Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. BP Measurements
2.3. Total Kidney Volume (TKV)
2.4. Urine and Blood Sample Collection
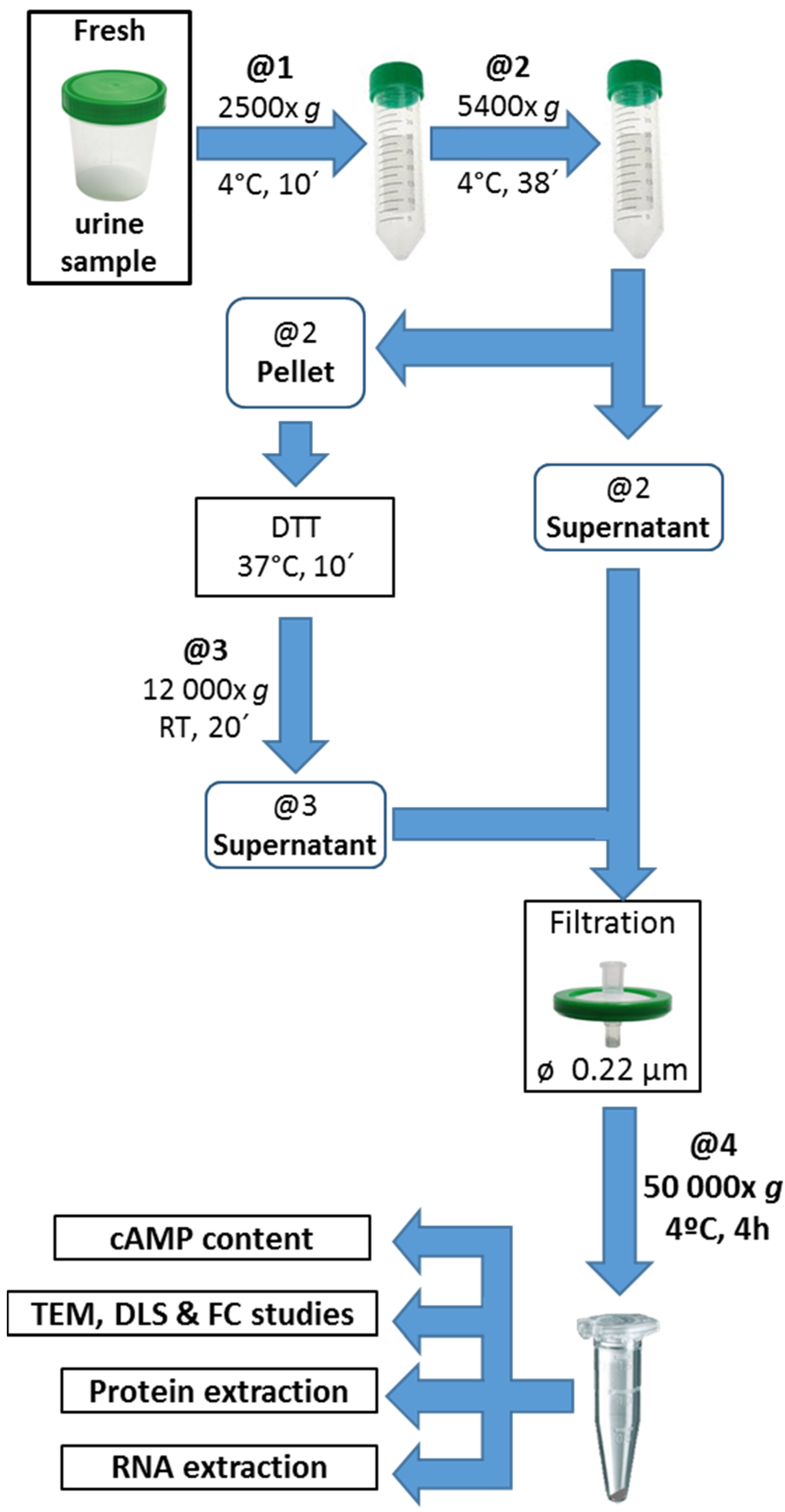
2.5. Urine EV Isolation
2.6. EV Characterization
2.7. Creatinine, Total Protein, and Albumin (UAE) Determination
2.8. Estimation of GFR
2.9. cAMP Determination
2.10. Statistical Procedures
3. Results
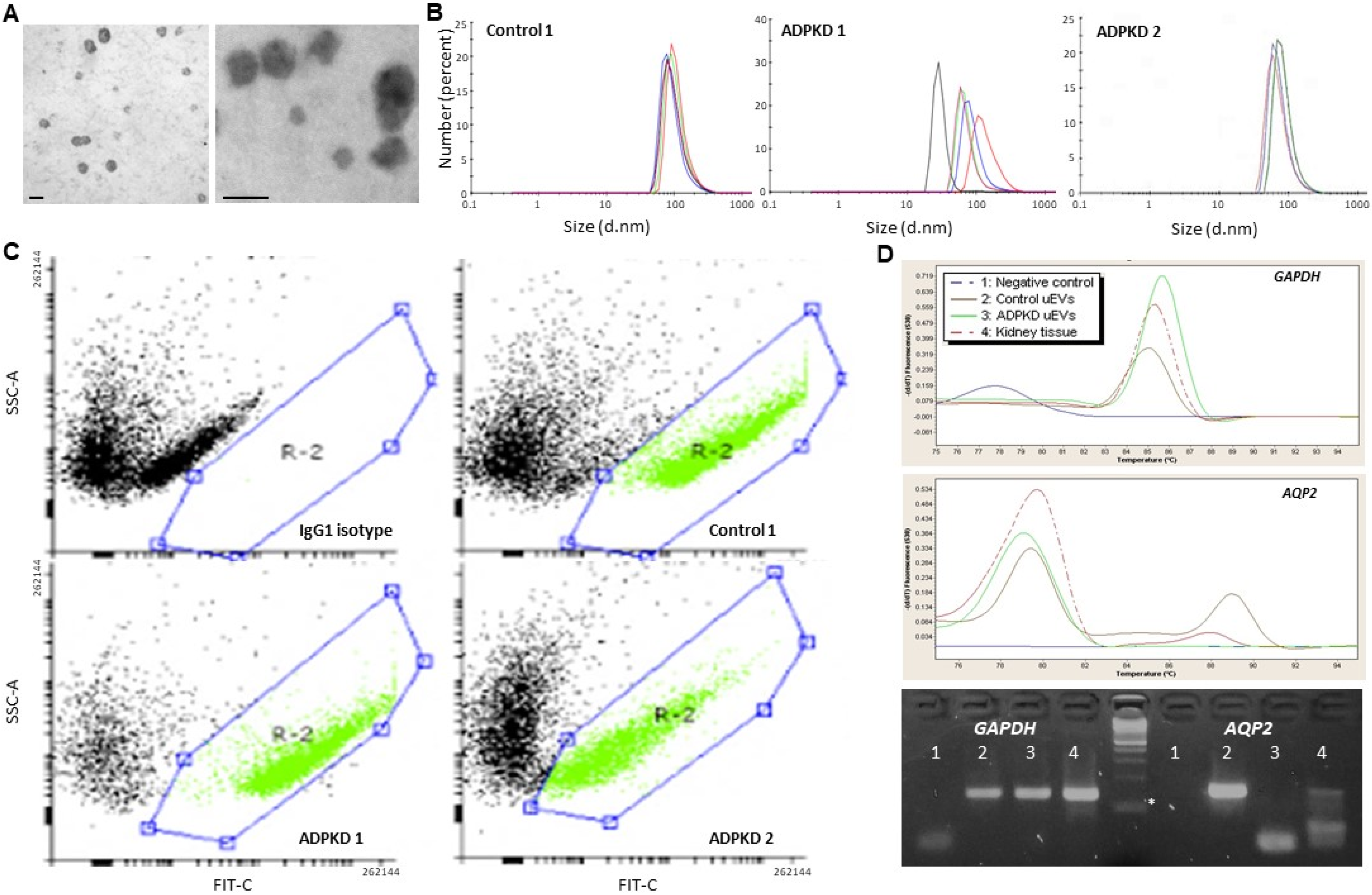
3.1. Urine EV Characterization
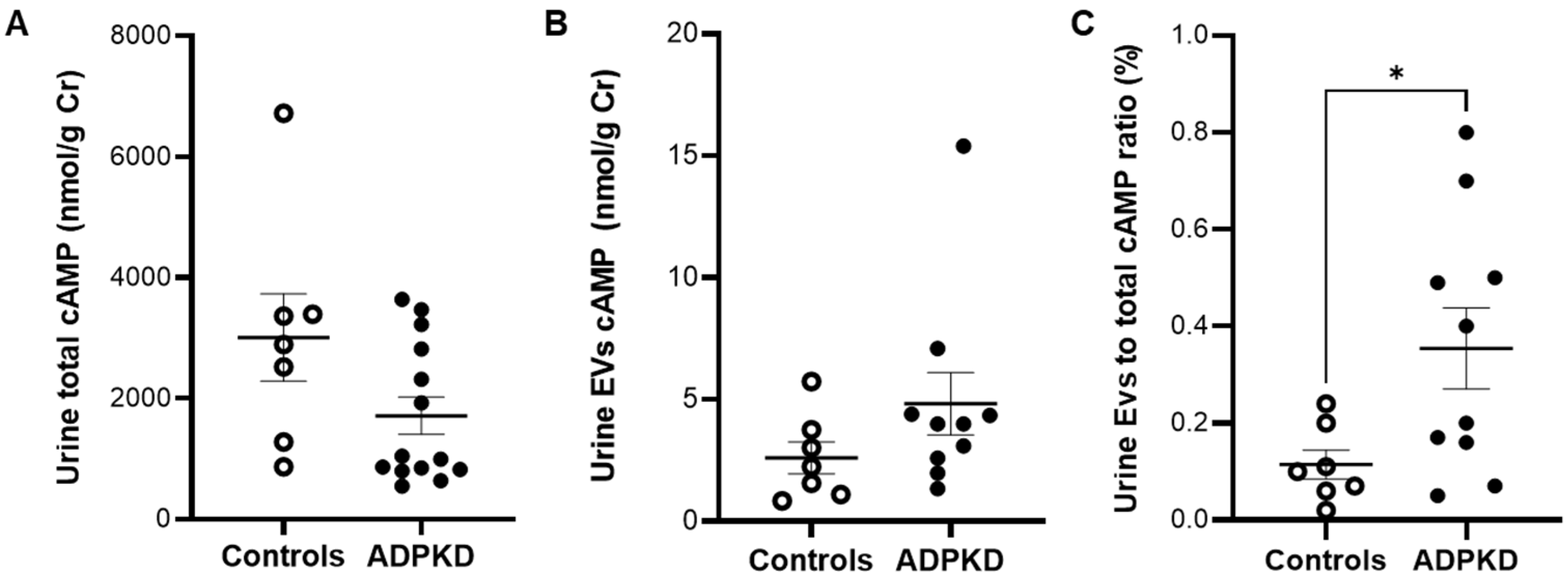
3.2. Urine cAMP in ADPKD and Controls
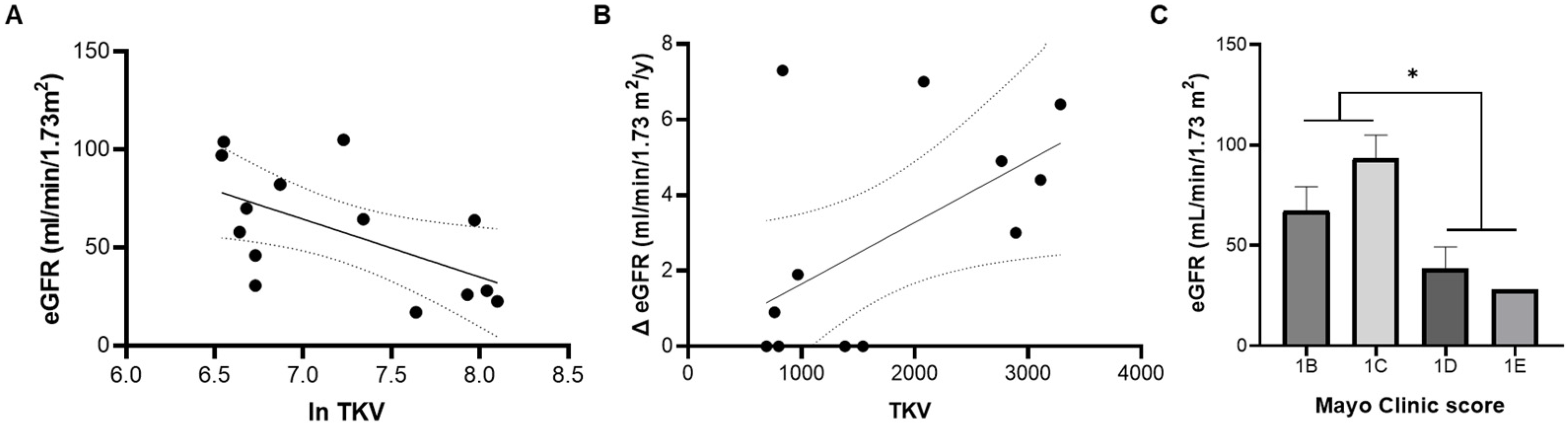
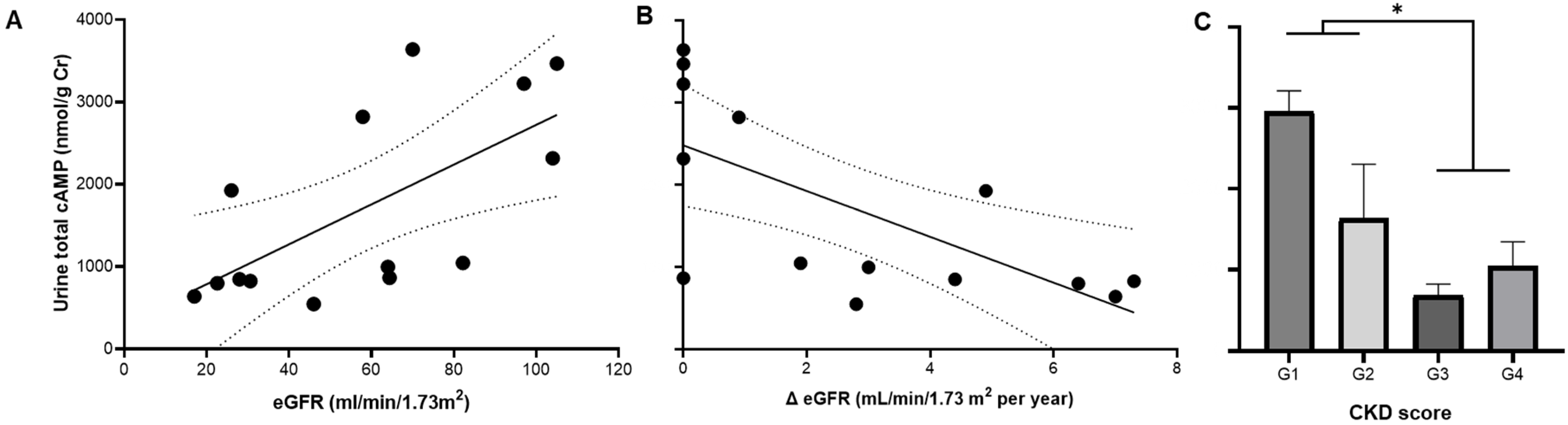
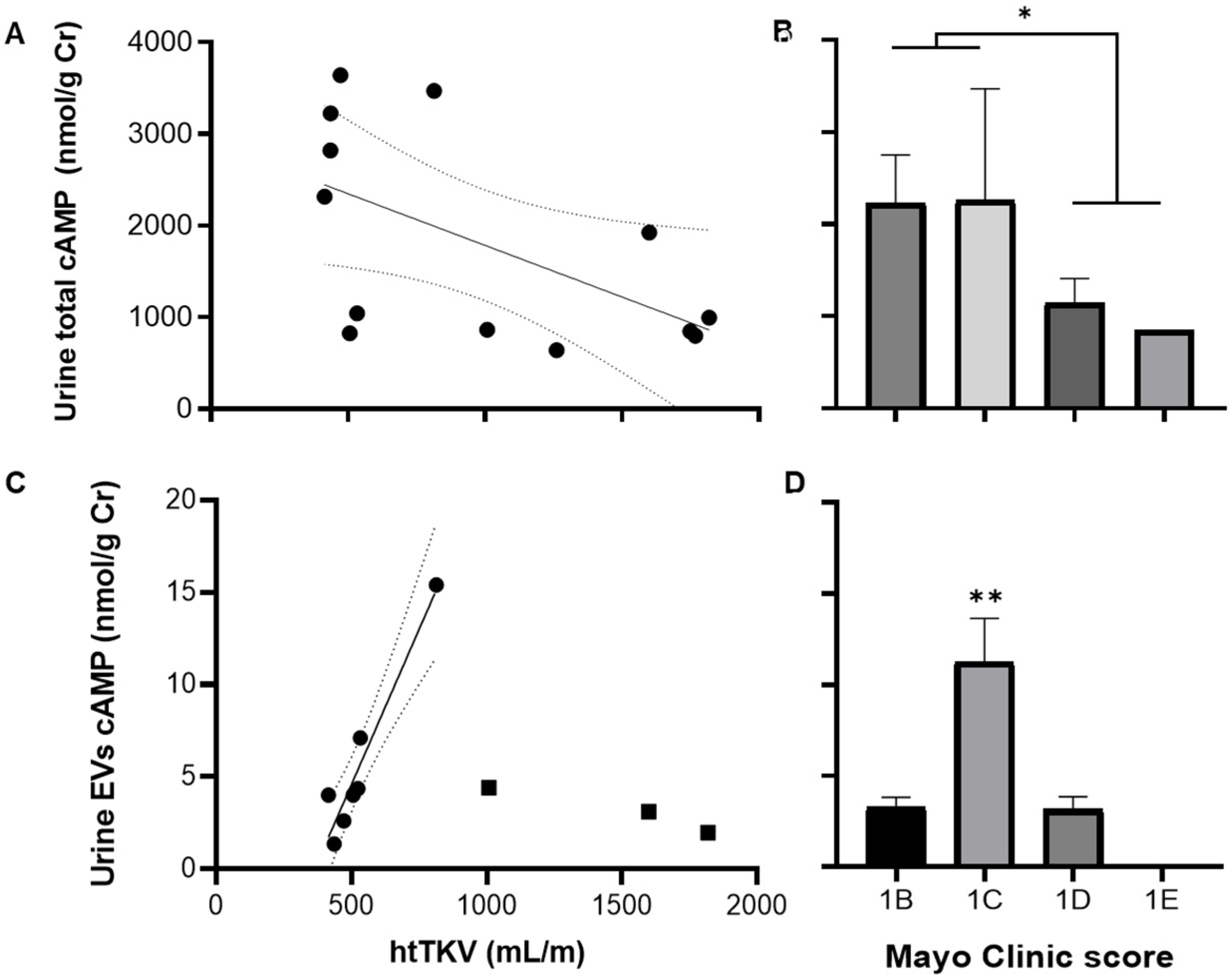
3.3. Total and EV cAMP Content in ADPKD Progression
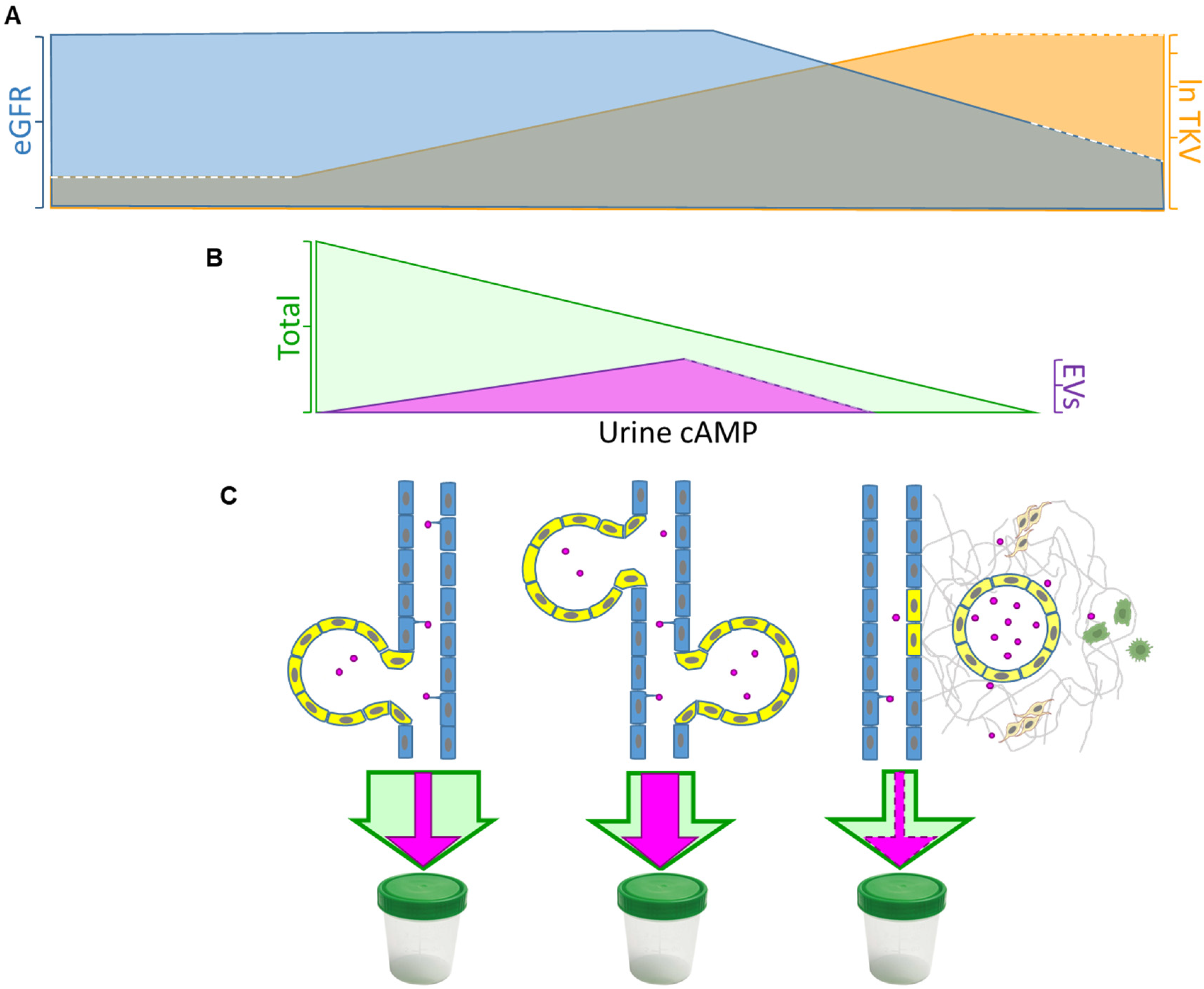
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grantham, J.J.; Mulamalla, S.; Swenson-Fields, K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2011, 7, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Franz, K.A.; Reubi, F.C. Rate of functional deterioration in polycystic kidney disease. Kidney Int. 1983, 23, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Tazón-Vega, B.; Vilardell, M.; Pérez-Oller, L.; Ars, E.; Ruiz, P.; Devuyst, O.; Lens, X.; Fernández-Llama, P.; Ballarín, J.; Torra, R. Study of candidate genes affecting the progression of renal disease in autosomal dominant polycystic kidney disease type 1. Nephrol. Dial. Transplant 2007, 22, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.; Watanabe, E.H.; Onuchic, L.F. Polycystins and Molecular Basis of Autosomal Dominant Polycystic Kidney Disease. In Polycystic Kidney Disease; Li, X., Ed.; Codon Publications: Brisbane, Australia, 2015; pp. 139–167. ISBN 978-0-9944381-0-2. [Google Scholar]
