A Food Matrix Triggers a Similar Allergic Immune Response in BALB/c Mice Sensitized with Native, Denatured, and Digested Ovalbumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
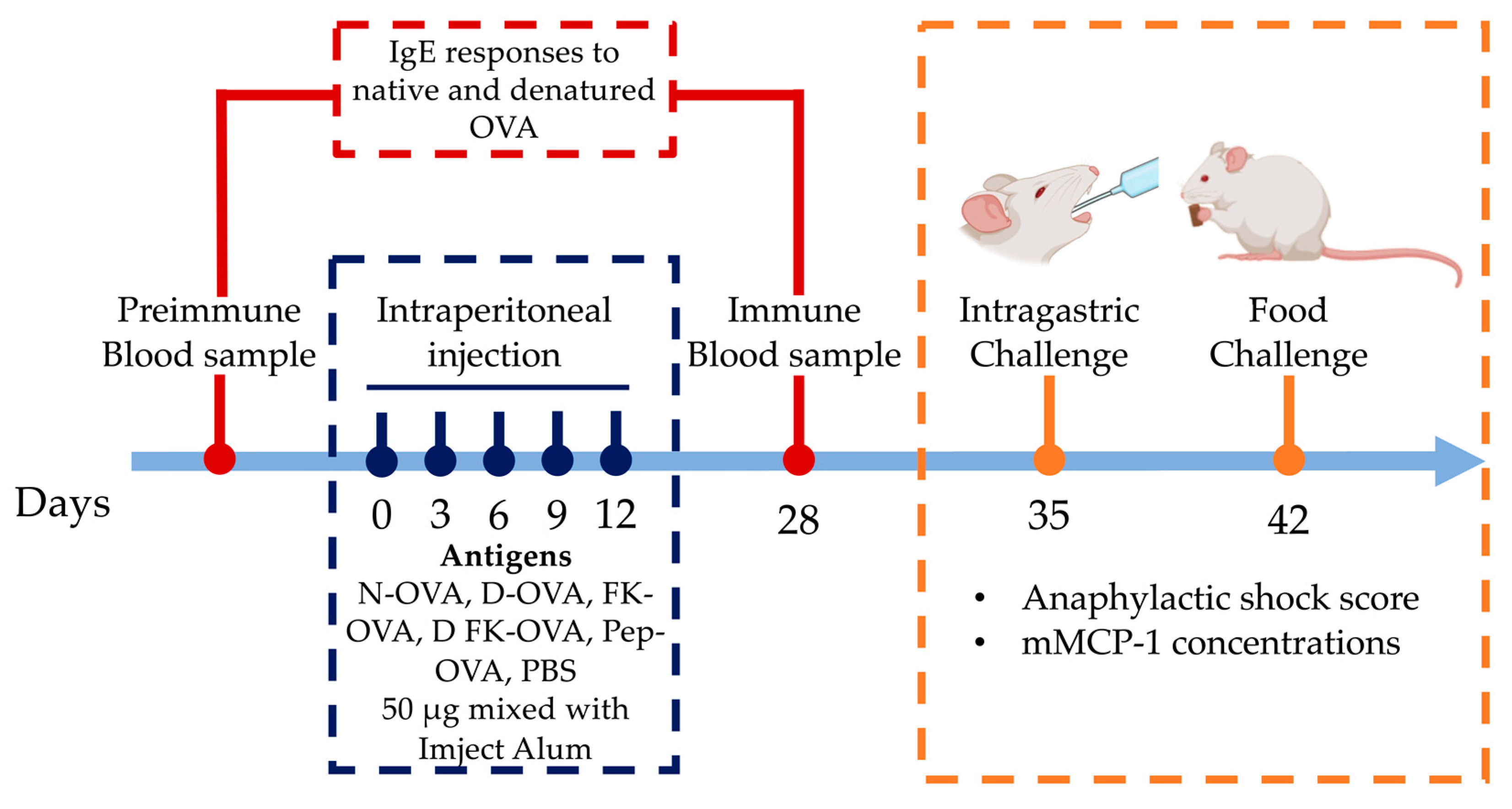
2.3. Preparation of Food Matrix Model
2.4. Antigen Preparation for Sensitization Protocols
2.5. Sensitization of Mice
2.6. Serum IgE Titers Evaluation
2.7. Intragastric and Oral Food Challenge
2.8. Statistical Analysis
3. Results
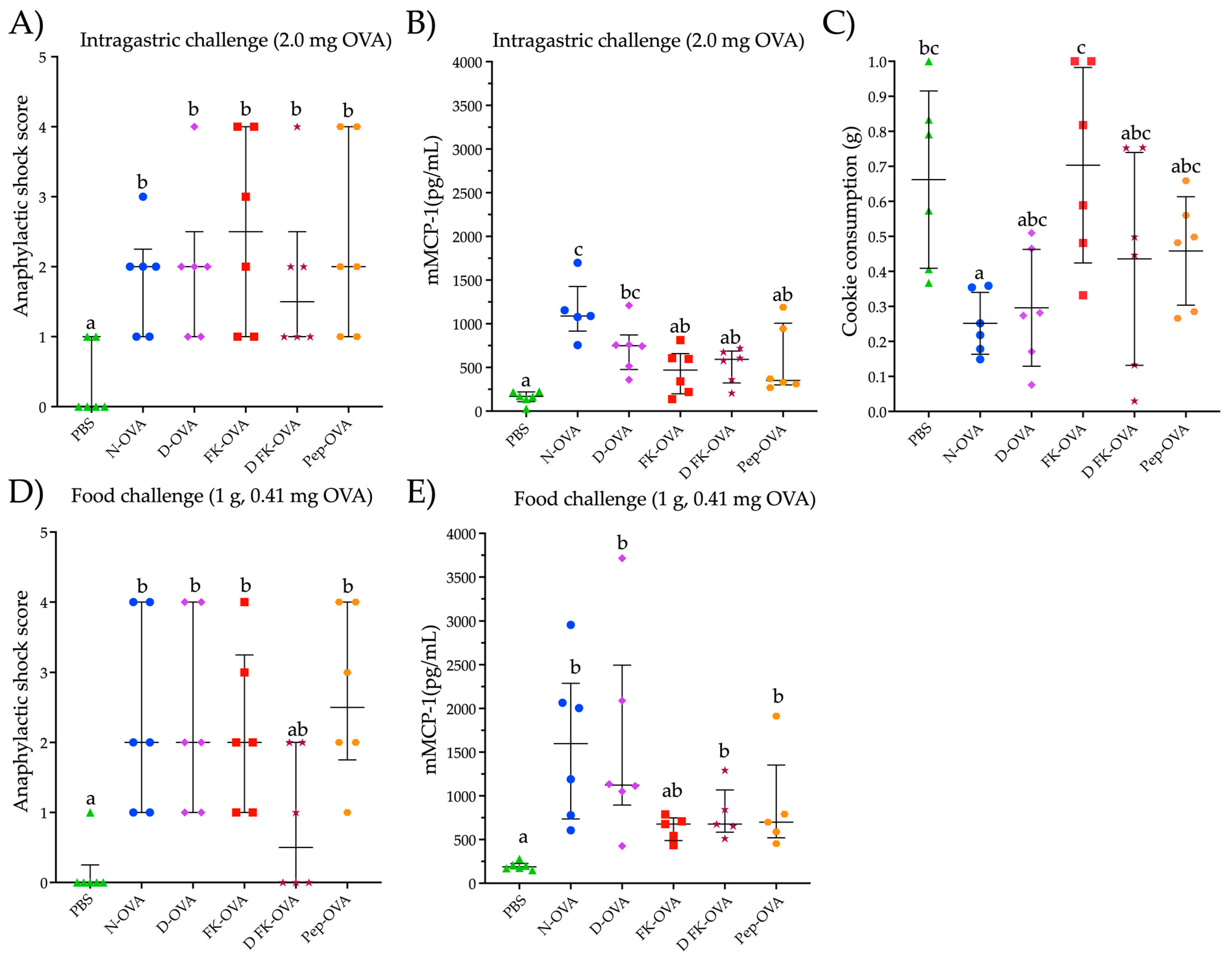
3.1. IgE Responses against Native and Denatured Ovalbumin
3.2. Intragastric and Oral Food Challenge Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Lin, S.; Sun, N. How Does Food Matrix Components Affect Food Allergies, Food Allergens and the Detection of Food Allergens? A Systematic Review. Trends Food Sci. Technol. 2022, 127, 280–290. [Google Scholar] [CrossRef]
- Vissers, Y.M.; Wichers, H.J.; Savelkoul, H.F.J. Influence of Food Processing, Digestion and the Food Matrix on Allergenicity & Cellular Measures of Allergenicity. In Multidisciplinary Approaches to Allergies; Gao, Z.-S., Zheng, M., Gilissen, L.J.W.J., Shen, H.-H., Frewer, L.J., Eds.; Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2012; pp. 203–227. ISBN 978-3-642-31609-8. [Google Scholar]
- Thomas, K.; Herouet-Guicheney, C.; Ladics, G.; Bannon, G.; Cockburn, A.; Crevel, R.; Fitzpatrick, J.; Mills, C.; Privalle, L.; Vieths, S. Evaluating the Effect of Food Processing on the Potential Human Allergenicity of Novel Proteins: International Workshop Report. Food Chem. Toxicol. 2007, 45, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Bøgh, K.L.; van Bilsen, J.; Głogowski, R.; López-Expósito, I.; Bouchaud, G.; Blanchard, C.; Bodinier, M.; Smit, J.; Pieters, R.; Bastiaan-Net, S.; et al. Current Challenges Facing the Assessment of the Allergenic Capacity of Food Allergens in Animal Models. Clin. Transl. Allergy 2016, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Castan, L.; Bøgh, K.L.; Maryniak, N.Z.; Epstein, M.M.; Kazemi, S.; O’Mahony, L.; Bodinier, M.; Smit, J.J.; van Bilsen, J.H.M.; Blanchard, C.; et al. Overview of in Vivo and Ex Vivo Endpoints in Murine Food Allergy Models: Suitable for Evaluation of the Sensitizing Capacity