AST and ALT APRI Scores and Dysglycemia in Saudi Arabia: A Retrospective Population Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Statistics
3. Results
3.1. Baseline Characteristics
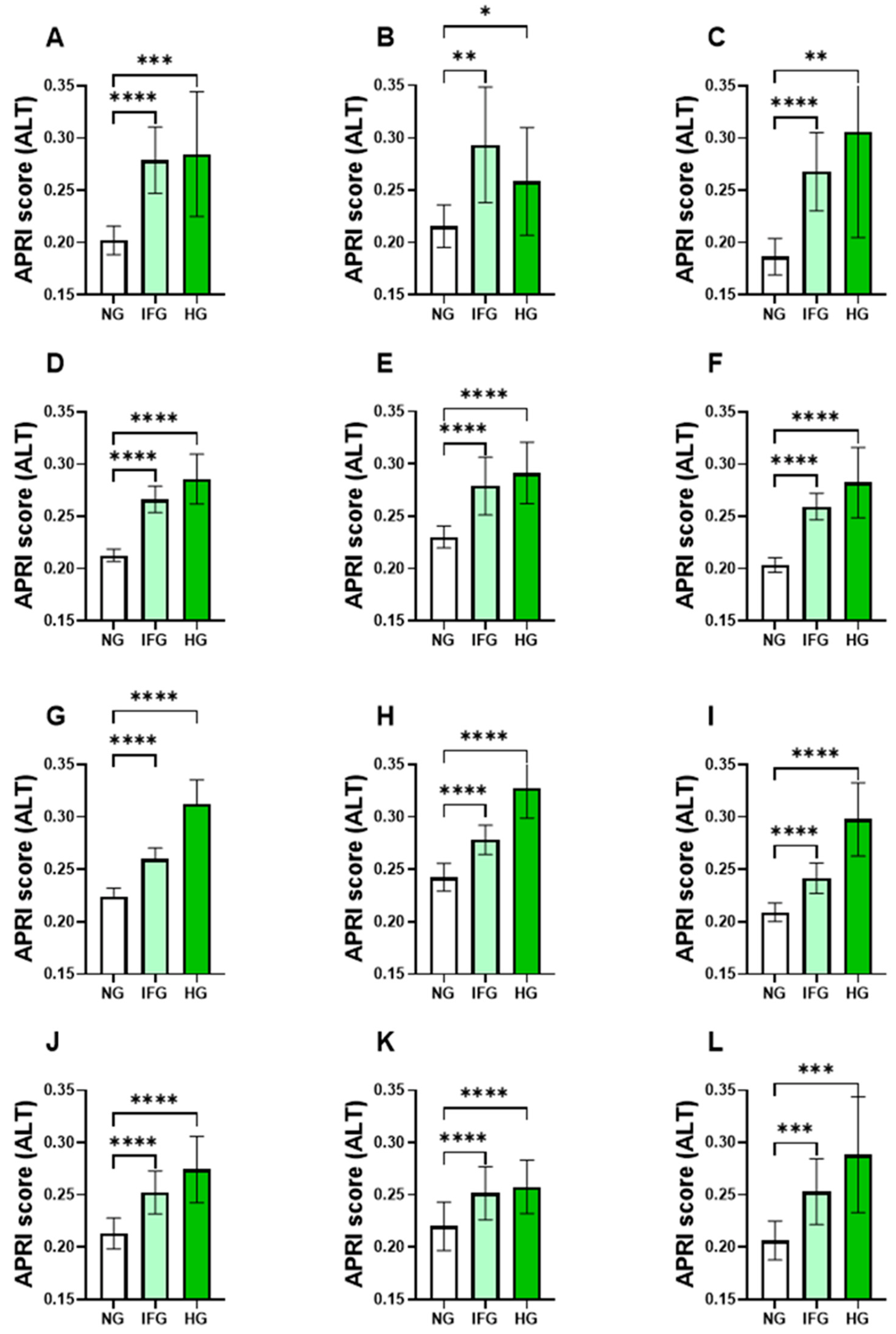
3.2. Levels of APRI Scores Were Elevated in the IFG and HG Groups
3.3. FBG Is Significantly Elevated in Subjects with High APRI Score
3.4. AST but Not ALT APRI Score Follows Distinct Age- and Gender-Specific Patterns
3.5. Prevalence of Hyperglycemia in High APRI Score
3.6. Association of APRI Scores with FBG Concentration
3.7. Risk Assessment of APRI Score in Light of Hyperglycemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100, 153952. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Stu. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, B.; Elnaggar, R.K.; Alshehri, M.M.; Khunti, K.; Alenazi, A. National and regional prevalence rates of diabetes in Saudi Arabia: Analysis of national survey data. Int. J. Diabetes Dev. Ctries. 2023, 43, 392–397. [Google Scholar] [CrossRef]
- Tolman, K.G.; Fonseca, V.; Dalpiaz, A.; Tan, M.H. Spectrum of Liver Disease in Type 2 Diabetes and Management of Patients With Diabetes and Liver Disease. Diabetes Care 2007, 30, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Targher, G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: Spotlight on nonalcoholic fatty liver disease. Ann. Transl. Med. 2017, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- Coman, L.I.; Coman, O.A.; Bădărău, I.A.; Păunescu, H.; Ciocîrlan, M. Association between Liver Cirrhosis and Diabetes Mellitus: A Review on Hepatic Outcomes. J. Clin. Med. 2021, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, K.; Tavares, C.D.J.; Rines, A.K.; Puigserver, P. Molecular Pathophysiology of Hepatic Glucose Production. Mol. Aspects Med. 2015, 46, 21. [Google Scholar] [CrossRef] [PubMed]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Van Beek, J.H.D.A.; De Moor, M.H.M.; De Geus, E.J.C.; Lubke, G.H.; Vink, J.M.; Willemsen, G.; Boomsma, D.I. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav. Genet. 2013, 43, 329–339. [Google Scholar] [CrossRef]
- Noroozi Karimabad, M.; Khalili, P.; Ayoobi, F.; Esmaeili-Nadimi, A.; La Vecchia, C.; Jamali, Z. Serum liver enzymes and diabetes from the Rafsanjan cohort study. BMC Endocr. Disord. 2022, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, C.Q.; Schooling, C.M.; Zhang, W.S.; Cheng, K.K.; Lam, T.H. Liver enzymes and incident diabetes in China: A prospective analysis of 10 764 participants in the Guangzhou Biobank Cohort Study. J. Epidemiol. Community Health 2015, 69, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Jalili, V.; Poorahmadi, Z.; Hasanpour Ardekanizadeh, N.; Gholamalizadeh, M.; Ajami, M.; Houshiarrad, A.; Hajipour, A.; Shafie, F.; Alizadeh, A.; Mokhtari, Z.; et al. The association between obesity with serum levels of liver enzymes, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma-glutamyl transferase in adult women. Endocrinol. Diabetes Metab. 2022, 5, e367. [Google Scholar] [CrossRef]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. Alanine Aminotransferase, γ-Glutamyltransferase, and Incident DiabetesThe British Women’s Heart and Health Study and meta-analysis. Diabetes Care 2009, 32, 741–750. [Google Scholar] [CrossRef]
