Severe Early-Onset Intrahepatic Cholestasis of Pregnancy Following Ovarian Hyperstimulation Syndrome with Pulmonary Presentation after In Vitro Fertilization: Case Report and Systematic Review of Case Reports
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Consent and Case Report
2.2. Systematic Review of Case Reports
2.2.1. Search Strategy and Eligibility Criteria
2.2.2. Data Extraction and Outcome Measures
- (1)
- clinical presentation of early-onset intrahepatic cholestasis of pregnancy (ICP) following ovarian hyperstimulation syndrome (OHSS),
- (2)
- clinical differences between early-onset intrahepatic cholestasis in spontaneous versus IVF pregnancies,
- (3)
- early-onset ICP treatments,
- (4)
- delivery and perinatal outcomes with early-onset ICP.
2.2.3. Statistical Analysis
3. Results
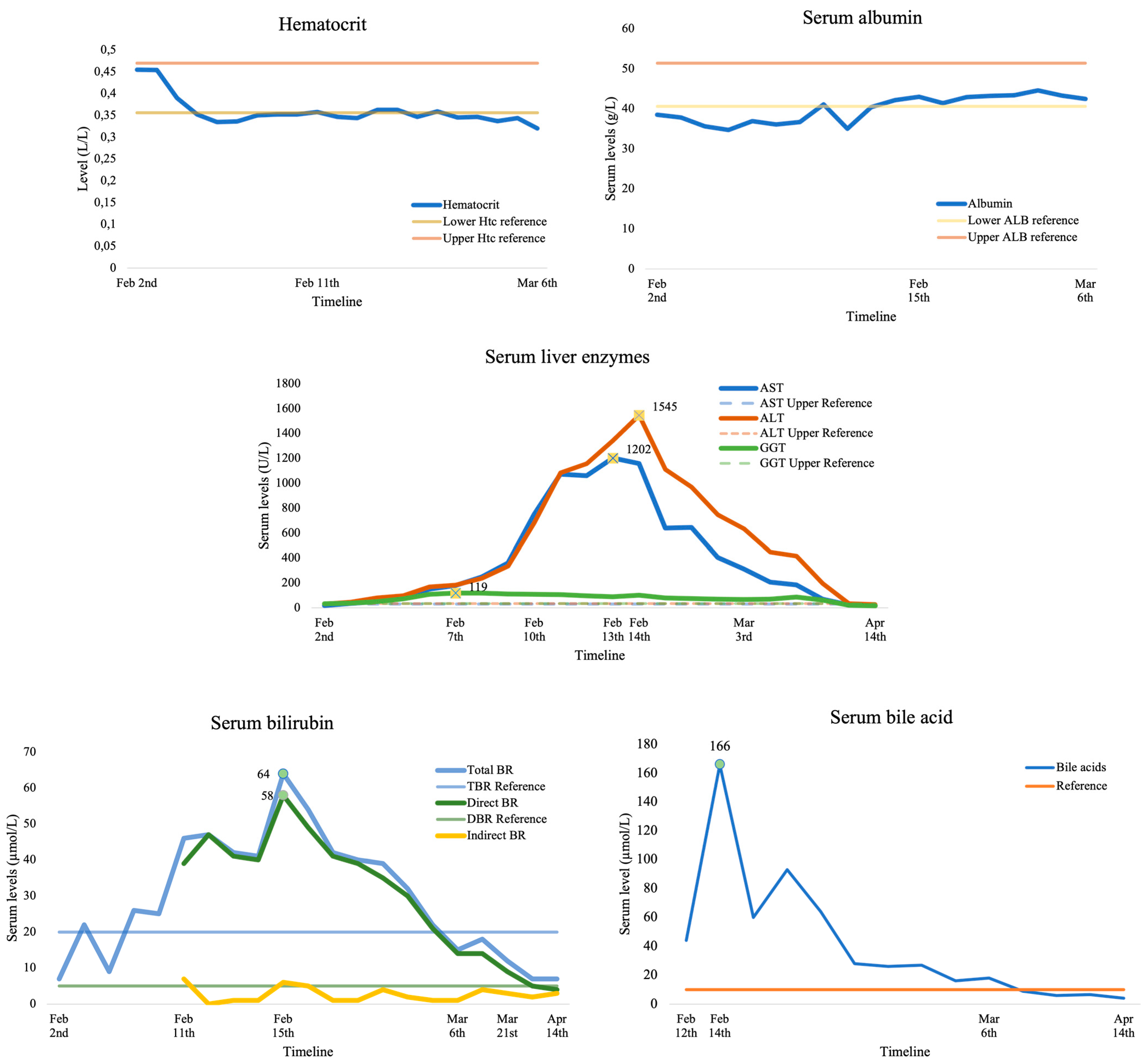
3.1. Case Presentation
3.2. Systematic Review of Case Reports
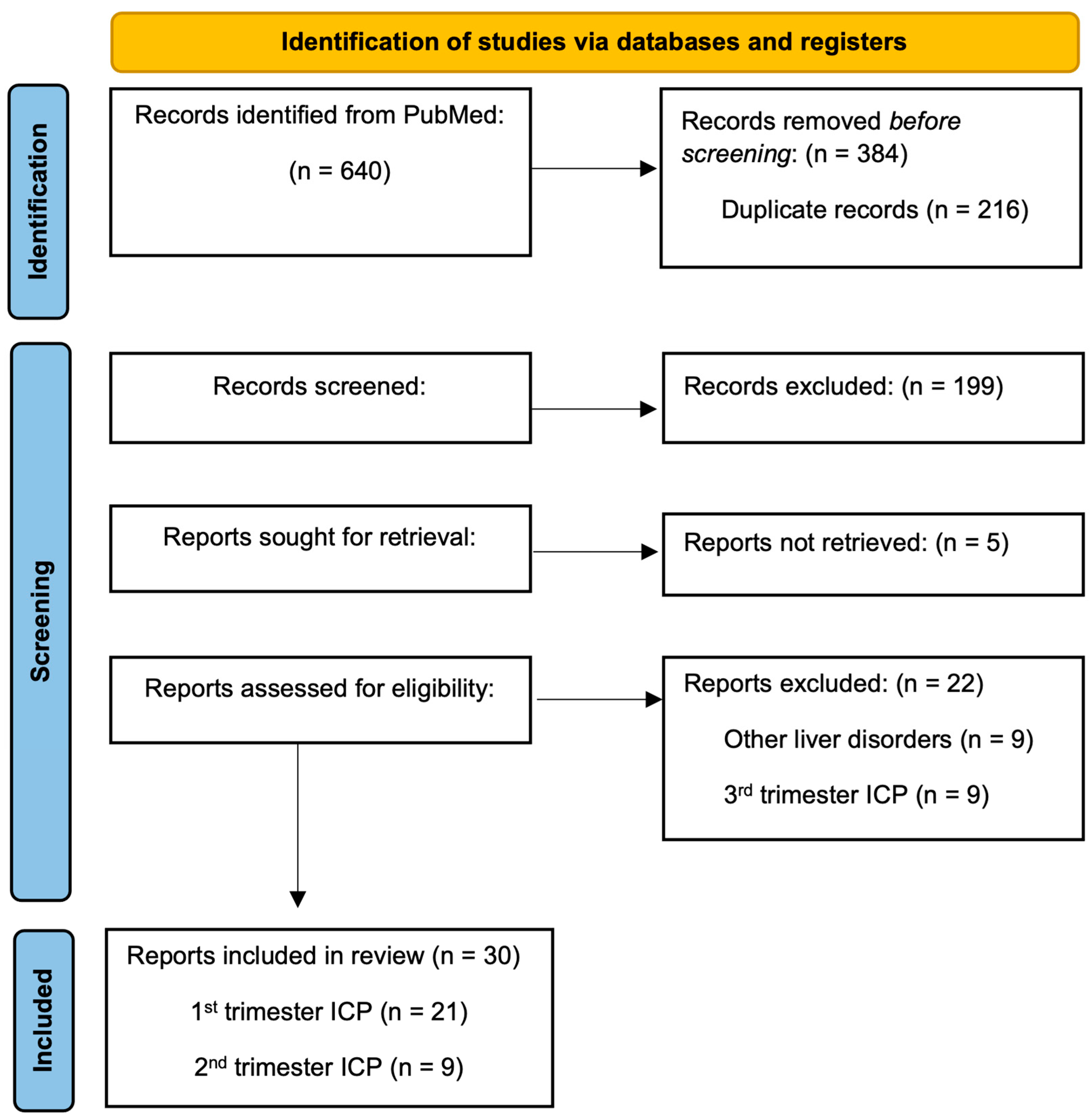
3.2.1. Literature Search and Data Extraction
3.2.2. First Trimester ICP following OHSS
3.2.3. Clinical Differences between Spontaneous vs. IVF Pregnancies with 1st Trimester ICP
3.2.4. Treatment of 1st Trimester ICP
3.2.5. Pregnancy Outcomes with 1st Trimester ICP
3.2.6. Pooled 1st and 2nd Trimester ICP Cases Regarding Early-Onset ICP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williamson, C.; Geenes, V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2014, 124, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Gu, W.; Hou, Y. Diagnosis and prognosis of early-onset intrahepatic cholestasis of pregnancy: A prospective study. J. Matern.-Fetal Neonatal Med. 2019, 32, 997–1003. [Google Scholar] [CrossRef]
