Understanding the Mode of Action of a Micro-Immunotherapy Formulation: Pre-Clinical Evidence from the Study of 2LEBV® Active Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Items and Experimental Controls
2.2. Evaluation of the Phagocytosis Capabilities of Human Granulocytes
2.3. Assessment of the Cytokine-Secretion Profile in Peripheral Blood Mononuclear Cells
2.4. Evaluation of the Intracellular Expression of IL-2 and the Viability of Human Peripheral Blood Mononuclear Cells
2.5. Evaluation of the Expression of Human Leukocyte Antigen-II
2.5.1. In HUVEC Cells
2.5.2. In Human Peripheral Blood Mononuclear Cell-Derived M1-Macrophages
2.6. Statistical Analysis
3. Results
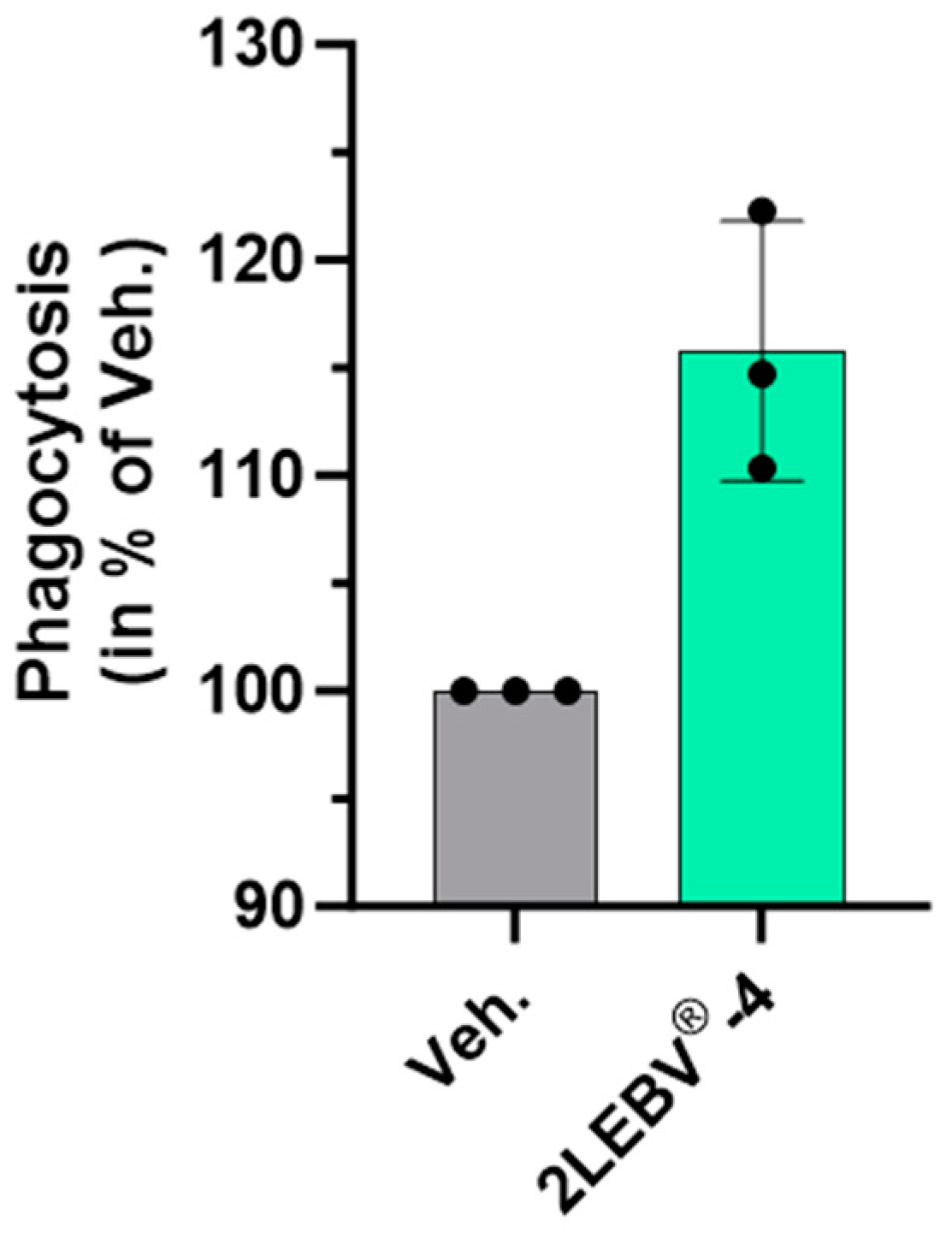
3.1. Actives from 2LEBV® Enhance the Phagocytosis Capabilities of Human Granulocytes
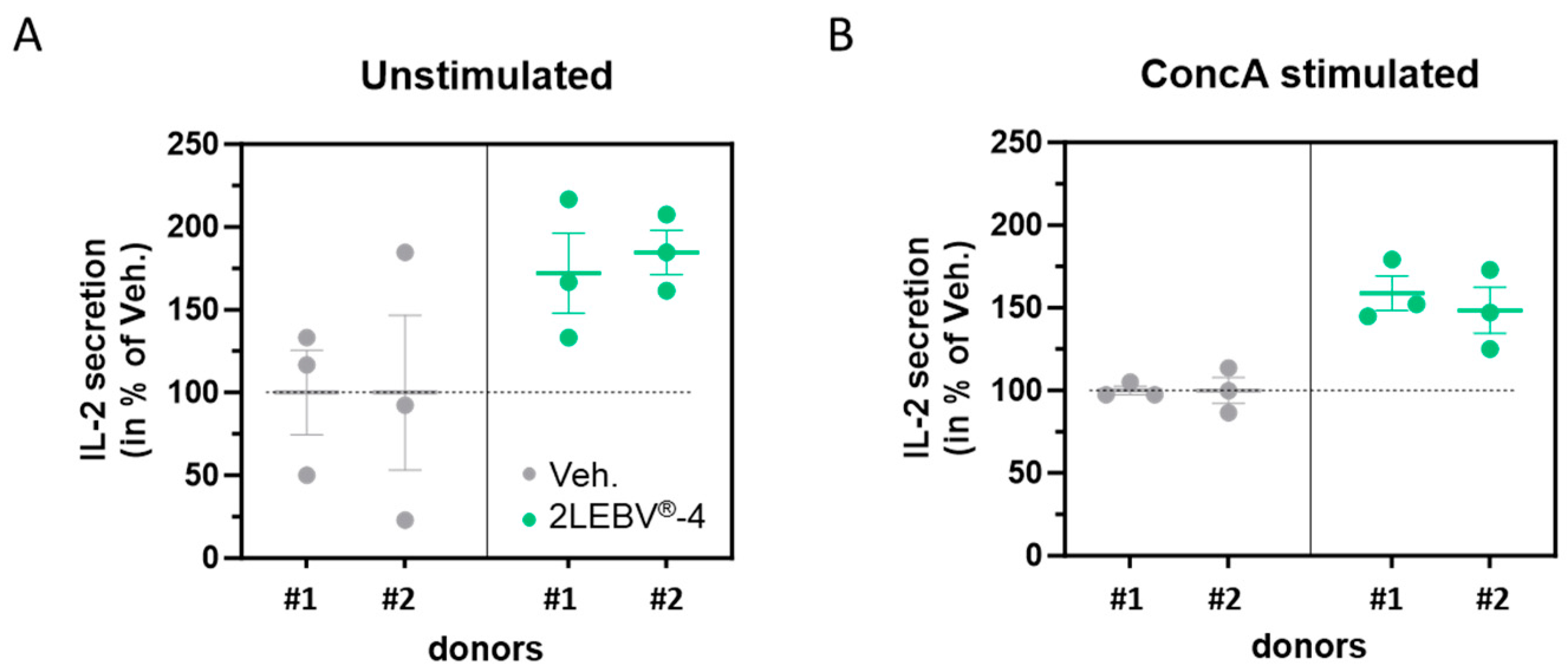
3.2. Actives from 2LEBV® Enhance the Secretion of Interleukin-2 in Unstimulated and Concanavalin A-Stimulated Human Peripheral Blood Mononuclear Cells
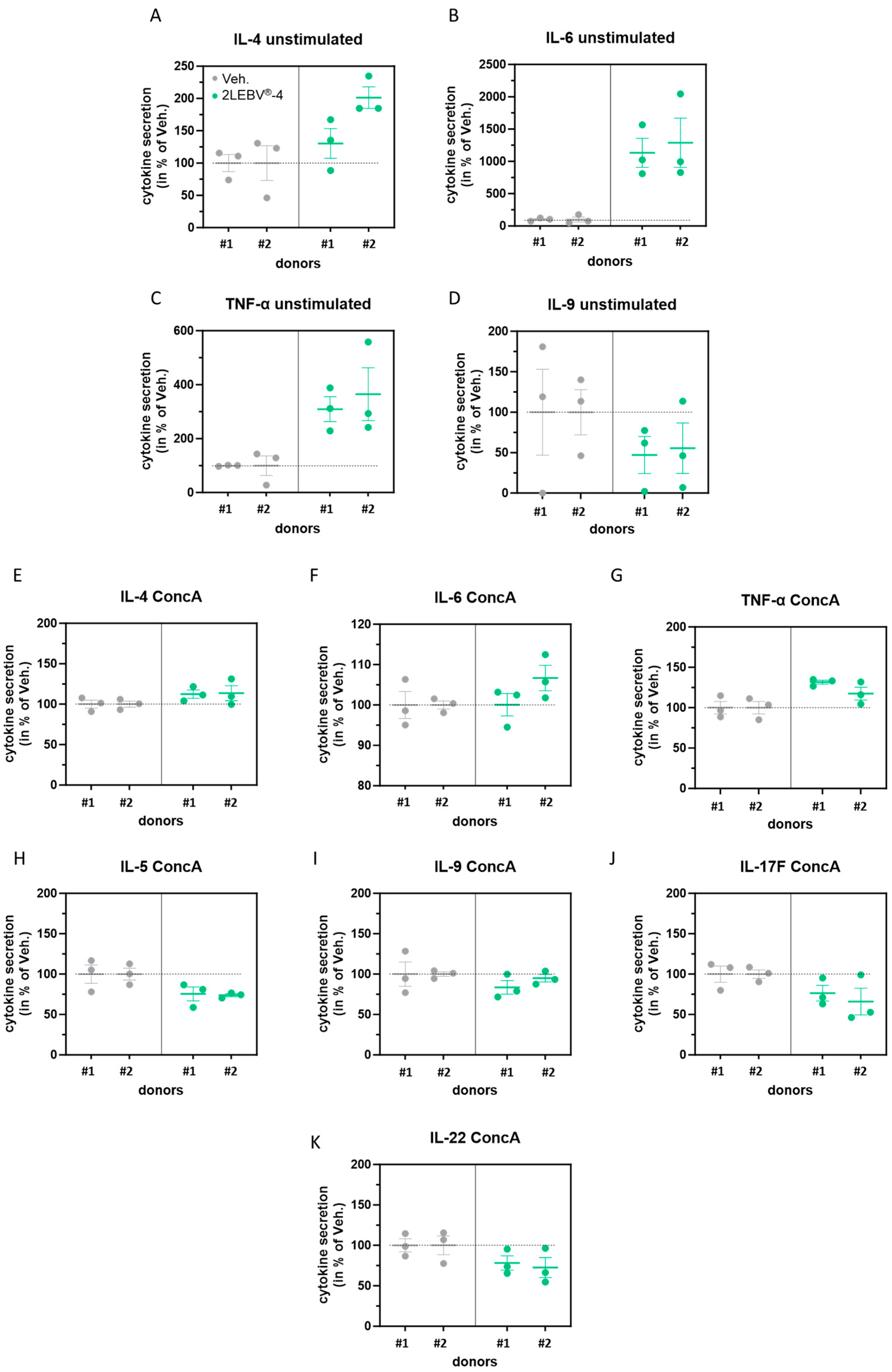
3.3. Actives from 2LEBV® Modulate the Secretion of Several Cytokines in Unstimulated and Concanavalin A-Stimulated Human Peripheral Blood Mononuclear Cells
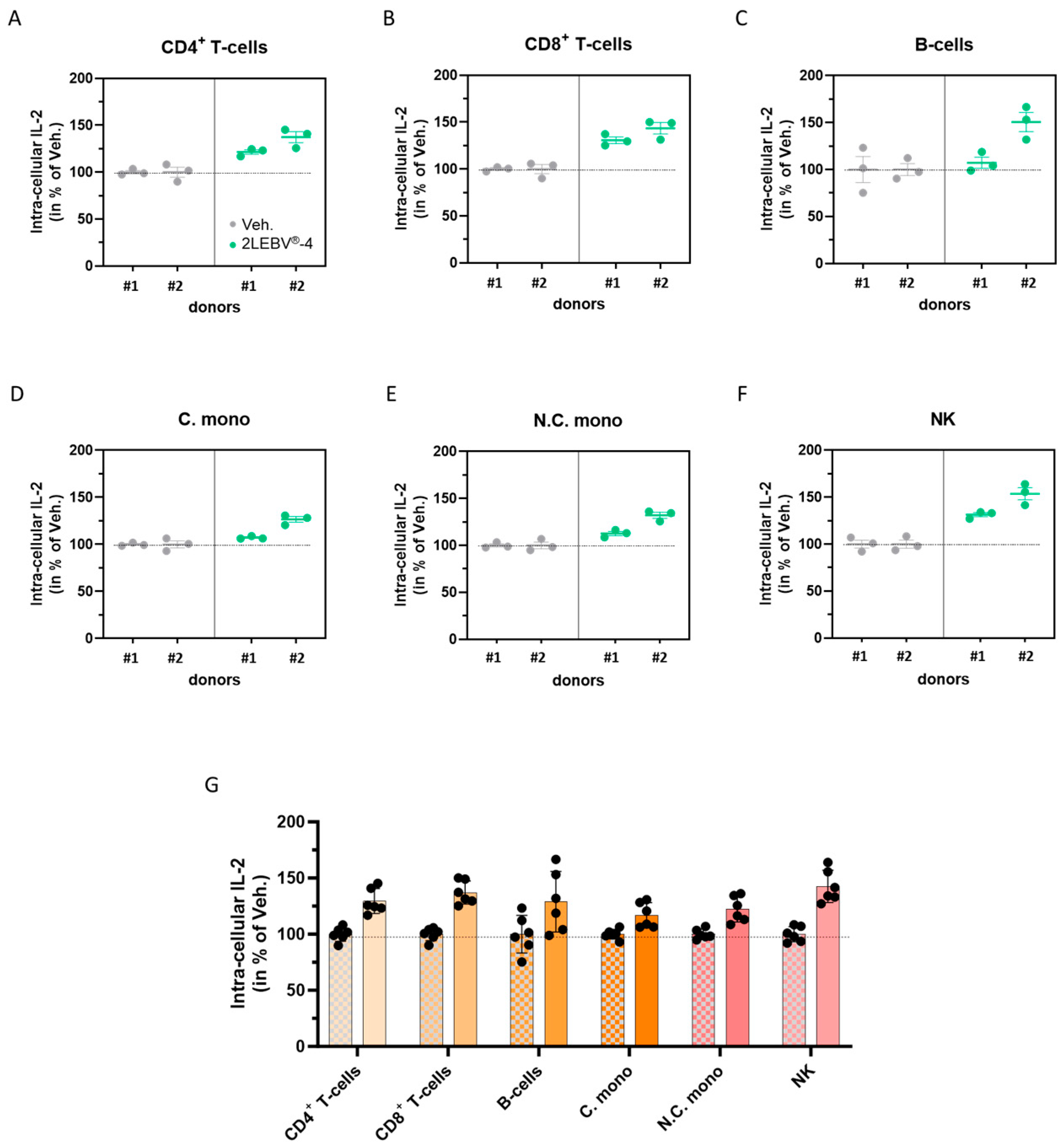
3.4. Actives from 2LEBV® Enhance the Intracellular Production of Interleukin-2 in a Wide Range of Concanavalin A-Stimulated Human Peripheral Blood Mononuclear Cell Sub-Populations
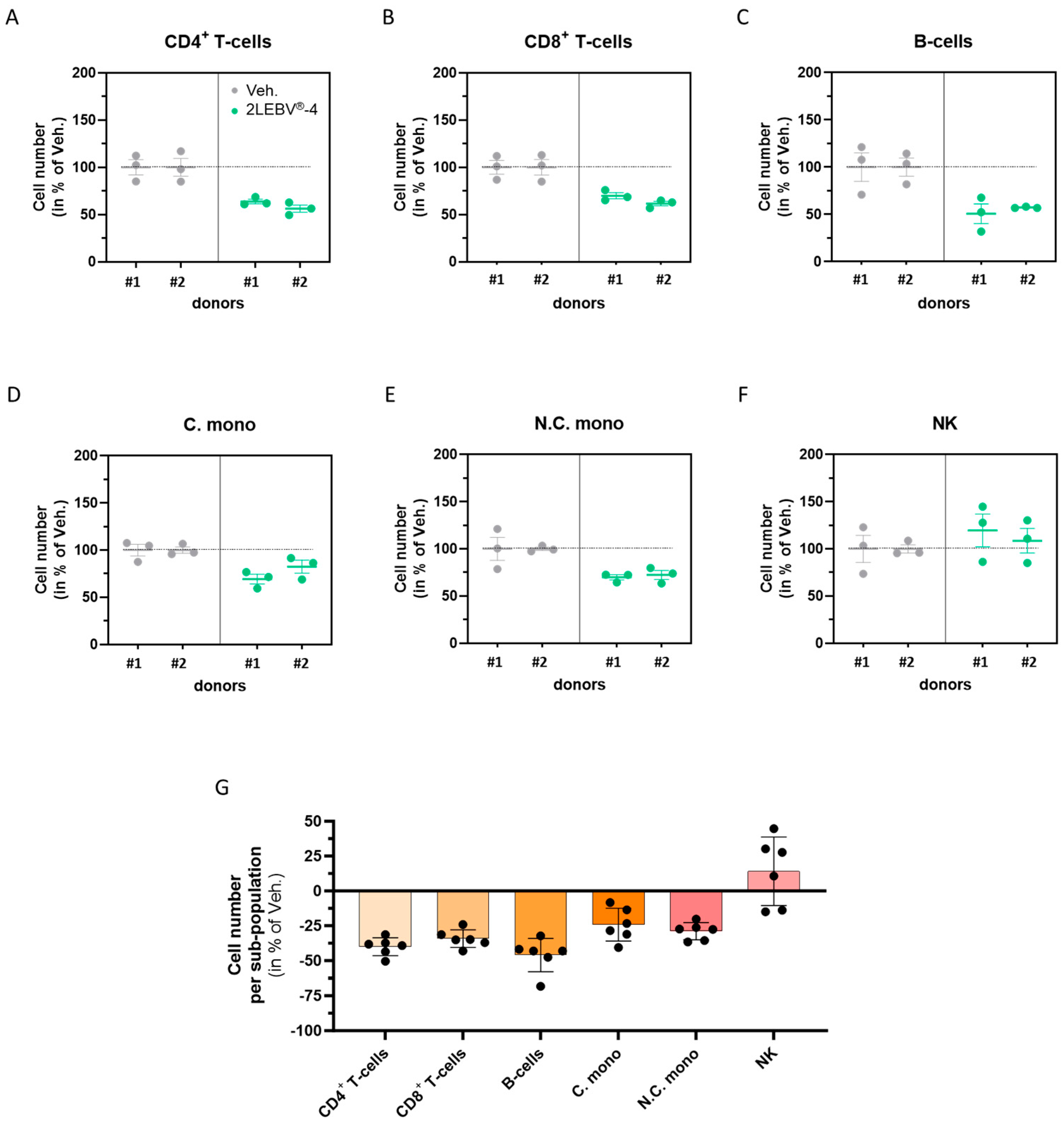
3.5. Actives from 2LEBV® Reduce the Overall Proliferation of Several Concanavalin A-Stimulated Human Peripheral Blood Mononuclear Cell Sub-Populations
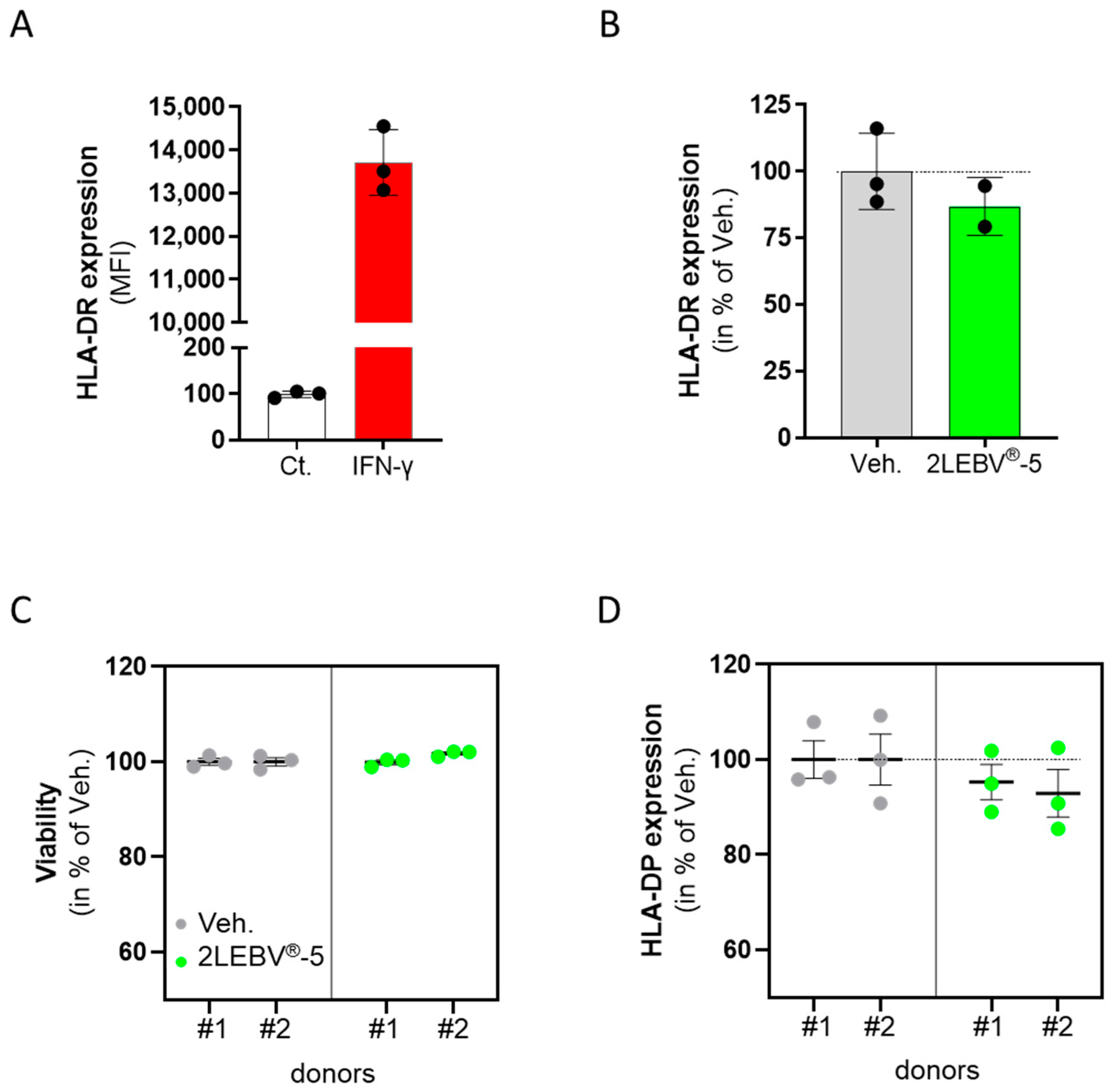
3.6. Actives from 2LEBV® Reduce the Expression of Human Leukocyte Antigen-II in Two Models of Human Immune-Related Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H. Primary Epstein-Barr virus infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Palendira, U.; Edwards, E.S.J. Human immunity against EBV—Lessons from the clinic. J. Exp. Med. 2017, 214, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Crombie, J.L.; LaCasce, A.S. Epstein Barr Virus Associated B-Cell Lymphomas and Iatrogenic Lymphoproliferative Disorders. Front. Oncol. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Khan, G. Epstein-Barr virus, cytokines, and inflammation: A cocktail for the pathogenesis of Hodgkin’s lymphoma? Exp. Hematol. 2006, 34, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.; To, K.F.; Huang, D.P. Focus on nasopharyngeal carcinoma. Cancer Cell 2004, 5, 423–428. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Houen, G.; Trier, N.H. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front. Immunol. 2021, 11, 587380. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deveau, T.-M.; Munter, S.E.; Ryder, D.; Buck, A.; Beck-Engeser, G.; Chan, F.; Lu, S.; Goldberg, S.A.; Hoh, R.; et al. Impact of Pre-Existing Chronic Viral Infection and Reactivation on the Development of Long COVID. medRxiv 2022. medRxiv:2022.06.21.22276660. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2021, 23, 110. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Brulefert, A.; Floris, I. The Unitary Micro-Immunotherapy Medicine Interferon-γ (4 CH) Displays Similar Immunostimulatory and Immunomodulatory Effects than Those of Biologically Active Human Interferon-γ on Various Cell Types. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef]
- Floris, I.; Rose, T.; Rojas, J.A.C.; Appel, K.; Roesch, C.; Lejeune, B. Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Dose-Response Publ. Int. Hormesis Soc. 2020, 18, 1559325820961723. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Appel, K.; Rose, T.; Lejeune, B. 2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion. J. Inflamm. Res. 2018, 11, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Chenuet, P.; Togbe, D.; Volteau, C.; Lejeune, B. Potential Role of the Micro-Immunotherapy Medicine 2LALERG in the Treatment of Pollen-Induced Allergic Inflammation. Dose-Response Publ. Int. Hormesis Soc. 2020, 18, 1559325820914092. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I.; Lejeune, B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment. Int. J. Mol. Sci. 2021, 22, 6717. [Google Scholar] [CrossRef]
- Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A Micro-Immunotherapy Sequential Medicine MIM-seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. Int. J. Mol. Sci. 2022, 23, 6059. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I. Special Focus on the Cellular Anti-Inflammatory Effects of Several Micro-Immunotherapy Formulations: Considerations Regarding Intestinal-, Immune-Axis-Related- and Neuronal-Inflammation Contexts. J. Inflamm. Res. 2022, 15, 6695–6717. [Google Scholar] [CrossRef]
- Jacques, C.; Floris, I. How an Immune-Factor-Based Formulation of Micro-Immunotherapy Could Interfere with the Physiological Processes Involved in the Atopic March. Int. J. Mol. Sci. 2023, 24, 1483. [Google Scholar] [CrossRef]
- Merlo, A.; Turrini, R.; Dolcetti, R.; Martorelli, D.; Muraro, E.; Comoli, P.; Rosato, A. The interplay between Epstein-Barr virus and the immune system: A rationale for adoptive cell therapy of EBV-related disorders. Haematologica 2010, 95, 1769–1777. [Google Scholar] [CrossRef]
- Sausen, D.G.; Bhutta, M.S.; Gallo, E.S.; Dahari, H.; Borenstein, R. Stress-Induced Epstein-Barr Virus Reactivation. Biomolecules 2021, 11, 1380. [Google Scholar] [CrossRef]
- Bendickova, K.; Fric, J. Roles of IL-2 in bridging adaptive and innate immunity, and as a tool for cellular immunotherapy. J. Leukoc. Biol. 2020, 108, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R. Concanavalin A triggers T lymphocytes by directly interacting with their receptors for activation. J. Immunol. 1982, 128, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L. Research methods: Know when your numbers are significant. Nature 2012, 492, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Landais, E.; Saulquin, X.; Houssaint, E. The human T cell immune response to Epstein-Barr virus. Int. J. Dev. Biol. 2003, 49, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; Noelle, R.J. Interleukin-9 as a T helper type 17 cytokine. Immunology 2010, 131, 169–173. [Google Scholar] [CrossRef]
- Lakhanpal, S.; Gonchoroff, N.J.; Handwerger, B.S. Interleukin 2 induces proliferation of normal “resting” human T cells in the absence of other known external stimulation. Cell. Immunol. 1987, 106, 62–75. [Google Scholar] [CrossRef]