- Monami, M.; Bardini, G.; Lamanna, C.; Pala, L.; Cresci, B.; Francesconi, P.; Buiatti, E.; Rotella, C.M.; Mannucci, E. Liver enzymes and risk of diabetes and cardiovascular disease: Results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism 2008, 57, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; Massaro, J.M.; Vasan, R.S.; D’Agostino, R.B.; Ellison, R.C.; Fox, C.S. Aminotransferase Levels and 20-Year Risk of Metabolic Syndrome, Diabetes, and Cardiovascular Disease. Gastroenterology 2008, 135, 1935–1944. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Roberts, C.K.; Crespi, C.M.; Zhang, Z.F. Elevated liver enzymes in individuals with undiagnosed diabetes in the U.S. J. Diabetes Complications 2013, 27, 333–339. [Google Scholar] [CrossRef]
- Kwon, S.S.; Lee, S.G. A high alanine aminotransferase/aspartate aminotransferase ratio determines insulin resistance and metabolically healthy/unhealthy obesity in a general adult population in korea: The korean national health and nutritional examination survey 2007–2010. Exp. Clin. Endocrinol. Diabetes 2019, 127, 677–684. [Google Scholar] [CrossRef]
- Alzahrani, S.H.; Baig, M.; Bashawri, J.I.; Aashi, M.M.; Shaibi, F.K.; Alqarni, D.A. Prevalence and Association of Elevated Liver Transaminases in Type 2 Diabetes Mellitus Patients in Jeddah, Saudi Arabia. Cureus 2019, 11, 2–9. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Şaşmaz, M.İ.; Ayvaz, M.A.; Dülger, A.C.; Kuday Kaykısız, E.K.; Güven, R. Aspartate-aminotransferase to platelet ratio index score for predicting HELLP syndrome. Am. J. Emerg. Med. 2020, 38, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Eyisoy, Ö.G.; Taşdemir, Ü.; Eriç Özdemir, M.; Eyisoy, S.; Kahramanoğlu, Ö.; Öçal, A.; Demirci, O. Aspartate aminotransferase to platelet ratio index (APRI) score: Is it useful in patients with intrahepatic cholestasis of pregnancy? J. Matern. Fetal. Neonatal Med. 2022, 35, 10137–10142. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, C.; Li, W.; Huang, Y.; Chen, L. The gamma-glutamyl transpeptidase to platelet ratio for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B and non-alcoholic fatty liver disease. Oncotarget 2017, 8, 28641. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Q.; Li, J.; Liao, Y.; Chen, Q.; Liao, W.J.; Huang, J. The preoperative alkaline phosphatase-to-platelet ratio index is an independent prognostic factor for hepatocellular carcinoma after hepatic resection. Medicine 2016, 95, e5734. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alsughayyir, J.; Basudan, A.M.; Alsubki, R.; Alqahtani, S.; Awan, Z.A.; Algethami, M.R.; Al-Sheikh, Y.A. Monocyte–Lymphocyte Ratio and Dysglycemia: A Retrospective, Cross-Sectional Study of the Saudi Population. Healthcare 2022, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alsughayyir, J.; Basudan, A.; Ghneim, H.K.; Aboul-Soud, M.A.M.; Marie, M.; Dera, A.; Alfaifi, M.; Alkhathami, A.G.; Awan, Z.A.; et al. Isolated and Combined Effect of Age and Gender on Neutrophil–Lymphocyte Ratio in the Hyperglycemic Saudi Population. Medicina 2022, 58, 1040. [Google Scholar] [CrossRef]