- Torres, V.E.; Harris, P.C. Strategies Targeting cAMP Signaling in the Treatment of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2014, 25, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Belibi, F.A.; Reif, G.; Wallace, D.P.; Yamaguchi, T.; Olsen, L.; Li, H.; Helmkamp, G.M.; Grantham, J.J. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004, 66, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.P. Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Wang, X.; Qian, Q.; Somlo, S.; Harris, P.C.; Gattone, V.H. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat. Med. 2004, 10, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Gattone, V.H.; Maser, R.L.; Tian, C.; Rosenberg, J.M.; Branden, M.G. Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev. Genet. 1999, 24, 309–318. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015, 26, 160–172. [Google Scholar] [CrossRef]
- Jackson, E.K.; Raghvendra, D.K. The Extracellular Cyclic AMP-Adenosine Pathway in Renal Physiology. Annu. Rev. Physiol. 2004, 66, 571–599. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Azurmendi, P.J.; Fraga, A.R.; Galan, F.M.; Kotliar, C.; Arrizurieta, E.E.; Valdez, M.G.; Forcada, P.J.; Stefan, J.S.S.; Martin, R.S. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol. Dial. Transplant. 2009, 24, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.F.; Mazzuoccolo, L.D.; Oddo, E.M.; Iscoff, P.V.; Muchnik, C.; Neumann, H.P.H.; Martin, R.S.; Fraga, A.R.; Azurmendi, P.J. Co-Inheritance of Autosomal Dominant Polycystic Kidney Disease and Naevoid Basal Cell Carcinoma Syndrome: Effects on Renal Progression. Nephron 2018, 140, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ravine, D.; Sheffield, L.; Danks, D.M.; Gibson, R.N.; Walker, R.G.; Kincaid-Smith, P. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994, 343, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef] [PubMed]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.T.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Li, Y.-J.; Hu, X.-B.; Huang, S.; Xiang, D.-X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef]
- Balseiro, C.A.; Bruchhausen, A.; Gayone, E.; Granada, M.; Pascual-Winter, F.; Serquis, A.; Usaj, G. NANO 2014. In Proceedings of the XIV Encuentro de Superficies y Materiales Nanoestructurados, San Carlos de Bariloche, Argentina, 14–16 May 2014; Abstract #P56. p. 47. [Google Scholar]
- Martinez, M.; Romano, M.; Martinez, A.; González, A.; Muchnik, C.; Stengel, F.; Mazzuoccolo, L.; Azurmendi, P. Nevoid Basal Cell Carcinoma Syndrome: PTCH1 Mutation Profile and Expression of Genes Involved in the Hedgehog Pathway in Argentinian Patients. Cells 2019, 8, 144. [Google Scholar] [CrossRef]
- Supplements, K.I. Chapter 2: Definition, identification, and prediction of CKD progression. In KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 63–72. [Google Scholar]