- Bolukbas, F.F.; Bolukbas, C.; Balaban, H.Y.; Aygun, C.; Ignak, S.; Ergul, E.; Yazicioglu, M.; Ersahin, S.S. Intrahepatic Cholestasis of Pregnancy: Spontaneous vs in vitro Fertilization. Euroasian J. Hepato-Gastroenterol. 2017, 7, 126–129. [Google Scholar] [CrossRef]
- Estiú, M.C.; Frailuna, M.A.; Otero, C.; Dericco, M.; Williamson, C.; Marin, J.J.G.; Macias, R.I. Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid. PLoS ONE 2017, 12, e0176504. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, M.F.; Aslan, K.; Guler, I.; Mutlu, I.; Erdem, M.; Bozkurt, N.; Erdem, A. Two cases of first onset intrahepatic cholestasis of pregnancy associated with moderate ovarian hyperstimulation syndrome after IVF treatment and review of the literature. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2017, 37, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Ambrosini, G.; Nuzzi, L.; Buzzaccarini, G.; Esposito, F.; Capobianco, G.; Chiantera, V.; Laganà, A.S.; Andrisani, A. Intrahepatic cholestasis of pregnancy after ovarian hyperstimulation syndrome with wild-type ABCB4 gene: A peculiar case and literature review. BMC Womens Health 2023, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Edoo, A.W.I.; Imcha, M.; Ali, A.; Burke, G.; Cotter, A.; Skehan, M. A case report of early onset intrahepatic cholestasis of pregnancy. Arch. Dis. Child.-Fetal Neonatal Ed. 2012, 97 (Suppl. S1), A63. [Google Scholar] [CrossRef]
- Shimono, J.; Tsuji, H.; Azuma, K.; Hashiguchi, M.; Fujishima, M. A rare case of hepatic injury associated with ovarian hyperstimulation syndrome. Am. J. Gastroenterol. 1998, 93, 123–124. [Google Scholar] [CrossRef]
- Ayyash, M.; Smith, N.; Keerthy, M.; Singh, A.; Shaman, M. Benign Recurrent Intrahepatic Cholestasis in Pregnancy: Fetal Death at 36 Weeks of Gestation. Case Rep. Obstet. Gynecol. 2021, 2021, 5086846. [Google Scholar] [CrossRef] [PubMed]