- Li, Q.; Cohen, J.I. Epstein-Barr Virus and the Human Leukocyte Antigen Complex. Curr. Clin. Microbiol. Rep. 2019, 6, 175–181. [Google Scholar] [CrossRef]
- Haan, K.M.; Kwok, W.W.; Longnecker, R.; Speck, P. Epstein-Barr Virus Entry Utilizing HLA-DP or HLA-DQ as a Coreceptor. J. Virol. 2000, 74, 2451–2454. [Google Scholar] [CrossRef]
- Spriggs, M.K.; Armitage, R.J.; Comeau, M.R.; Strockbine, L.; Farrah, T.; Macduff, B.; Ulrich, D.; Alderson, M.R.; Müllberg, J.; Cohen, J.I. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR beta chain and inhibits antigen presentation. J. Virol. 1996, 70, 5557–5563. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Matsuoka, T.; Mitsuya, H.; Nishimura, Y.; Matsushita, S. Cross-linking HLA-DR molecules on Th1 cells induces anergy in association with increased level of cyclin-dependent kinase inhibitor p27(Kip1). Immunol. Lett. 2002, 81, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Araake, M.; Uchiyama, T.; Imanishi, K.; Yan, X.-J. Activation of human vascular endothelial cells by IFN-γ: Acquisition of HLA class II expression, TSST-1-binding activity and accessory activity in T cell activation by the toxin. Int. Arch. Allergy Appl. Immunol. 1991, 96, 55–61. [Google Scholar] [CrossRef]
- Valenzuela, N.M. IFNγ, and to a Lesser Extent TNFα, Provokes a Sustained Endothelial Costimulatory Phenotype. Front. Immunol. 2021, 12, 648946. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Hanafusa, T.; Miyagawa, J.-I.; Kono, N.; Tarui, S. Nicotinamide and 3-Aminobenzamide Reduce Interferon-γ-Induced Class II MHC (HLA-DR and -DP) Molecule Expression on Cultured Human Endothelial Cells and Fibroblasts. Immunopharmacol. Immunotoxicol. 1991, 13, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, E.I.; Macal, M.; Lewis, G.M.; Harker, J.A. Innate and Adaptive Immune Regulation During Chronic Viral Infections. Annu. Rev. Virol. 2015, 2, 573–597. [Google Scholar] [CrossRef]
- Lucas, M.; Karrer, U.; Lucas, A.; Klenerman, P. Viral escape mechanisms—Escapology taught by viruses. Int. J. Exp. Pathol. 2001, 82, 269–286. [Google Scholar] [CrossRef]