of Novel Proteins? Allergy 2020, 75, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Association of Cereal Chemists. Approved Methods of Analysis, 11th ed.; Method 10–50.05. Baking Quality of Cookie Flour; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- Koch, C.; Jensen, S.S.; Øster, A.; Houen, G. A Comparison of the Immunogenicity of the Native and Denatured Forms of a Protein. APMIS 1996, 104, 115–125. [Google Scholar] [CrossRef]
- Holm, B.E.; Bergmann, A.C.; Hansen, P.R.; Koch, C.; Houen, G.; Trier, N.H. Antibodies with Specificity for Native and Denatured Forms of Ovalbumin Differ in Reactivity between Enzyme-Linked Immunosorbent Assays. APMIS 2015, 123, 136–145. [Google Scholar] [CrossRef]
- Thomas, K.; Aalbers, M.; Bannon, G.A.; Bartels, M.; Dearman, R.J.; Esdaile, D.J.; Fu, T.J.; Glatt, C.M.; Hadfield, N.; Hatzos, C.; et al. A Multi-Laboratory Evaluation of a Common in Vitro Pepsin Digestion Assay Protocol Used in Assessing the Safety of Novel Proteins. Regul. Toxicol. Pharmacol. 2004, 39, 87–98. [Google Scholar] [CrossRef]
- Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Arvizu-Flores, A.A.; Flores-Mendoza, L.K.; Lopez-Teros, V.; Astiazaran-Garcia, H.; Gracia-Valenzuela, M.H.; Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Ontiveros, N. Assessment of the Route of Exposure to Ovalbumin and Cow’s Milk Proteins on the Induction of IgE Responses in BALB/c Mice. Biology 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Arámburo-Galvez, J.G.; Sotelo-Cruz, N.; Flores-Mendoza, L.K.; Gracia-Valenzuela, M.H.; Chiquete-Elizalde, F.I.R.; Espinoza-Alderete, J.G.; Trejo-Martínez, H.; Canizalez-Román, V.A.; Ontiveros, N.; Cabrera-Chávez, F. Assessment of the Sensitizing Potential of Proteins in BALB/c Mice: Comparison of Three Protocols of Intraperitoneal Sensitization. Nutrients 2018, 10, 903. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas-Torres, F.I.; Reyes-Moreno, C.; de Jesús Vergara-Jiménez, M.; Cuevas-Rodríguez, E.O.; Milán-Carrillo, J.; Gutiérrez-Dorado, R.; Arámburo-Gálvez, J.G.; Ontiveros, N.; Cabrera-Chávez, F. Assessing the Sensitizing and Allergenic Potential of the Albumin and Globulin Fractions from Amaranth (Amaranthus Hypochondriacus) Grains