- De Matteis, C.; Cariello, M.; Graziano, G.; Battaglia, S.; Suppressa, P.; Piazzolla, G.; Sabbà, C.; Moschetta, A. AST to Platelet Ratio Index (APRI) is an easy-to-use predictor score for cardiovascular risk in metabolic subjects. Sci. Rep. 2021, 11, 14834. [Google Scholar] [CrossRef]
- Bussler, S.; Vogel, M.; Pietzner, D.; Harms, K.; Buzek, T.; Penke, M.; Händel, N.; Körner, A.; Baumann, U.; Kiess, W.; et al. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase): Effects of age, sex, body mass index, and pubertal stage. Hepatology 2018, 68, 1319–1330. [Google Scholar] [CrossRef]
- Corbin, K.D.; Pittas, A.G.; Desouza, C.; Grdinovac, K.K.; Herzig, K.H.; Kashyap, S.R.; Kim, S.H.; Nelson, J.; Rasouli, N.; Vickery, E.M.; et al. Indices of hepatic steatosis and fibrosis in prediabetes and association with diabetes development in the vitamin D and type 2 diabetes study. J. Diabetes Complicat. 2023, 37, 108475. [Google Scholar] [CrossRef]
- Drazilova, S.; Janicko, M.; Skladany, L.; Kristian, P.; Oltman, M.; Szantova, M.; Krkoska, D.; Mazuchova, E.; Piesecka, L.; Vahalova, V.; et al. Glucose Metabolism Changes in Patients with Chronic Hepatitis C Treated with Direct Acting Antivirals. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6095097. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Parrinello, C.M.; Rudolph, B.J.; Lazo, M.; Gallo, L.C.; Thyagarajan, B.; Cotler, S.J.; Qi, Q.; Seeherunvong, T.; Vidot, D.C.; Strickler, H.D.; et al. Associations of Insulin Resistance and Glycemia with Liver Enzymes in Hispanic/Latino Youths. J. Clin. Gastroenterol. 2019, 53, E46–E53. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Apekey, T.A.; Walley, J. Liver aminotransferases and risk of incident type 2 diabetes: A systematic review and meta-analysis. Am. J. Epidemiol. 2013, 178, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Guo, D.H.; Xu, H.Y.; Tang, S.T.; Wang, X.X.; Jin, Y.P.; Wang, P. Association of liver enzymes levels with fasting plasma glucose levels in Southern China: A cross-sectional study. BMJ Open 2019, 9, e025524. [Google Scholar] [CrossRef] [PubMed]
- Vozarova, B.; Stefan, N.; Lindsay, R.S.; Saremi, A.; Pratley, R.E.; Bogardus, C.; Antonio Tataranni, P. High Alanine Aminotransferase Is Associated With Decreased Hepatic Insulin Sensitivity and Predicts the Development of Type 2 Diabetes. Diabetes 2002, 51, 1889–1895. [Google Scholar] [CrossRef]
- Sung, K.C.; Jeong, W.S.; Wild, S.H.; Byrne, C.D. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012, 35, 717–722. [Google Scholar] [CrossRef]
