Performance of Deep-Learning Solutions on Lung Nodule Malignancy Classification: A Systematic Review
Abstract
:1. Background
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Synthesis
3. Results
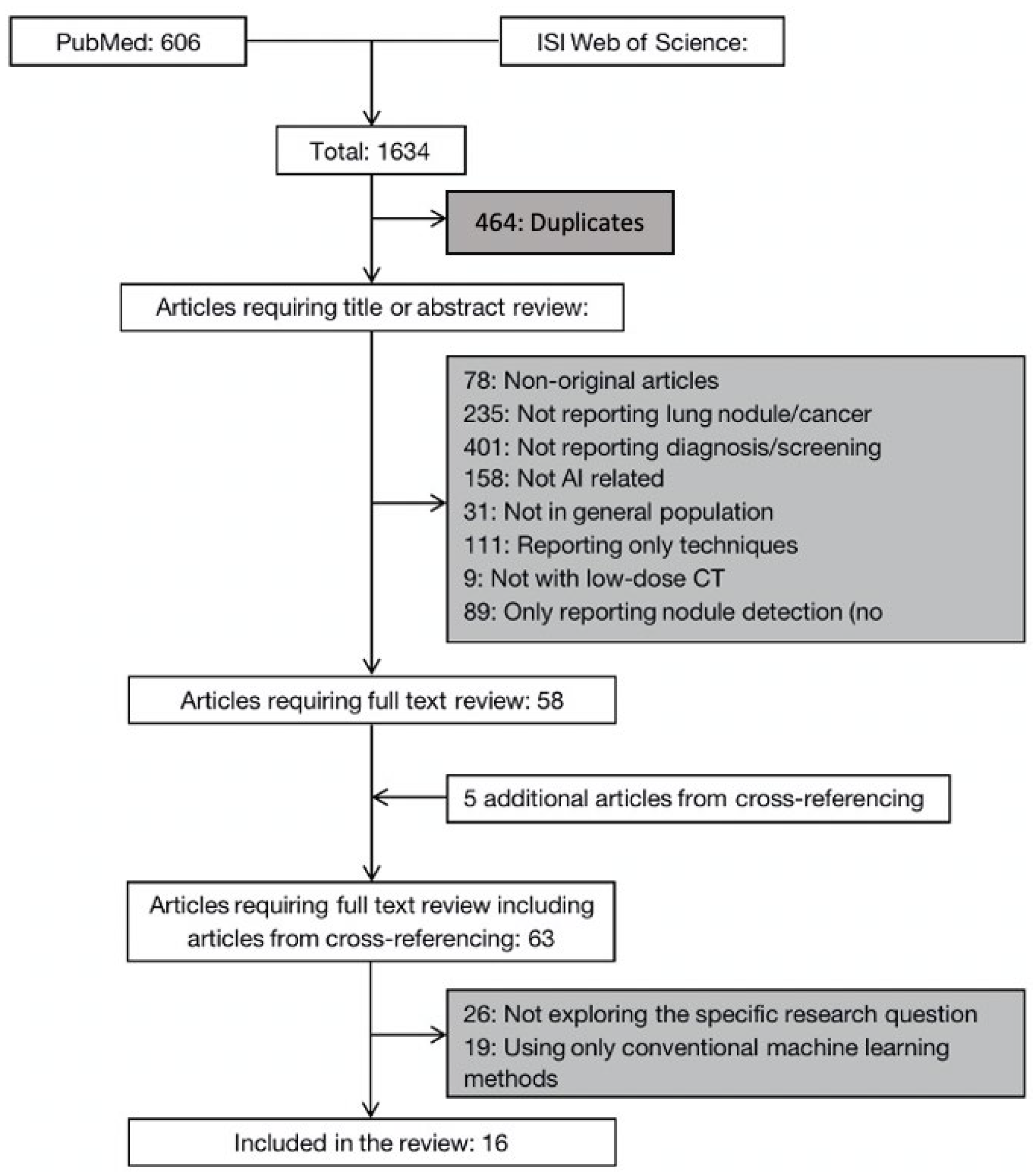
3.1. Literature Search Results
3.2. Deep-Learning Solutions on Lung Nodule Malignancy Classification
3.3. Convolutional Neural Network (CNN)
3.4. Autoencoder (AE)
3.5. Deep Belief Network (DBN)
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
Appendix A. Search Strategy
- (1)
- (CT or CAT or (computed and tomography)) OR ((CT or CAT or (computed and tomography)) and (scan or scanning or screen or screening)) OR ((spiral or helix or helical) and CT or CAT or (computed and tomography)) OR (((spiral or helix or helical) and CT or CAT or (computed and tomography)) and (scan or scanning or screen or screening))
- (2)
- (low and dose) or low-dose or LDCT or (ultralow and dose) or ultralow-dose or ULDCT
- (3)
- (artificial and intelligence) or AI or (computer and assisted) or computer-assisted or (neural and network) or (machine and learning) or (deep and learning)
- (4)
- ((lung or pulmonary or bronchial) AND (neoplasm or nodule or lesion or cancers or neoplasms or nodules or lesions or cancer or carcinoma or carcinomas))
- (5)
- 1 and 2 and 3 and 4
- (6)
- Limit 5 to English language
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. JNCI J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef]
- Aberle, D.; The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- Wender, R.; Fontham, E.T.H.; Barrera, E., Jr.; Colditz, G.; Church, T.R.; Ettinger, D.S.; Etzioni, R.; Flowers, C.R.; Gazelle, G.S.; Kelsey, D.K.; et al. American Cancer Society lung cancer screening guidelines. CA Cancer J. Clin. 2013, 63, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xu, D.; Liu, S.; Cham, M.D. The Added Value of Computer-aided Detection of Small Pulmonary Nodules and Missed Lung Cancers. J. Thorac. Imaging 2018, 33, 390–395. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, A.; Beache, G.M.; Gimel’Farb, G.; Suzuki, K.; Okada, K.; Elnakib, A.; Soliman, A.; Abdollahi, B. Computer-aided diagnosis systems for lung cancer: Challenges and methodologies. Int. J. Biomed. Imaging 2013, 2013, 942353. [Google Scholar] [CrossRef]
- Greenspan, H.; van Ginneken, B.; Summers, R.M. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Trans. Med. Imaging 2016, 35, 1153–1159. [Google Scholar] [CrossRef]
- Nishio, M.; Sugiyama, O.; Yakami, M.; Ueno, S.; Kubo, T.; Kuroda, T.; Togashi, K. Computer-aided diagnosis of lung nodule classification between benign nodule, primary lung cancer, and metastatic lung cancer at different image size using deep convolutional neural network with transfer learning. PLoS ONE 2018, 13, e0200721. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose. Chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, C.T.; Li, Y.; Tammemagi, M.C.; Brock, M.V.; Atkar-Khattra, S.; Xu, Y.; Hu, P.; Mayo, J.R.; Schmidt, H.; et al. Prediction of lung cancer risk at follow-up screening with low-dose CT: A training and validation study of a deep learning method. Lancet Digit. Health 2019, 1, e353–e362. