Foley Catheter as a Tourniquet for Hemorrhage Prevention during Peripartum Hysterectomy in Patients with Placenta Accreta Spectrum (PAS)—A Hospital-Based Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Settings
2.2. Data Sources
2.3. Participants
2.4. Bias
2.5. Surgical Technique
- Skin incision: When conducting surgery for placenta accreta syndrome, a midline incision that avoids the umbilicus is preferred. If a primary lower segment incision is present, it should be extended upwards along the midline. The choice of skin incision technique should be based on the patient’s medical history and the surgeon’s experience and preferences.
- Access to the uterus: At this stage, we also use monopolar instruments to dissect the subcutaneous tissues. The rectus sheath is separated along its fibers. The rectus muscles are separated by pulling. The peritoneum is opened by stretching with index fingers.
- Opening the uterus: After gaining access to the abdominal cavity and visualizing the uterus, in cases of PAS, the uterine incision should be made above the intrauterine margins of the placenta to minimize bleeding. Prior to making the incision, it is advisable to perform an ultrasound to determine the optimal site for the uterine incision. The uterus is opened with an index finger and the hole enlarged between the index finger of one hand and the thumb on the other.
- Delivery of the baby
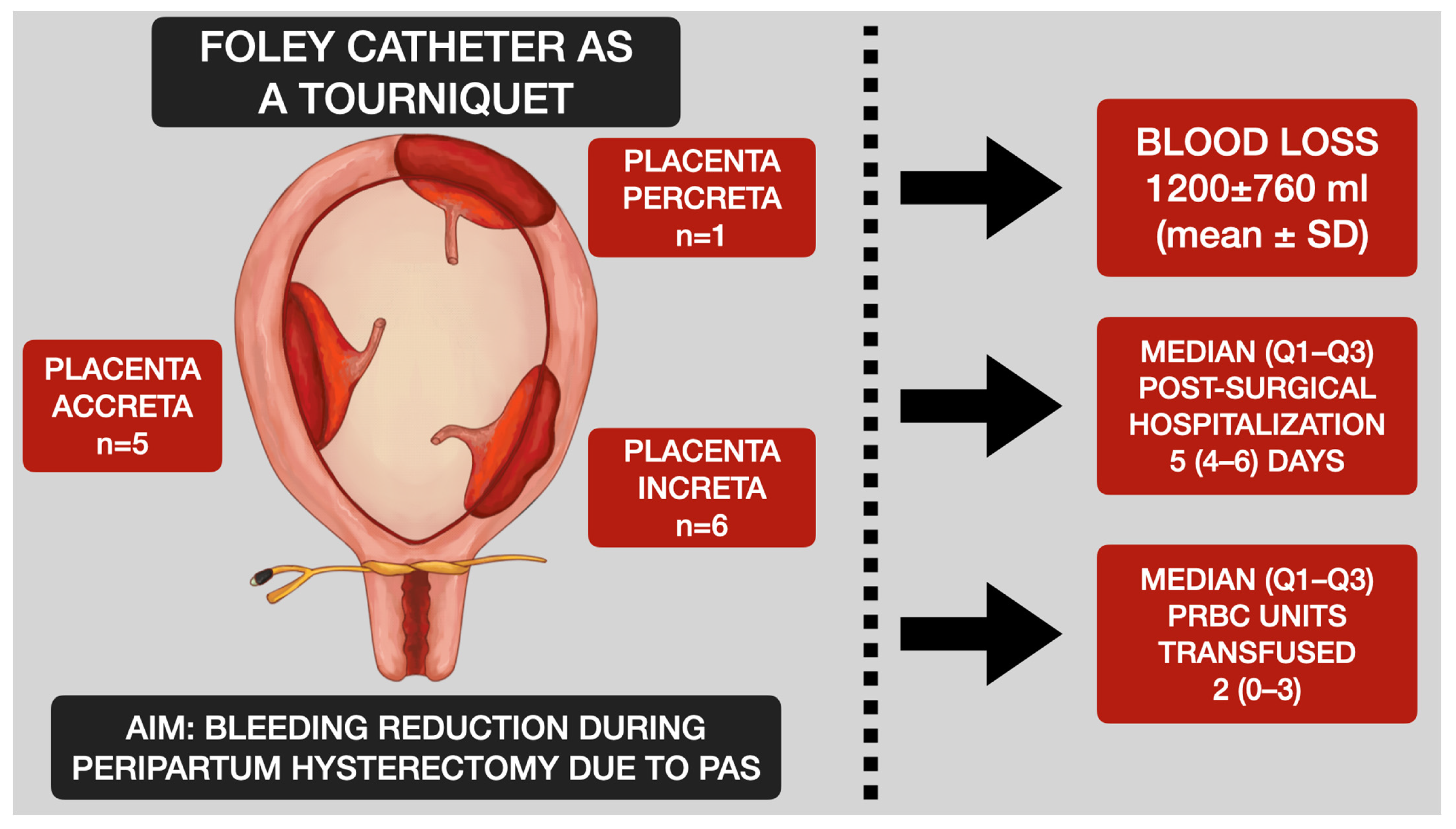
- Evaluation of the placenta and bleeding: After the baby is delivered, the uterus is extracted from the abdominal cavity, along with the placenta. In cases of a planned hysterectomy due to placenta accreta spectrum (PAS), the placenta is not detached from the uterus. If the placenta has been manually extracted and hemorrhage ensues, the procedure for inserting a Foley catheter remains the same, irrespective of whether the placenta remains in the uterus or not. The initial step involves releasing the uterine appendages. This is accomplished by manipulating the uterus in a horizontal manner. Subsequently, an assistant employs a sterile Foley catheter (Ch 16/18 French) to guide it caudally to the most inferior point and then secures it “en bloc” around the cervix (our technique avoids perforating the broad ligament) at the level of the uterosacral ligaments, approximately 3–4 cm below the incision line. Once positioned, the catheter is tightened and secured using Kocher forceps in preparation for the subsequent stages of the hysterectomy. The tourniquet technique facilitates hemostasis, granting the surgeon time to contemplate the potential for uterine preservation or the surgical approach to adhesions with neighboring organs. Given its straightforward and reversible placement, the tourniquet can be momentarily loosened to assess active bleeding, and then retightened to proceed with the operation. At this point, once the Foley catheter is clamped, both the surgical and anesthesia teams can ready themselves for subsequent phases of the procedure, especially if a hemorrhage occurs or if PAS is identified intraoperatively. Employing a Foley catheter as a tourniquet does not preclude the utilization of other techniques, encompassing both pharmacological and compression approaches. The specific surgical and Foley catheter insertion techniques are depicted in Figure 1 (graphical illustration) and Figure 2 (intraoperative image).