- Gómez-Sámano, M.T.; Cuevas-Ramos, D.; Mehta, R.; Brau-Figueroa, H.; Meza-Arana, C.E.; Gulias-Herrero, A. Association of Alanine Aminotransferase Levels (ALT) with the Hepatic Insulin Resistance Index (HIRI): A cross-sectional study. BMC Endocr. Disord. 2012, 12, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Hong, J.; Chao, M.; Gu, W.; Wang, W.; Ning, G. Positive correlations of liver enzymes with metabolic syndrome including insulin resistance in newly diagnosed type 2 diabetes mellitus. Endocrine 2010, 38, 181–187. [Google Scholar] [CrossRef]
- Hanley, A.J.G.; Williams, K.; Festa, A.; Wagenknecht, L.E.; D’Agostino, R.B.; Kempf, J.; Zinman, B.; Haffner, S.M.; D’Agostino, R.B.; Kempf, J.; et al. Elevations in Markers of Liver Injury and Risk of Type 2 Diabetes. Diabetes 2004, 53, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Rahman, S.; Haque, T.; Sumon, A.H.; Ahmed, A.M.; Ali, N. Prevalence of elevated liver enzymes and its association with type 2 diabetes: A cross-sectional study in Bangladeshi adults. Endocrinol. Diabetes Metab. 2020, 3, e00116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Han, X.; Hu, H.; Wang, F.; Yu, C.; Yuan, J.; Yao, P.; Li, X.; Yang, K.; et al. Serum alanine transaminase levels predict type 2 diabetes risk among a middle-aged and elderly Chinese population. Ann. Hepatol. 2019, 18, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Higgins, T.; Cembrowski, G.S. Limiting the testing of AST: A diagnostically nonspecific enzyme. Am. J. Clin. Pathol. 2015, 144, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Quirós Cognuck, S.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; de Paula, F.J.A.; Rodrigues Franci, C.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs. Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.J.; Eiras, S.; Piñeiro, R.; López-Otero, D.; Gallego, R.; Fernández, A.L.; Lago, F.; González-Juanatey, J.R. Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. Findings in patients undergoing cardiac surgery. Rev. Esp. Cardiol. 2006, 59, 1252–1260. [Google Scholar] [CrossRef]
- Tu, T.; Chen, J.; Chen, L.; Stiles, B.L. Dual-specific protein and lipid phosphatase pten and its biological functions. Cold Spring Harb. Perspect. Med. 2020, 10, a036301. [Google Scholar] [CrossRef]
- Ishihara, H.; Sasaoka, T.; Kagawa, S.; Murakami, S.; Fukui, K.; Kawagishi, Y.; Yamazaki, K.; Sato, A.; Iwata, M.; Urakaze, M.; et al. Association of the polymorphisms in the 5′-untranslated region of PTEN gene with type 2 diabetes in a Japanese population. FEBS Lett. 2003, 554, 450–454. [Google Scholar] [CrossRef]
- Samaan, M.C.; Anand, S.S.; Sharma, A.M.; Samjoo, I.A.; Tarnopolsky, M.A. Sex differences in skeletal muscle Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) levels: A cross-sectional study. Sci. Rep. 2015, 5, 9154. [Google Scholar] [CrossRef]



| Gender | No. of Subjects (%) |
|---|---|
| Male | 5928 (41.81) |
| Young | 432 (3.05) |
| Young adults | 2358 (16.63) |
| Adults | 2580 (18.20) |
| Elderlies | 558 (3.94) |
| Female | 8210 (57.91) |
| Young | 427 (3.01) |
| Young adults | 4304 (30.36) |
| Adults | 2855 (20.14) |