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, F.; Zhou, Z.; Zhang, F.; Wang, Q.; Peng, Z.; Su, D.; Fan, Y.; Wang, Y. Deep Learning Based Effectiveness Evaluation of Chest CT Pulmonary Nodules Detection with Artificial Intelligence. Zhongguo Fei Ai Za Zhi 2019, 22, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Asuntha, A.; Srinivasan, A. Deep learning for lung Cancer detection and classification. Multimed. Tools Appl. 2020, 79, 7731–7762. [Google Scholar] [CrossRef]
- Lei, Y.; Tian, Y.; Shan, H.; Zhang, J.; Wang, G.; Kalra, M.K. Shape and margin-aware lung nodule classification in low-dose CT images via soft activation mapping. Med. Image Anal. 2020, 60, 101628. [Google Scholar] [CrossRef]
- Ozdemir, O.; Russell, R.L.; Berlin, A.A. A 3D Probabilistic Deep Learning System for Detection and Diagnosis of Lung Cancer Using Low-Dose CT Scans. IEEE Trans. Med. Imaging 2020, 39, 1419–1429. [Google Scholar] [CrossRef]
- Paul, R.; Schabath, M.B.; Gillies, R.; Hall, L.O.; Goldgof, D.B. Hybrid models for lung nodule malignancy prediction utilizing convolutional neural network ensembles and clinical data. J. Med. Imaging 2020, 7, 024502. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Li, D.; Niu, J. Combining multi-scale feature fusion with multi-attribute grading, a CNN model for benign and malignant classification of pulmonary nodules. J. Digit. Imaging 2020, 33, 869–878. [Google Scholar] [CrossRef]
- Hua, K.-L.; Hsu, C.-H.; Hidayati, S.C.; Cheng, W.-H.; Chen, Y.-J. Computer-aided classification of lung nodules on computed tomography images via deep learning technique. Onco Targets Ther. 2015, 8, 2015–2022. [Google Scholar]
- Liu, K.; Kang, G. Multiview Convolutional Neural Networks for Lung Nodule Classification. Imaging Syst. Technol. 2017, 27, 12–22. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, J.; Xia, Y.; Michael, F.; Zhang, Y. Fusing texture, shape and deep model-learned information at decision level for automated classification of lung nodules on chest CT. Inf. Fusion 2018, 42, 102–110. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Yang, F.; Yu, D.; Dong, D.; Yang, C.; Zang, Y.; Tian, J. Multi-crop Convolutional Neural Networks for lung nodule malignancy suspiciousness classification. Pattern Recognit. 2017, 61, 663–673. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, Y.; Zhang, J.; Song, Y.; Feng, D.; Fulham, M.; Cai, W. Knowledge-based Collaborative Deep Learning for Benign-Malignant Lung Nodule Classification on Chest CT. IEEE Trans. Med. Imaging 2019, 38, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, B.; Qian, W. Automatic Feature Learning Using Multichannel ROI Based on Deep Structured Algorithms for Computerized Lung Cancer Diagnosis. Comput. Biol. Med. 2017, 89, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Ling, S.H. Using Multi-level Convolutional Neural Network for Classification of Lung Nodules on CT images. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 686–689. [Google Scholar]