- Wander, G.; Neuberger, F.; Dhanjal, M.K.; Nelson-Piercy, C.; Soh, M.C. Cytomegalovirus may mimic the presentation of intrahepatic cholestasis and hemolysis, elevated liver enzymes and low platelets in immunosuppressed pregnant women. Obstet. Med. 2016, 9, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Cemortan, M.; Stavinskaia, L.; Sagaidac, I.; Cernetchi, O. Early onset of intrahepatic cholestasis of pregnancy: A case report. J. Gastrointest. Liver Dis. 2022, 31, 145–146. [Google Scholar] [CrossRef]
- Keitel, V.; Dröge, C.; Stepanow, S.; Fehm, T.; Mayatepek, E.; Köhrer, K.; Häussinger, D. Intrahepatic cholestasis of pregnancy (ICP): Case report and review of the literature. Z. Gastroenterol. 2016, 54, 1327–1333. [Google Scholar] [CrossRef]
- Covach, A.J.; Rose, W.N. Intrahepatic Cholestasis of Pregnancy Refractory to Multiple Medical Therapies and Plasmapheresis. AJP Rep. 2017, 7, e223–e225. [Google Scholar] [CrossRef][Green Version]
- Stulic, M.; Culafic, D.; Boricic, I.; Stojkovic Lalosevic, M.; Pejic, N.; Jankovic, G.; Milovanovic, T.; Culafic-Vojinovic, V.; Vlaisavljevic, Z.; Culafic, M. Intrahepatic Cholestasis of Pregnancy: A Case Study of the Rare Onset in the First Trimester. Medicina 2019, 55, 454. [Google Scholar] [CrossRef]
- Ryley, N.G.; Forman, R.; Barlow, D.; Fleming, K.A.; Trowell, J.M. Liver abnormality in ovarian hyperstimulation syndrome. Hum. Reprod. Oxf. Engl. 1990, 5, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Obrzut, B.; Kuczyński, W.; Grygoruk, C.; Putowski, L.; Kluz, S.; Skret, A. Liver dysfunction in severe ovarian hyperstimulation syndrome. Gynecol. Endocrinol. 2005, 21, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wongjarupong, N.; Bharmal, S.; Lim, N. Never Too Soon: An Unusual Case of Intrahepatic Cholestasis of Pregnancy at Five Weeks Gestation. Cureus 2020, 12, e10540. [Google Scholar] [CrossRef]