| Elderlies | 624 (4.40) |
| Unknown | 39 (0.28) |
| Characteristic | NG (n = 9079) | IFG (n = 3310) | HG (n = 1788) | p Value |
|---|---|---|---|---|
| Age (years) | 40.14 (±0.16) | 41.19 (±0.28) | 43.15 (±0.41) | <0.0001 |
| Male (%) | 41.44 | 42.36 | 42.73 | |
| Female (%) | 58.31 | 57.40 | 56.82 | |
| WBC count (×106/μL) | 5.91 (±0.02) | 6.03 (±0.03) | 6.61 (±0.05) | <0.0001 |
| RBC count (×106/μL) | 5.24 (±0.01) | 5.37 (±0.01) | 5.35 (±0.01) | <0.0001 |
| Hemoglobin (g/dL) | 14.06 (±0.02) | 14.51 (±0.03) | 14.64 (±0.05) | <0.0001 |
| Platelet Count (×106/mL) | 281.9 (±0.78) | 270.8 (±1.27) | 259.8 (±1.77) | <0.0001 |
| AST (U/L) | 20.31 (±0.13) | 21.3 (±0.20) | 21.01 (±0.29) | <0.0001 |
| ALT (U/L) | 22.18 (±0.19) | 26.25 (±0.37) | 27.27 (±0.50) | <0.0001 |
| Total Bilirubin | 0.66 (±0.01) | 0.63 (±0.01) | 0.58 (±0.01) | <0.0001 |
| Albumin (g/dL) | 4.23 (±0.01) | 4.20 (±0.01) | 4.12 (±0.01) | <0.0001 |
| Creatinine (mg/dL) | 0.75 (±0.01) | 0.81 (±0.01) | 0.84 (±0.01) | <0.0001 |
| Parameter | NG | HG |
|---|---|---|
| Both | ||
| N-APRI score (AST) | 98.24 | 95.64 |
| H-APRI score (AST) | 1.76 | 4.36 |
| N-APRI score (ALT) | 93.75 | 89.65 |
| H-APRI score (ALT) | 6.25 | 10.35 |
| Male | ||
| N-APRI score (AST) | 97.88 | 95.16 |
| H-APRI score (AST) | 2.12 | 4.84 |
| N-APRI score (ALT) | 92.39 | 88.48 |
| H-APRI score (ALT) | 7.61 | 11.52 |
| Female | ||
| N-APRI score (AST) | 98.51 | 95.96 |
| H-APRI score (AST) | 1.49 | 4.04 |
| N-APRI score (ALT) | 94.77 | 90.65 |
| H-APRI score (ALT) | 5.23 | 9.35 |
| Score | 95% CI | z Statistic | Significance Level | ||
|---|---|---|---|---|---|
| APRI score (AST) | PR | ||||
| Both | 2.48 | 1.90–3.23 | 6.69 | p < 0.0001 | |
| Male | 2.28 | 1.56–3.33 | 4.24 | p < 0.0001 | |
| Female | 2.71 | 1.87–3.92 | 5.26 | p < 0.0001 | |
| OR | |||||
| Both | 2.55 | 1.93–3.35 | 6.65 | p < 0.0001 | |
| Male | 2.35 | 1.58–3.49 | 4.21 | p < 0.0001 | |
| Female | 2.78 | 1.89–4.08 | 5.23 | p < 0.0001 | |
| APRI score (ALT) | PR | ||||
| Both | 1.66 | 1.41–1.94 | 6.26 | p < 0.0001 | |
| Male | 1.51 | 1.21–1.90 | 3.59 | p = 0.0003 | |
| Female | 1.79 | 1.43–2.24 | 5.11 | p < 0.0001 | |
| OR | |||||
| Both | 1.73 | 1.46–2.06 | 6.18 | p < 0.0001 | |
| Male | 1.58 | 1.23–2.03 | 3.55 | p = 0.0004 | |
| Female | 1.87 | 1.47–2.39 | 5.04 | p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshuweishi, Y.; Alfaifi, M.; Almoghrabi, Y.; Alfhili, M.A. AST and ALT APRI Scores and Dysglycemia in Saudi Arabia: A Retrospective Population Study. Life 2023, 13, 1881. https://doi.org/10.3390/life13091881
Alshuweishi Y, Alfaifi M, Almoghrabi Y, Alfhili MA. AST and ALT APRI Scores and Dysglycemia in Saudi Arabia: A Retrospective Population Study. Life. 2023; 13(9):1881. https://doi.org/10.3390/life13091881
Chicago/Turabian StyleAlshuweishi, Yazeed, Mohammed Alfaifi, Yousef Almoghrabi, and Mohammad A. Alfhili. 2023. "AST and ALT APRI Scores and Dysglycemia in Saudi Arabia: A Retrospective Population Study" Life 13, no. 9: 1881. https://doi.org/10.3390/life13091881