before and after an Extrusion Process. Med. Kaunas Lith. 2019, 55, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Esch, B.; van Bilsen, J.H.M.; Jeurink, P.V.; Garssen, J.; Penninks, A.H.; Smit, J.J.; Pieters, R.H.H.; Knippels, L.M.J. Interlaboratory Evaluation of a Cow’s Milk Allergy Mouse Model to Assess the Allergenicity of Hydrolysed Cow’s Milk Based Infant Formulas. Toxicol. Lett. 2013, 220, 95–102. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; Molina, E.; López-Fandiño, R. Egg Proteins as Allergens and the Effects of the Food Matrix and Processing. Food Funct. 2015, 6, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liu, Y.; Liu, K.; Wang, S.; Liu, Q.; Lin, S. Gastrointestinal Fate of Food Allergens and Its Relationship with Allergenicity. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3376–3404. [Google Scholar] [CrossRef]
- Claude, M.; Lupi, R.; Bouchaud, G.; Bodinier, M.; Brossard, C.; Denery-Papini, S. The Thermal Aggregation of Ovalbumin as Large Particles Decreases Its Allergenicity for Egg Allergic Patients and in a Murine Model. Food Chem. 2016, 203, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Bøgh, K.L.; Andreasen, M.S.; Madsen, C.B. The Use of Aluminium Hydroxide as Adjuvant Modulates the Specific Antibody Response—A Brown Norway Rat Study with Native and Denatured Cow’s Milk Allergens. Scand. J. Immunol. 2020, 92, e12891. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Sanders, S.E.; Cunningham, M.M.; Kelsoe, G. Disparate Adjuvant Properties among Three Formulations of “Alum”. Vaccine 2013, 31, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J. The Role of Allergen-Specific IgE, IgG and IgA in Allergic Disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef]
- Okamoto, S.; Taniuchi, S.; Sudo, K.; Hatano, Y.; Nakano, K.; Shimo, T.; Kaneko, K. Predictive Value of IgE/IgG4 Antibody Ratio in Children with Egg Allergy. Allergy Asthma Clin. Immunol. 2012, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Germundson, D.L.; Nagamoto-Combs, K. Isotype-Specific Detection of Serum Immunoglobulins against Allergens. In Animal Models of Allergic Disease: Methods and Protocols; Nagamoto-Combs, K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; pp. 159–167. ISBN 978-1-07-161001-5. [Google Scholar]
