022, 17, 5413. [Google Scholar] [CrossRef] [PubMed]
- Zan, C.; An, J.; Wu, Z.; Li, S. Engineering Molecular Nanoprobes to Target Early Atherosclerosis: Precise Diagnostic Tools and Promising Therapeutic Carriers. Nanotheranostics 2023, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; MacH, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
| Anti-Inflammatory Therapy | Action |
|---|---|
| Aspirin | COX-inhibition Inhibition of NF-κΒ pathway Activation of Thromboxane prostanoid receptor IL-6 and TNF-α reduction Macrophage-colony-stimulating factor inhibition Increased activity of NO synthase Increased the formation of 15-epi-LXA4 Decreased MRP8/14 levels |
| Statins | Reduction of endothelin-1 Reduction of asymmetrical dimethyl arginine Reduction of NO synthesis Reduction of TNF-α, IL-6, IFN-γ and MCP-1 levels Increase in IL-10 levels Increased macrocalcification |
| Colchicine | Inhibition of tubulin polymerization Reduction of chemotaxis Decreased expression of adhesion molecules Inhibition of NF-κΒ pathway Reduced formation of the inflammasome |
| IL-1β inhibitors | Inhibition of NF-κΒ pathway Reduction of IL-6 and TNF-α Reduced expression of ICAM-1, VCAM-1, P-selectin, E-selectin, MCP-1 Reduced transmigration of inflammatory cell |
| IL-6 inhibitors | Reduction of vascular inflammation Reduced expression of adhesion molecules Reduced production of TNF-α, IL-18, and IFN-γ Reduced production of fibrinogen Reduction of MMP-9 |
| TNF-α inhibitors | Reduced endothelial cell apoptosis Reduced expression of ICAM-1, VCAM-1 and MCP-1 |
| IL-1 receptor antagonist | Reduction of vascular NADPH oxidase Reduced activation of NF-Κb Reduced NO synthesis |
| gene transfers of mutant IFN-γ receptors | Decreased macrophage accumulation Reduced VSMC proliferation Reduced expression of cytokines, adhesion molecules, and MMPs |
| NLRP3 inflammasome inhibitors | Reduced expression of adhesion molecules Reduced macrophage accumulation |
| CCLR/CCR2 inhibitors | Reduced macrophage accumulation Reduced VSMC proliferation Improved endothelial function |
| CCL5 inhibitors | Reduced local expression of VCAM-1, ICAM-1, MCP-1 Reduced leukocyte infiltrations of the atheromas Reduced TNF-α |
| ROS-targeting nanoparticles | Reduced endothelial cell apoptosis Reduced vascular inflammation Reduced formation of foam cells |
| First Author/ Study Name | Anti-Inflammatory Therapy | Type of Study | Date | Main Findings |
|---|---|---|---|---|
| Ridker et al. [64] | Aspirin | Case control study | 1997 | Aspirin reduced the risk of MI in patients in the highest quartile of hs-CRP but not in those in the lowest quartile |
| Kronish et al. [65] | Aspirin | Prospective cohort | 2010 | Adherence to aspirin inversely correlated with hs-CRP levels |
| Ridker et al. JUPITER trial [66] | Statins | Randomized trial | 2008 | Rosuvastatin reduced CRP levels by 37% Rosuvastatin reduced the risk for cardiovascular events (HR: 0.56; 95% CI, 0.46 to 0.69; p < 0.001) and all-cause mortality |
| Sahebkar et al. [67] | Statins | Meta-analysis | 2015 | Statin therapy reduces endothelin-1 levels |
| Arabi et al. [68,69] | Statins | Meta-analysis | 2022 | Statin and ezetimibe combination therapy reduce levels of hs-CRP and various cytokines |
| Tardif et al. COLCOT trial [70] | Colchicine | Randomized trial | 2019 | Colchicine reduced the risk for cardiovascular mortality in patients recruited within 30 days after a MI (HR, 0.77; 95% CI, 0.61 to 0.96; p = 0.02) |
| Nidorf et al. LoDo-Co-2 trial [71] | Colchicine | Randomized trial | 2020 | Colchicine reduced the risk for cardiovascular events in patients with chronic CAD (HR: 0.69; 95% CI 0.57 to 0.83; p < 0.001) |
| Ridker et al. CIRT trial [72] | Methotrexate | Randomized trial | 2019 | Methotrexate did not reduce the risk for the primary endpoint (HR: 0.96; 95% CI 0.79 to 1.16; p = 0.67) |
| Ridker et al. CANTOS trial [73] | Canakinumab | Randomized trial | 2017 | Canakinumab achieved a dose-dependent reduction of CRP and a reduction of the hazard for nonfatal MI/stroke or cardiovascular death in patients with previous MI and hs-CRP > 2 mg/L (HR: 0.85 (95% CI, 0.74 to 0.98; p = 0.021 in the 150 mg dose group) Canakinumab associated with increased risk for fatal infection |
| Broch et al. ASSAIL-MI [74] | Tocilizumab | Randomized trial | 2021 | Tocilizumab increased myocardial salvage in patients with ST-elevation MI |
| Ridker et al. RESCUE trial [75] | Ziltivekimab | Randomized trial | 2021 | Ziltivekimab achieved dose-dependent reduction (77–92%) of hs-CRP levels in patients with increased cardiovascular risk, chronic kidney disease, and hs-CRP > 2 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitroglou, Y.; Aggeli, C.; Theofilis, P.; Tsioufis, P.; Oikonomou, E.; Chasikidis, C.; Tsioufis, K.; Tousoulis, D. Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes. Life 2023, 13, 1669. https://doi.org/10.3390/life13081669
Dimitroglou Y, Aggeli C, Theofilis P, Tsioufis P, Oikonomou E, Chasikidis C, Tsioufis K, Tousoulis D. Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes. Life. 2023; 13(8):1669. https://doi.org/10.3390/life13081669
Chicago/Turabian StyleDimitroglou, Yannis, Constantina Aggeli, Panagiotis Theofilis, Panagiotis Tsioufis, Evangelos Oikonomou, Christos Chasikidis, Konstantinos Tsioufis, and Dimitris Tousoulis. 2023. "Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes" Life 13, no. 8: 1669. https://doi.org/10.3390/life13081669
APA StyleDimitroglou, Y., Aggeli, C., Theofilis, P., Tsioufis, P., Oikonomou, E., Chasikidis, C., Tsioufis, K., & Tousoulis, D. (2023). Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes. Life, 13(8), 1669. https://doi.org/10.3390/life13081669