- Total vs. subtotal hysterectomy: In our center, the preferred method is the total removal of the uterus. Based on our experience and established scientific reports, total hysterectomy is associated with lower rates of reoperation and perioperative mortality and is less complicated than subtotal hysterectomy. We recommend retaining the cervix if hemorrhage can be effectively managed in this manner or if the surgeon is not confident in performing a total hysterectomy [22,23].
- Closing the Abdominal Wall: In our center, we consistently place abdominal drains following cesarean sections. For peripartum hysterectomies, we recommend inserting two drains—one above and one below the fascia. The rectus muscles are left unsutured. The fascia is closed using a continuous suture.
- Skin Closure: The skin can be closed using staples, sutures, or adhesive strips, depending on the surgeon’s preference.
2.6. Statistics
3. Results
Participants, Descriptive Data and Periprocedural Characteristics and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APH | Antepartum hemorrhage |
| CS | Cesarean section |
| FFP | Fresh frozen plasma |
| GA | General anesthesia |
| Hb | Hemoglobin |
| IVF | In vitro fertilization |
| PAS | Placenta accreta spectrum |
| PIH | Pregnancy induced hypertension |
| PPH | Postpartum hemorrhage |
| PRBC | Packed red blood cells |
| SA | Spinal anesthesia |
References
- Jauniaux, E.; Bunce, C.; Grønbeck, L.; Langhoff-Roos, J. Prevalence and main outcomes of placenta accreta spectrum: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Pri-Paz, S.; Herzog, T.J.; Shah, M.; Bonanno, C.; Lewin, S.N.; Simpson, L.L.; Gaddipati, S.; Sun, X.; D’Alton, M.E.; et al. Predictors of massive blood loss in women with placenta accreta. Am. J. Obstet. Gynecol. 2011, 205, 38.e1–38.e6. [Google Scholar] [CrossRef]
- Bartels, H.C.; Postle, J.D.; Downey, P.; Brennan, D.J. Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis. Markers 2018, 2018, 1507674. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Devine, P.; Shah, M.; Gaddipati, S.; Lewin, S.N.; Simpson, L.L.; Bonanno, C.; Sun, X.; D’Alton, M.E.; Herzog, T.J. Morbidity and Mortality of Peripartum Hysterectomy. Obstet. Gynecol. 2010, 115, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstet. Gynecol. 2018, 132, e259–e275. [Google Scholar] [CrossRef] [PubMed]
- Miseljic, N.; Ibrahimovic, S. Health Implications of Increased Cesarean Section Rates. Mater. Socio Med. 2020, 32, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Stănculescu, R.V.; Brătilă, E.; Socolov, D.G.; Russu, M.C.; Bauşic, V.; Chirculescu, R.; Coroleucă, C.A.; Pristavu, A.I.; Dragomir, R.E.; Papuc, P.; et al. Update on placenta accreta spectrum disorders by considering epidemiological factors, ultrasound diagnosis and pathological exam—Literature review and authors’ experience. Rom. J. Morphol. Embryol. 2022, 63, 293–305. [Google Scholar] [CrossRef]