- Xu, H.; Heyman, B. IgG-Mediated Suppression of Antibody Responses: Hiding or Snatching Epitopes? Scand. J. Immunol. 2020, 92, e12921. [Google Scholar] [CrossRef]
- Gonipeta, B.; Kim, E.; Gangur, V. Mouse Models of Food Allergy: How Well Do They Simulate the Human Disorder? Crit. Rev. Food Sci. Nutr. 2015, 55, 437–452. [Google Scholar] [CrossRef]
- Giannetti, A.; Meglio, P.; Ricci, G. Skin Prick Test: The Only Predictive Tool of Anaphylaxis? A Case Report. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 49–52. [Google Scholar]
- Marrs, T.; Brough, H.A.; Kwok, M.; Lack, G.; Santos, A.F. Basophil CD63 Assay to Peanut Allergens Accurately Diagnoses Peanut Allergy in Patient with Negative Skin Prick Test and Very Low Specific IgE. Pediatr. Allergy Immunol. 2022, 33, e13739. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M. The Very Low IgE Producer: Allergology, Genetics, Immunodeficiencies, and Oncology. Biomedicines 2023, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.H.; Holm, J.; Lund, G.; Riise, E.; Lund, K. Several Distinct Properties of the IgE Repertoire Determine Effector Cell Degranulation in Response to Allergen Challenge. J. Allergy Clin. Immunol. 2008, 122, 298–304. [Google Scholar] [CrossRef]
- Nakano, N.; Kitaura, J. Mucosal Mast Cells as Key Effector Cells in Food Allergies. Cells 2022, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S. Current Codex Guidelines for Assessment of Potential Protein Allergenicity. Food Chem. Toxicol. 2008, 46, S20–S23. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms (GMO); Mullins, E.; Bresson, J.-L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; George Firbank, L.; Guerche, P.; Hejatko, J.; Naegeli, H.; et al. Scientific Opinion on Development Needs for the Allergenicity and Protein Safety Assessment of Food and Feed Products Derived from Biotechnology. EFSA J. 2022, 20, e07044. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Villa, C.; Verhoeckx, K.; Cirkovic-Velickovic, T.; Schrama, D.; Roncada, P.; Rodrigues, P.M.; Piras, C.; Martín-Pedraza, L.; Monaci, L.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens? Clin. Rev. Allergy Immunol. 