- Tagawa, T.; Albanese, M.; Bouvet, M.; Moosmann, A.; Mautner, J.; Heissmeyer, V.; Zielinski, C.; Lutter, D.; Hoser, J.; Hastreiter, M.; et al. Epstein-Barr viral miRNAs inhibit antiviral CD4+ T cell responses targeting IL-12 and peptide processing. J. Exp. Med. 2016, 213, 2065–2080. [Google Scholar] [CrossRef]
- Vistarop, A.G.; Cohen, M.; De Matteo, E.; Preciado, M.V.; Chabay, P.A. Analysis of Epstein-Barr virus infection models in a series of pediatric carriers from a developing country. Sci. Rep. 2016, 6, 23303. [Google Scholar] [CrossRef]
- Savard, M.; Bélanger, C.; Tardif, M.; Gourde, P.; Flamand, L.; Gosselin, J. Infection of Primary Human Monocytes by Epstein-Barr Virus. J. Virol. 2000, 74, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Oxenius, A. Interleukin 2: From immunostimulation to immunoregulation and back again. EMBO Rep. 2007, 8, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Im, S.J.; Araki, K.; Ahmed, R. Cytokine-Mediated Regulation of CD8 T-Cell Responses During Acute and Chronic Viral Infection. Cold Spring Harb. Perspect. Biol. 2019, 11, a028464. [Google Scholar] [CrossRef]
- Tian, H.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Interactions of interleukin 2 (IL-2) and IL-2 receptors mediate the activities of B lymphocytes in flounder (Paralichthys olivaceus). Int. J. Biol. Macromol. 2023, 227, 113–123. [Google Scholar] [CrossRef]
- Wen, Q.; Xiong, W.; He, J.; Zhang, S.; Du, X.; Liu, S.; Wang, J.; Zhou, M.; Ma, L. Fusion cytokine IL-2-GMCSF enhances anticancer immune responses through promoting cell-cell interactions. J. Transl. Med. 2016, 14, 41. [Google Scholar] [CrossRef]
- Molloy, M.J.; Zhang, W.; Usherwood, E.J. Cutting Edge: IL-2 Immune Complexes as a Therapy for Persistent Virus Infection 1. J. Immunol. 2009, 182, 4512–4515. [Google Scholar] [CrossRef]
- West, E.E.; Jin, H.-T.; Rasheed, A.-U.; Penaloza-MacMaster, P.; Ha, S.-J.; Tan, W.G.; Youngblood, B.; Freeman, G.J.; Smith, K.A.; Ahmed, R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Investig. 2013, 123, 2604–2615. [Google Scholar] [CrossRef]
- Baiocchi, R.A.; Caligiuri, M.A. Low-dose interleukin 2 prevents the development of Epstein-Barr virus (EBV)-associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 5577–5581. [Google Scholar] [CrossRef]