- Usta, I.M.; Hobeika, E.M.; Abu Musa, A.A.; Gabriel, G.E.; Nassar, A.H. Placenta previa-accreta: Risk factors and complications. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 1045–1049. [Google Scholar] [CrossRef]
- Cantwell, R.; Cluttonbrock, T.; Cooper, G.M.; Dawson, A.; Drife, J.; Garrod, D.; Harper, A.; Hulbert, D.; Lucas, S.; Mcclure, J.H.; et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1–203, Erratum in: BJOG 2015, 122, e1. [Google Scholar] [CrossRef]
- O’brien, D.; Babiker, E.; O’Sullivan, O.; Conroy, R.; McAuliffe, F.; Geary, M.; Byrne, B. Prediction of peripartum hysterectomy and end organ dysfunction in major obstetric haemorrhage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 153, 165–169. [Google Scholar] [CrossRef]
- El Gelany, S.; Ibrahim, E.M.; Mohammed, M.; Abdelraheim, A.R.; Khalifa, E.M.; Abdelhakium, A.K.; Yousef, A.M.; Hassan, H.; Goma, K.; Khairy, M. Management of bleeding from morbidly adherent placenta during elective repeat caesarean section: Retrospective-record-based study. BMC Pregnancy Childbirth 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Enste, R.; Cricchio, P.; Dewandre, P.-Y.; Braun, T.; Leonards, C.O.; Niggemann, P.; Spies, C.; Henrich, W.; Kaufner, L. Placenta Accreta Spectrum Part I: Anesthesia considerations based on an extended review of the literature. J. Périnat. Med. 2022, 51, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Enste, R.; Cricchio, P.; Dewandre, P.-Y.; Braun, T.; Leonards, C.O.; Niggemann, P.; Spies, C.; Henrich, W.; Kaufner, L. Placenta Accreta Spectrum Part II: Hemostatic considerations based on an extended review of the literature. J. Périnat. Med. 2022, 51, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.E.; AbdelQadir, Y.H.; Abdelghafar, Y.A.; Kashbour, M.O.; Salem, N.; Abdelkhalek, A.N.; Nourelden, A.Z.; Eshag, M.M.E.; Shah, J. Therapeutic effect of Internal iliac artery ligation and uterine artery ligation techniques for bleeding control in placenta accreta spectrum patients: A meta-analysis of 795 patients. Front. Surg. 2022, 9, 983297. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.T.; Ahmed, H.A.; Mohamed, Y.A.-R. A Novel Torniquet to reduce blood loss during surgical treatment of postpartum hemorrhage in cesarean section. J. Am. Sci. 2012, 8, 100–103. [Google Scholar]
- Meng, J.-L.; Gong, W.-Y.; Wang, S.; Ni, X.-J.; Zuo, C.-T.; Gu, Y.-Z. Two-tourniquet sequential blocking as a simple intervention for hemorrhage during cesarean delivery for placenta previa accreta. Int. J. Gynecol. Obstet. 2017, 138, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, X.; Liu, L.; Duan, S.; Pei, C.; Zhao, Y.; Liu, R.; Wang, W.; Jian, Y.; Liu, Y.; et al. Placenta Accreta Spectrum Outcomes Using Tourniquet and Forceps for Vascular Control. Front. Med. 2021, 8, 557678. [Google Scholar] [CrossRef] [PubMed]
- Altal, O.F.; Qudsieh, S.; Ben-Sadon, A.; Hatamleh, A.; Bataineh, A.; Halalsheh, O.; Amarin, Z. Cervical tourniquet during cesarean section to reduce bleeding in morbidly adherent placenta: A pilot study. Future Sci. OA 2022, 8, FSO789. [Google Scholar] [CrossRef]
- Ikeda, T.; Sameshima, H.; Kawaguchi, H.; Yamauchi, N.; Ikenoue, T. Tourniquet technique prevents profuse blood loss in placenta accreta cesarean section. J. Obstet. Gynaecol. Res. 2005, 31, 27–31. [Google Scholar] [CrossRef]