- Davio, C.A.; Cricco, G.P.; Maria Bergoc, R.; Rivera, E.S. H1 and H2 Histamine Receptors in N-Nitroso-N-Methylurea (NMU)-Induced Carcinomas with Atypical Coupling to Signal Transducers. Biochem. Pharmacol. 1995, 50, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mangoo-Karim, R.; Uchic, M.; Lechene, C.; Grantham, J.J. Renal epithelial cyst formation and enlargement in vitro: Dependence on cAMP. Proc. Natl. Acad. Sci. USA 1989, 86, 6007–6011. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.S.; Reif, G.A.; Nivens, E.; White, C.; Wallace, D.P. Calmodulin-sensitive adenylyl cyclases mediate AVP-dependent cAMP production and Cl− secretion by human autosomal dominant polycystic kidney cells. Am. J. Physiol. Physiol. 2012, 303, F1412–F1424. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.S.; Raman, A.; Reif, G.A.; Magenheimer, B.S.; White, C.; Calvet, J.P.; Wallace, D.P. Phosphodiesterase Isoform Regulation of Cell Proliferation and Fluid Secretion in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Kakeshita, K.; Koike, T.; Imamura, T.; Fujioka, H.; Yamazaki, H.; Kinugawa, K. Prognostic impact of urine cyclic AMP levels in patients with chronic kidney disease. Clin. Exp. Nephrol. 2022, 26, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Kakeshita, K.; Koike, T.; Imamura, T.; Fujioka, H.; Yamazaki, H.; Kinugawa, K. Altered arginine vasopressin-cyclic AMP-aquaporin 2 pathway in patients with chronic kidney disease. Clin. Exp. Nephrol. 