- Tambussi, G.; Ghezzi, S.; Nozza, S.; Vallanti, G.; Magenta, L.; Guffanti, M.; Brambilla, A.; Vicenzi, E.; Carrera, P.; Racca, S.; et al. Efficacy of Low-Dose Intermittent Subcutaneous Interleukin (IL)–2 in Antiviral Drug–Experienced Human Immunodeficiency Virus–Infected Persons with Detectable Virus Load: A Controlled Study of 3 IL-2 Regimens with Antiviral Drug Therapy. J. Infect. Dis. 2001, 183, 1476–1484. [Google Scholar] [CrossRef]
- DE Sanctis, J.B.; Blanca, I.; Bianco, N.E. Secretion of cytokines by natural killer cells primed with interleukin-2 and stimulated with different lipoproteins. Immunology 1997, 90, 526–533. [Google Scholar] [CrossRef]
- Setoguchi, R.; Hori, S.; Takahashi, T.; Sakaguchi, S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005, 201, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.; Sarkar, S. Regulation of Effector and Memory CD8 T Cell Differentiation by IL-2—A Balancing Act. Front. Immunol. 2018, 9, 2987. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lauder, S.N.; Jones, E.; Smart, K.; Bloom, A.; Williams, A.S.; Hindley, J.P.; Ondondo, B.; Taylor, P.R.; Clement, M.; Fielding, C.; et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 2013, 43, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L.; Wang, C.-T.; Yang, S.-J.; Leu, C.-H.; Chen, S.-H.; Wu, C.-L.; Shiau, A.-L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017, 7, 43829. [Google Scholar] [CrossRef] [PubMed]
- Yarilina, A.; Park-Min, K.-H.; Antoniv, T.; Hu, X.; Ivashkiv, L.B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon–response genes. Nat. Immunol. 2008, 9, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Solomon, D.H. Tumor necrosis factor blockade and the risk of viral infection. Nat. Rev. Rheumatol. 2010, 6, 165–174. [Google Scholar] [CrossRef]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.; Hennighausen, L.; et al. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity 2007, 26, 371–381. [Google Scholar] [CrossRef]
- Paiva, I.A.; Badolato-Corrêa, J.; Familiar-Macedo, D.; De-Oliveira-Pinto, L.M. Th17 Cells in Viral Infections—Friend or Foe? Cells 2021, 10, 1159. [Google Scholar] [CrossRef]