- Staniczek, J.; Manasar-Dyrbuś, M.; Skowronek, K.; Winkowska, E.; Stojko, R. Foley Catheter as a Tourniquet for Peripartum Hemorrhage Prevention in Patients with Placenta Accreta Spectrum—A Two Case Report and a Review of the Literature. Medicina 2023, 59, 641. [Google Scholar] [CrossRef]
- Holmgren, G.; Sjöholm, L.; Stark, M. The Misgav Ladach method for cesarean section: Method description. Acta Obstet. Gynecol. Scand. 1999, 78, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Imudia, A.N.; Hobson, D.T.; Awonuga, A.O.; Diamond, M.P.; Bahado-Singh, R.O. Determinants and complications of emergent cesarean hysterectomy: Supracervical vs total hysterectomy. Am. J. Obstet. Gynecol. 2010, 203, 221.e1–221.e5. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, M.; Karacor, T.; Peker, N.; Nacar, M.C.; Okutucu, G. The effect of surgical procedure on surgical outcomes in patients undergoing emergency peripartum hysterectomy: A retrospective multicenter study. J. Matern. Fetal Neonatal Med. 2022, 35, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.; Thomson, A.; Papworth, S.; the Royal College of Obstetricians and Gynaecologists. Antenatal corticosteroids to reduce neonatal morbidity and mortality. BJOG Int. J. Obstet. Gynaecol. 2022, 129, e35–e60. [Google Scholar] [CrossRef] [PubMed]
- Wielgos, M.; Bomba-Opoń, D.; Breborowicz, G.H.; Czajkowski, K.; Debski, R.; Leszczynska-Gorzelak, B.; Oszukowski, P.; Radowicki, S.; Zimmer, M. Recommendations of the Polish Society of Gynecologists and Obstetricians regarding caesarean sections. Ginekol. Pol. 2018, 89, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Visconti, F.; Quaresima, P.; Rania, E.; Palumbo, A.R.; Micieli, M.; Zullo, F.; Venturella, R.; Di Carlo, C. Difficult caesarean section: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.F.; Nassar, A.H.; Theron, G.; Barnea, E.R.; Ramasauskaite, D.; Lloyd, I.; Chandraharan, E.; Miller, S.; Burke, T.; Ossanan, G.; et al. FIGO recommendations on the management of postpartum hemorrhage 2022. Int. J. Gynecol. Obstet. 2022, 157 (Suppl. S1), 3–50. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S. Complications of allergies to latex urinary catheters. Br. J. Nurs. 1997, 6, 786–793. [Google Scholar] [CrossRef]


| Patient | Maternal Age (Years) | Gravidity | Parity | Previous CS (n) | Gestational Age (Weeks) | Associated Conditions | Cause of CS | Birth Weight (g) | APGAR Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 3 | 3 | 2 | 37 | None | Placenta percreta, 2 previous CS | 3210 | 9 |
| 2 | 38 | 3 | 3 | 1 | 38 | None | Placenta increta, 1 previous CS | 3070 | 8 |
| 3 | 32 | 1 | 1 | 0 | 38 | PIH | Fetal tachycardia/relaparotomy (Uterine atony) | 3650 | 10 |
| 4 | 37 | 1 | 1 | 0 | 38 | Lumbosacral discopathy, IVF | Lumbosacral discopathy/relaparotomy (Uterine atony) | 3350 | 10 |
| 5 | 28 | 2 | 2 | 0 | 39 | None | Marginal placenta praevia | 3830 | 10 |
| 6 | 30 | 1 | 1 | 0 | 38 | None | Marginal placenta praevia | 3270 | 10 |
| 7 | 36 | 2 | 2 | 1 | 34 | Myomectomy | Placenta increta | 2470 | 9 |
| 8 | 31 | 2 | 1 | 0 | 38 | None | Placenta praevia | 3430 | 10 |