2022, 26, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Barash, I.; Ponda, M.P.; Goldfarb, D.S.; Skolnik, E.Y. A Pilot Clinical Study to Evaluate Changes in Urine Osmolality and Urine cAMP in Response to Acute and Chronic Water Loading in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 693–697. [Google Scholar] [CrossRef]
- Graffe, C.C.; Bech, J.N.; Lauridsen, T.G.; Pedersen, E.B. Urinary excretion of AQP2 and ENaC in autosomal dominant polycystic kidney disease during basal conditions and after a hypertonic saline infusion. Am. J. Physiol. Physiol. 2012, 302, F917–F927. [Google Scholar] [CrossRef]
- Hallows, K.R.; Althouse, A.D.; Li, H.; Saitta, B.; Abebe, K.Z.; Bae, K.T.; Miskulin, D.C.; Perrone, R.D.; Seliger, S.L.; Watnick, T.J. Association of Baseline Urinary Metabolic Biomarkers with ADPKD Severity in TAME-PKD Clinical Trial Participants. Kidney360 2021, 2, 795–808. [Google Scholar] [CrossRef]
- Messchendorp, A.L.; Spithoven, E.M.; Casteleijn, N.F.; Dam, W.A.; van den Born, J.; Tonnis, W.F.; Gaillard, C.A.J.M.; Meijer, E. Association of plasma somatostatin with disease severity and progression in patients with autosomal dominant polycystic kidney disease. BMC Nephrol. 2018, 19, 368. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Lu, Y.; Kawano, H.; Horie, S.; Muto, S. Association of arginine vasopressin surrogate marker urinary copeptin with severity of autosomal dominant polycystic kidney disease (ADPKD). Clin. Exp. Nephrol. 