2022, 62, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Bøgh, K.L.; Dupont, D.; Egger, L.; Gadermaier, G.; Larré, C.; Mackie, A.; Menard, O.; Adel-Patient, K.; Picariello, G.; et al. The Relevance of a Digestibility Evaluation in the Allergenicity Risk Assessment of Novel Proteins. Opinion of a Joint Initiative of COST Action ImpARAS and COST Action INFOGEST. Food Chem. Toxicol. 2019, 129, 405–423. [Google Scholar] [CrossRef]
- Hoh, R.A.; Boyd, S.D. Gut Mucosal Antibody Responses and Implications for Food Allergy. Front. Immunol. 2018, 9, 2221. [Google Scholar] [CrossRef]
- Steele, L.; Mayer, L.; Cecilia Berin, M. Mucosal Immunology of Tolerance and Allergy in the Gastrointestinal Tract. Immunol. Res. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Vickery, B.P.; Chin, S.; Burks, A.W. Pathophysiology of Food Allergy. Pediatr. Clin. N. Am. 2011, 58, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Pekar, J.; Ret, D.; Untersmayr, E. Stability of Allergens. Mol. Immunol. 2018, 100, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Martos, G.; Lopez-Exposito, I.; Bencharitiwong, R.; Berin, M.C.; Nowak-Węgrzyn, A. Mechanisms Underlying Differential Food Allergy Response to Heated Egg. J. Allergy Clin. Immunol. 2011, 127, 990–997.e2. [Google Scholar] [CrossRef] [Green Version]
- Mine, Y.; Yang, M. Epitope Characterization of Ovalbumin in BALB/c Mice Using Different Entry Routes. Biochim. Biophys. Acta BBA-Proteins Proteom. 2007, 1774, 200–212. [Google Scholar] [CrossRef]
- Arámburo-Gálvez, J.G.; Figueroa-Salcido, O.G.; Cárdenas-Torres, F.I.; Ontiveros, N. Report of In Silico Prediction of Ovalbumin Hydrolysis by Pepsin 2023. Figshare. Dataset. Available online: https://doi.org/10.6084/m9.figshare.23563941.v1 (accessed on 4 July 2023). [CrossRef]
- Selby-Pham, S.N.B.; Miller, R.B.; Howell, K.; Dunshea, F.; Bennett, L.E. Physicochemical Properties of Dietary Phytochemicals Can Predict Their Passive Absorption in the Human Small Intestine. Sci. Rep. 2017, 7, 1931. [Google Scholar] [CrossRef] [Green Version]
- Akkerdaas, J.H.; Cianferoni, A.; Islamovic, E.; Kough, J.; Ladics, G.S.; McClain, S.; Poulsen, L.K.; Silvanovich, A.; Pereira Mouriès, L.; van Ree, R. Impact of Food Matrices on Digestibility of Allergens and Poorly Allergenic Homologs. Front. Allergy 2022, 3, 909410. [Google Scholar] [CrossRef] [PubMed]