- Espinoza-Delgado, I.; Bosco, M.C.; Musso, T.; Gusella, G.L.; Longo, D.L.; Varesio, L. Interleukin-2 and human monocyte activation. J. Leukoc. Biol. 1995, 57, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gearing, A.; Thorpe, R.; Bird, C.; Spitz, M. Human B cell proliferation is stimulated by interleukin 2. Immunol. Lett. 1985, 9, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Atitey, K.; Anchang, B. Mathematical Modeling of Proliferative Immune Response Initiated by Interactions between Classical Antigen-Presenting Cells Under Joint Antagonistic IL-2 and IL-4 Signaling. Front. Mol. Biosci. 2022, 9, 777390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Das, A. IL-2 mediates NK cell proliferation but not hyperactivity. Immunol. Res. 2018, 66, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.; Lindemann, M.; Baiocchi, R.; Linett, M.; Tan, J.; Chou, C.; Narula, S.; Caligiuri, M. The Functional Characterization of Interleukin-10 Receptor Expression on Human Natural Killer Cells. Blood 1995, 85, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Blattman, J.N.; Grayson, J.M.; Wherry, E.J.; Kaech, S.M.; Smith, K.A.; Ahmed, R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003, 9, 540–547. [Google Scholar] [CrossRef] [PubMed]
- D’souza, W.N.; Schluns, K.S.; Masopust, D.; Lefrançois, L. Essential Role for IL-2 in the Regulation of Antiviral Extralymphoid CD8 T Cell Responses. J. Immunol. 2002, 168, 5566–5572. [Google Scholar] [CrossRef]
- Ito, S.; Bollard, C.M.; Carlsten, M.; Melenhorst, J.J.; Biancotto, A.; Wang, E.; Chen, J.; Kotliarov, Y.; Cheung, F.; Xie, Z.; et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1388–1395. [Google Scholar] [CrossRef]
- Huldani, H.; Rashid, A.I.; Turaev, K.N.; Opulencia, M.J.C.; Abdelbasset, W.K.; Bokov, D.O.; Mustafa, Y.F.; Al-Gazally, M.E.; Hammid, A.T.; Kadhim, M.M.; et al. Concanavalin A as a promising lectin-based anti-cancer agent: The molecular mechanisms and therapeutic potential. Cell Commun. Signal. 2022, 20, 167. [Google Scholar] [CrossRef]
- Gonnella, R.; Granato, M.; Farina, A.; Santarelli, R.; Faggioni, A.; Cirone, M. PKC theta and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2015, 1853, 1586–1595. [Google Scholar] [CrossRef]