2015, 19, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Rigor & Standardization Subcommittee; Urine Task Force (R). Program and abstract book uEV2023 Virtual symposium on Urinary Extracellular Vesicles. In Proceedings of the 2nd Virtual Symposium on Urinary Extracellular Vesicles, International Society of Extracellular Vesicles-ISEV), Internet, 16 February 2023; p. 21. [Google Scholar]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.M.; Bastos, A.P.; Amaral, A.G.; Sousa, M.F.; Souza, L.E.; Malheiros, D.M.; Piontek, K.; Irigoyen, M.C.; Watnick, T.J.; Onuchic, L.F. Renal cyst growth is the main determinant for hypertension and concentrating deficit in Pkd1 -deficient mice. Kidney Int. 2014, 85, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.M.; Lefkimmiatis, K. Extracellular calcium and cAMP: Second messengers as ‘third messengers’? Physiology 2007, 22, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, A.; Scruggs, A.K.; Leavesley, S.J.; Annamdevula, N.; George, A.H.; Britain, A.L.; Francis, C.M.; Knighten, J.M.; Rich, T.C.; Bauer, N.N. Extracellular vesicle-induced cyclic AMP signaling. Cell. Signal. 2022, 95, 110348. [Google Scholar] [CrossRef] [PubMed]
- Sayner, S.L.; Choi, C.-S.; Maulucci, M.E.; Ramila, K.C.; Zhou, C.; Scruggs, A.K.; Yarbrough, T.; Blair, L.A.; King, J.A.; Seifert, R.; et al. Extracellular vesicles: Another compartment for the second messenger, cyclic adenosine monophosphate. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L691–L700. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T.; Zhou, W.; Shen, C.; Landsittel, D.P.; Wu, Z.; Tao, C.; Chapman, A.B.; Torres, V.E.; Yu, A.S.L.; Mrug, M.; et al. Growth pattern of kidney cyst number and volume in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 823–833. [Google Scholar] [CrossRef]