- Polovic, N.; Blanusa, M.; Gavrovic-Jankulovic, M.; Atanaskovic-Markovic, M.; Burazer, L.; Jankov, R.; Velickovic, T.C. A Matrix Effect in Pectin-Rich Fruits Hampers Digestion of Allergen by Pepsin in Vivo and in Vitro. Clin. Exp. Allergy 2007, 37, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; López-Expósito, I.; Molina, E.; López-Fandiño, R. IgE-Binding and in Vitro Gastrointestinal Digestibility of Egg Allergens in the Presence of Polysaccharides. Food Hydrocoll. 2013, 30, 597–605. [Google Scholar] [CrossRef]
- Mattar, H.; Padfield, P.; Simpson, A.; Mills, E.N.C. The Impact of a Baked Muffin Matrix on the Bioaccessibility and IgE Reactivity of Egg and Peanut Allergens. Food Chem. 2021, 362, 129879. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arámburo-Gálvez, J.G.; Tinoco-Narez-Gil, R.; Arvizu-Flores, A.A.; Figueroa-Salcido, O.G.; Mora-Melgem, J.A.; Islas-Rubio, A.R.; Flores-Mendoza, L.K.; Lopez-Teros, V.; Astiazaran-Garcia, H.; Cárdenas-Torres, F.I.; et al. A Food Matrix Triggers a Similar Allergic Immune Response in BALB/c Mice Sensitized with Native, Denatured, and Digested Ovalbumin. Life 2023, 13, 1733. https://doi.org/10.3390/life13081733
Arámburo-Gálvez JG, Tinoco-Narez-Gil R, Arvizu-Flores AA, Figueroa-Salcido OG, Mora-Melgem JA, Islas-Rubio AR, Flores-Mendoza LK, Lopez-Teros V, Astiazaran-Garcia H, Cárdenas-Torres FI, et al. A Food Matrix Triggers a Similar Allergic Immune Response in BALB/c Mice Sensitized with Native, Denatured, and Digested Ovalbumin. Life. 2023; 13(8):1733. https://doi.org/10.3390/life13081733
Chicago/Turabian StyleArámburo-Gálvez, Jesús Gilberto, Raúl Tinoco-Narez-Gil, Aldo Alejandro Arvizu-Flores, Oscar Gerardo Figueroa-Salcido, José Antonio Mora-Melgem, Alma Rosa Islas-Rubio, Lilian Karem Flores-Mendoza, Veronica Lopez-Teros, Humberto Astiazaran-Garcia, Feliznando Isidro Cárdenas-Torres, and et al. 2023. "A Food Matrix Triggers a Similar Allergic Immune Response in BALB/c Mice Sensitized with Native, Denatured, and Digested Ovalbumin" Life 13, no. 8: 1733. https://doi.org/10.3390/life13081733