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Mondragón, M.G.; Torres, J.; Sánchez-Zauco, N.; Gómez-Delgado, A.; Camorlinga-Ponce, M.; Maldonado-Bernal, C.; Fuentes-Pananá, E.M. Elevated Levels of Interferon-γ Are Associated with High Levels of Epstein-Barr Virus Reactivation in Patients with the Intestinal Type of Gastric Cancer. J. Immunol. Res. 2017, 2017, e7069242. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Spriggs, M.K.; Kovats, S.; Turk, S.M.; Comeau, M.R.; Nepom, B.; Hutt-Fletcher, L.M. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 1997, 71, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Rivera, C.; Sgadari, C.; Franklin, J.; Max, E.E.; Bhatia, K.; Tosato, G. Infection of human endothelial cells with Epstein-Barr virus. J. Exp. Med. 1995, 182, 1213–1221. [Google Scholar] [CrossRef]
| Ingredients (CH) | 2LEBV®-4 | 2LEBV®-5 |
|---|---|---|
| hr-IL-1β | 10 | 10 |
| hr-IL-2 | 7 | 10 |
| DNA | 10 | 8 |
| RNA | 10 | 10 |
| SNA®-EBV | 10 | 10 |
| SNA®-HLA-II | 10 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacques, C.; Marchand, F.; Chatelais, M.; Brulefert, A.; Floris, I. Understanding the Mode of Action of a Micro-Immunotherapy Formulation: Pre-Clinical Evidence from the Study of 2LEBV® Active Ingredients. Life 2024, 14, 102. https://doi.org/10.3390/life14010102
Jacques C, Marchand F, Chatelais M, Brulefert A, Floris I. Understanding the Mode of Action of a Micro-Immunotherapy Formulation: Pre-Clinical Evidence from the Study of 2LEBV® Active Ingredients. Life. 2024; 14(1):102. https://doi.org/10.3390/life14010102
Chicago/Turabian StyleJacques, Camille, Flora Marchand, Mathias Chatelais, Adrien Brulefert, and Ilaria Floris. 2024. "Understanding the Mode of Action of a Micro-Immunotherapy Formulation: Pre-Clinical Evidence from the Study of 2LEBV® Active Ingredients" Life 14, no. 1: 102. https://doi.org/10.3390/life14010102
APA StyleJacques, C., Marchand, F., Chatelais, M., Brulefert, A., & Floris, I. (2024). Understanding the Mode of Action of a Micro-Immunotherapy Formulation: Pre-Clinical Evidence from the Study of 2LEBV® Active Ingredients. Life, 14(1), 102. https://doi.org/10.3390/life14010102