- Ding, H.; Li, L.X.; Harris, P.C.; Yang, J.; Li, X. Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat. Commun. 2021, 12, 4548. [Google Scholar] [CrossRef]
- Grantham, J.J.; Cook, L.T.; Torres, V.E.; Bost, J.E.; Chapman, B.; Harris, P.C.; Guay-Woodford, L.M.; Bae, K.T. Determinants of Renal Volume in Autosomal-Dominant Polycystic Kidney Disease. Kidney Int. 2008, 73, 108–116. [Google Scholar] [CrossRef]
- Mikkaichi, T.; Suzuki, T.; Onogawa, T.; Tanemoto, M.; Mizutamari, H.; Okada, M.; Chaki, T.; Masuda, S.; Tokui, T.; Eto, N.; et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc. Natl. Acad. Sci. USA 2004, 101, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.-L.; Zhu, H.; McNamara, P.J.; Leggas, M. Localization and Functional Characterization of the Rat Oatp4c1 Transporter in an In Vitro Cell System and Rat Tissues. PLoS ONE 2012, 7, e39641. [Google Scholar] [CrossRef] [PubMed]
- Pablo, J.; Colavita, M.; Santiago Todaro, J.; de Sousa, M.; May, M.; Gómez, N.; Yaneff, A.; Di Siervi, N.; Aguirre, V.; Guijas, C.; et al. Multidrug resistance protein 4 (MRP4/ABCC4) is overexpressed in clear cell renal cell carcinoma (ccRCC) and is essential to regulate cell proliferation. Int. J. Biol. Macromol. 2020, 161, 836–847. [Google Scholar] [CrossRef]