- Li, S.; Xu, P.; Li, B.; Chen, L.; Zhou, Z.; Hao, H.; Duan, Y.; Folkert, M.; Ma, J.; Huang, S.; et al. Predicting lung nodule malignancies by combining deep convolutional neural network and handcrafted features. Phys. Med. Biol. 2019, 64, 175012. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, N.; Suzuki, K. Comparing two classes of end-to-end machine-learning models in lung nodule detection and classification: MTANNs vs. CNNs. Pattern Recognit. 2017, 63, 476–486. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, L.; Luo, X.; Dou, X. Using Deep Learning for Classification of Lung Nodules on Computed Tomography Images. J. Healthc. Eng. 2017, 2017, 8314740. [Google Scholar] [CrossRef]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the Dimensionality of Data with Neural Networks. Science 2006, 313, 504–507. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, B.F.; Chao, X.Y.; Mao, F. Sentiment analysis of product reviews based on contrastive divergence-restricted Boltzmann machine deep learning. J. Comput. Appl. 2016, 36, 1045–1049. [Google Scholar]
- Liu, H.; Rashid, T.; Ware, J.; Jensen, P.; Austin, T.; Nasrallah, I.; Bryan, R.; Heckbert, S.; Habes, M. Adaptive Squeeze-and-Shrink Image Denoising for Improving Deep Detection of Cerebral Microbleeds. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2021, Proceedings of the 24th International Conference, Strasbourg, France, 27 September–1 October 2021; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Wang, J.; Lin, L.; Zhao, S.; Summary, X.W.; Wu, S. Review, Research progress of deep learning-based CT image detection and classification of pulmonary nodules. J. Biomed. Eng. 2019, 36, 670–676. [Google Scholar]
- Wang, H.; Li, Y.; Liu, S.; Yue, X. Design Computer-Aided Diagnosis System Based on Chest CT Evaluation of Pulmonary Nodules. Comput. Math. Methods Med. 2022, 2022, 7729524. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Fang, X. The clinical application and research progress of CT lung nodule detection based on artificial intelligence are reviewed. Chin. J. Radiol. 2019, 53, 522–525. (In Chinese) [Google Scholar]
- Taye, M.M. Understanding of Machine Learning with Deep Learning: Architectures, Workflow, Applications and Future Directions. Computers 2023, 12, 91. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, J.; Wang, Z.; Zhang, K.; Zhang, L.; Sun, Q. Deep learning for image-based cancer detection and diagnosis—A survey. Pattern Recognit. 2018, 83, 134–149. [Google Scholar] [CrossRef]
- Thanoon, M.A.; Zulkifley, M.A.; Mohd Zainuri, M.A.A.; Abdani, S.R. A Review of Deep Learning Techniques for Lung Cancer Screening and Diagnosis Based on CT Images. Diagnostics 2023, 13, 2617. [Google Scholar] [CrossRef]
- Wang, S.; Yang, D.M.; Rong, R.; Zhan, X.; Xiao, G. Pathology Image Analysis Using Segmentation Deep Learning Algorithms. Am. J. Pathol. 2019, 189, 1686–1698. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- EL-Bana, S.; Al-Kabbany, A.; Sharkas, M. A Two-Stage Framework for Automated Malignant Pulmonary Nodule Detection in CT Scans. Diagnostics 2020, 10, 131. [Google Scholar] [CrossRef]