| 9 | 32 | 3 | 2 | 1 | 33 | None | Placenta praevia, 1 previous CS | 2130 | 9 |
| 10 | 35 | 3 | 3 | 2 | 37 | Hypothyroidism | PROM, 2 previous CS | 2880 | 10 |
| 11 | 29 | 3 | 2 | 1 | 31 | Cholecystectomy | Placenta praevia, antepartum hemorrhage | 1660 | 8 |
| 12 | 40 | 2 | 2 | 1 | 40 | None | Ophthalmic, 2 previous CS | 3660 | 10 |
| Patient | CS Insicion | Anesthesia | Hysterectomy | Operation Time (min) | Preoperative Hb (g/dL) | Postoperative Hb (g/dL) | Blood Loss (mL) | PRBC (n) | FFP (n) | Length of Stay (Days) | Histopathological Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Midline vertical incision | SA | Total | 109 | 10.0 | 10.8 * | 400 | 2 | 0 | 5 | Placenta percreta |
| 2 | Midline vertical incision | SA | Total | 86 | 12.8 | 8.4 | 1200 | 0 | 0 | 5 | Placenta increta |
| 3 | Low transverse cesarean section | SA/GA (relaparotomy) | Total | 111 | 10,6 | 5.4 | 2000 | 5 | 2 | 4 | Placenta accreta |
| 4 | Low transverse cesarean section | SA/GA (relaparotomy) | Total | 71 | 12.5 | 5.5 | 2100 | 6 | 2 | 6 | Placenta increta |
| 5 | Low transverse cesarean section | SA | Total | 86 | 14.3 | 12.0 | 800 | 2 | 0 | 4 | Placenta accreta |
| 6 | Low transverse cesarean section | SA | Total | 113 | 13.5 | 9.6 | 1300 | 2 | 2 | 4 | Placenta accreta |
| 7 | Low transverse cesarean section | SA | Total | 80 | 12.6 | 9.7 | 650 | 0 | 0 | l0 * | Placenta increta |
| 8 | Low transverse cesarean section | GA | Total | 89 | 10.8 | 4.9 | 2500 | 3 | 2 | 5 | Placenta increta |
| 9 | Midline vertical incision | GA | Total | 110 | 12.0 | 9.3 | 1200 | 2 | 1 | 16 * | Placenta accreta |
| 10 | Low transverse cesarean section | SA | Total | 77 | 15.2 | 10.9 | 2600 | 0 | 0 | 2 | Placenta increta |
| 11 | Midline vertical incision | GA | Total | 78 | 12.3 | 8.2 | 900 | 3 | 2 | 3 | Placenta increta |
| 12 | Low transverse cesarean section | SA | Subtotal | 58 | 10.7 | 9.9 | 600 | 0 | 0 | 3 | Placenta accreta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniczek, J.; Manasar-Dyrbuś, M.; Winkowska, E.; Skowronek, K.; Stojko, R. Foley Catheter as a Tourniquet for Hemorrhage Prevention during Peripartum Hysterectomy in Patients with Placenta Accreta Spectrum (PAS)—A Hospital-Based Study. Life 2023, 13, 1774. https://doi.org/10.3390/life13081774
Staniczek J, Manasar-Dyrbuś M, Winkowska E, Skowronek K, Stojko R. Foley Catheter as a Tourniquet for Hemorrhage Prevention during Peripartum Hysterectomy in Patients with Placenta Accreta Spectrum (PAS)—A Hospital-Based Study. Life. 2023; 13(8):1774. https://doi.org/10.3390/life13081774
Chicago/Turabian StyleStaniczek, Jakub, Maisa Manasar-Dyrbuś, Ewa Winkowska, Kaja Skowronek, and Rafał Stojko. 2023. "Foley Catheter as a Tourniquet for Hemorrhage Prevention during Peripartum Hysterectomy in Patients with Placenta Accreta Spectrum (PAS)—A Hospital-Based Study" Life 13, no. 8: 1774. https://doi.org/10.3390/life13081774
APA StyleStaniczek, J., Manasar-Dyrbuś, M., Winkowska, E., Skowronek, K., & Stojko, R. (2023). Foley Catheter as a Tourniquet for Hemorrhage Prevention during Peripartum Hysterectomy in Patients with Placenta Accreta Spectrum (PAS)—A Hospital-Based Study. Life, 13(8), 1774. https://doi.org/10.3390/life13081774