- Sassi, Y.; Abi-Gerges, A.; Fauconnier, J.; Mougenot, N.; Reiken, S.; Haghighi, K.; Kranias, E.G.; Marks, A.R.; Lacampagne, A.; Engelhardt, S.; et al. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes; Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. Res. Commun. 2012, 26, 1009–1017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenberg, M.L.; Yaneff, A.; Ferradás, G.M.; Villafañe Tapia, M.P.; Davio, C.A.; Goette, N.P.; Vlachovsky, S.G.; Peroni, R.N.; Oddo, E.M.; Azurmendi, P.J. Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression. Life 2023, 13, 1817. https://doi.org/10.3390/life13091817
Rosenberg ML, Yaneff A, Ferradás GM, Villafañe Tapia MP, Davio CA, Goette NP, Vlachovsky SG, Peroni RN, Oddo EM, Azurmendi PJ. Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression. Life. 2023; 13(9):1817. https://doi.org/10.3390/life13091817
Chicago/Turabian StyleRosenberg, María Lucía, Agustín Yaneff, Gonzalo Manuel Ferradás, Margarita Paz Villafañe Tapia, Carlos Alberto Davio, Nora Paula Goette, Sandra Gabriela Vlachovsky, Roxana Noemí Peroni, Elisabet Mónica Oddo, and Pablo Javier Azurmendi. 2023. "Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression" Life 13, no. 9: 1817. https://doi.org/10.3390/life13091817
APA StyleRosenberg, M. L., Yaneff, A., Ferradás, G. M., Villafañe Tapia, M. P., Davio, C. A., Goette, N. P., Vlachovsky, S. G., Peroni, R. N., Oddo, E. M., & Azurmendi, P. J. (2023). Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression. Life, 13(9), 1817. https://doi.org/10.3390/life13091817