| Author (Year) | Country | Method (System Structure) | Dimension | Data Set | Effects Performance | Theme | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Location | Size | AUC | Accuracy | Sensitivity | Specificity | Diag. or Predc.? | ||||
| Nishio (2018) [10] | Japan | DCNN Classification: deep CNN modified via VGG-16 CNN | 2D | Hospital Patients data | Japan | 412 benign nodules 571 primary lung cancers 253 metastatic lung cancers | Image of 56,112,224, the best accuracies were 66.7%, 64.7%, 68.0%. | Lung nodule classification | |||
| Ardila (2019) [11] | US | Deep-learningCNN Cancer risk prediction: a deep-learning algorithm that uses a patient’s current and prior CT volumes | 3D | NLST | US | 42,290 CT cases from 14,851 patients, training set (70%), tuning set (15%), test set (15%) | AUC of 94.4% (95% confidence interval, 91.1–97.3) in 1 year | End-to-end lung cancer screening | |||
| Huang (2019) [12] | US | Deep-learningalgorithm Cancer incidence prediction at 1–3 years: compare accuracy of DeepLR scores and volume doubling time | / | NLST PanCan | US | Training set: 25,097 participants from NLST Validation set:2294 individuals from PanCan | AUC of 0.968 (SD 0.013) with 1-year AUC of 0.946 (SD 0.013) with 2-year AUC of 0.899 (SD 0.017) with 3-year | Prediction of lung cancer risk | |||
| Li (2019) [13] | China | DCNN and handcrafted features Propose fusion algorithm that combines handcrafted features into the features learned at the output layer of a 3D deep CNN | 3D | LIDC IDRI | US | 431 malignant nodules 795 benign nodules | AUC of 0.9303, accuracy of 88.58%, sensitivity of 82.60%, specificity of 91.82% | Predicting Nodule Malignancy | |||
| Asuntha (2020) [14] | India | FPSOCNN Feature extraction:HoG, wavelet transform-based features, LBP, SIFT, Zernike Moment Classification: use 7 methods, the best if FPSOCNN | / | Arthi Scan Hospital Data | India | 1000 malignant nodules | Accuracy of 94.97%, Sensitivity of 96.68%, Specificity of 95.89% | Lung cancer classification | |||
| Lei (2020) [15] | China | HESAM:classify nodules through shape and margin Features extraction: SAM to enable LNSM feature analysis with CNN; HESAM to localize LNSM features. | LIDC IDRI | US | 510 malignant nodules 635 benign nodules | Accuracy of 99.13%, Sensitivity of 0.9705, Specificity of 0.9921 | Lung nodule classification | ||||
| Ozdemir (2020) [16] | US | CNN Classification: MIL framework to train malignant classification network | 3D | LIDC-IDRI Kaggle stage-2 LUNA16 | US | LUNA16: 888 patients + 1186 annotated nodules Kaggle stage-2: 153 malignant + 353 benign nodules | AUC of 0.87 | Lung cancer Diagnosis | |||
| Paul (2020) [17] | US | CNN ensembles Malignant prediction made a hybrid model using an ensemble with CNN models of clinical and size information to enhance malignancy prediction. | 2D | COCO NLST | US | 82 malignant nodules 152 benign nodules | AUC of 0.9, accuracy of 83.12% | lung nodule malignancy prediction | |||
| Zhao (2020) [18] | China | MSMT Classification: A multi-stream multi-task network | 3D | LIDC-IDRI | US | 450 malignant nodules 554 benign nodules | AUC of 0.979, Accuracy of 93.92%, Sensitivity of 92.60%, Specificity | benign and malignant classification of nodules | |||
| Hua (2015) [19] | China (Taiwan) | DBN Classification: Deep Belief Network and CNN to classify | 2D | LIDC | US | 2545 nodules from 1010 scans | Malignant nodules: sensitivity of 73.4% specificity of 82.2% | classification of lung nodules | |||
| Liu (2017) [20] | China | MV-CNN Classification: Multi-view Convolutional Neural Networks, it takes multiple views of each entered nodule | 2D | LIDC-IDRI | US | 96 patients 3540 malignant nodules 764 benign nodules | AUC of 0.981, Sensitivity of 0.9049, Specificity of 0.9991 | Lung Nodule Classification | |||
| Xie (2018) [21] | China/Australia | Features Extraction: co-occurrence matrix, Fourier shape descriptor and deep CNN Classification: AdaBoosted back propagation neural network | 2D | LIDC-IDRI | US | The first data set contains 1324 benign and 648 malignant; the second contains 2021 benign and 648 malignant; the third contains 1324 benign and 1345 malignant. | AUCs of 0.9665, 0.9445, and 0.8124 for three data sets, Accuracies of 89.53%, 87.74%, 71.93% for three data sets Malignant nodules: Sensitivities of 84.19%, 81.11%, and 59.22% with specificity of 92.02%, 89.67%, and 84.85% for three data sets | Classification of lung nodules | |||
| Shen (2017) [22] | China/US | Classification: multi-crop CNN | 2D, 3D | LIDC-IDRI | US | 880 benign nodules 495 malignant nodules 1243 uncertain nodules | AUC of 0.93 Accuracy of 87.14% | Lung nodule malignancy suspiciousness classification | |||
| Xie (2019) [23] | China/Australia | MC-CNN Classification: Multi-view knowledge-based collaborative (MV-KBC) deep model to separate malignant from benign nodules | 3D | LIDC-IDRI | US | 644 malignant nodules 1301 benign nodules | AUC of 0.957 Accuracy of 91.76% | Benign-Malignant Lung Nodule Classification | |||
| Sun (2017) [24] | China | DBA and SDAE Classification: LeCun CNN, deep belief network, and stacked denoising autoencoder (SDAE) | 2D | LIDC | US | 1018 scans (41,372 benign nodules and 47,576 malignant nodules) | AUC of 0.899 ± 0.018 via CNN 0.852 ± 0.025 via SDAE | Computerized Lung Cancer Diagnosis | |||
| Lyu (2018) [25] | China Australia | ML-CNN Classification: use multi-level convolutional neural network | 2D | LIDC-IDRI | US | 1018 cases from 1010 patients | Accuracy 84.81% | Lung Nodules Classification | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Hu, M.; Ma, Y.; Yang, L.; Chen, J.; Lou, L.; Chen, C.; Xiao, Y. Performance of Deep-Learning Solutions on Lung Nodule Malignancy Classification: A Systematic Review. Life 2023, 13, 1911. https://doi.org/10.3390/life13091911
Liang H, Hu M, Ma Y, Yang L, Chen J, Lou L, Chen C, Xiao Y. Performance of Deep-Learning Solutions on Lung Nodule Malignancy Classification: A Systematic Review. Life. 2023; 13(9):1911. https://doi.org/10.3390/life13091911
Chicago/Turabian StyleLiang, Hailun, Meili Hu, Yuxin Ma, Lei Yang, Jie Chen, Liwei Lou, Chen Chen, and Yuan Xiao. 2023. "Performance of Deep-Learning Solutions on Lung Nodule Malignancy Classification: A Systematic Review" Life 13, no. 9: 1911. https://doi.org/10.3390/life13091911