- Liu, T.F.; He, J.J.; Wang, L.; Zhang, L.Y. Novel ABCB4 mutations in an infertile female with progressive familial intrahepatic cholestasis type 3: A case report. World J. Clin. Cases 2022, 10, 1998–2006. [Google Scholar] [CrossRef]
- Johnston, R.C.; Stephenson, M.L.; Nageotte, M.P. Novel heterozygous ABCB4 gene mutation causing recurrent first-trimester intrahepatic cholestasis of pregnancy. J. Perinatol. 2014, 34, 711–712. [Google Scholar] [CrossRef]
- Sarıkaya, E.; Deveer, R.; Kilic, S.; Batioglu, S. Ongoing Twin Pregnancy in an Obese, Polycystic Patient with Early Critical Ovarian Hyperstimulation Syndrome and Severe Liver Dysfunction: Case Report. Turk. Klin. J. Med. Sci. 2012, 32, 226–230. [Google Scholar] [CrossRef]
- Warren, J.E.; Blaylock, R.C.; Silver, R.M. Plasmapheresis for the treatment of intrahepatic cholestasis of pregnancy refractory to medical treatment. Am. J. Obstet. Gynecol. 2005, 192, 2088–2089. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.T.; Sheffield, J.S. Primary dermatologic findings with early-onset intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2011, 117 Pt 2, 456–458. [Google Scholar] [CrossRef]
- Koh, K.; Kathirvel, R.; Mathur, M. Rare case of obstetric cholestasis presenting in the first trimester following in vitro fertilisation. BMJ Case Rep. 2021, 14, e244254. [Google Scholar] [CrossRef]
- Midgley, D.Y.; Khalaf, Y.; Braude, P.R.; Nelson-Piercy, C. Recurrent cholestasis following ovarian hyperstimulation syndrome: Case report. Hum. Reprod. Oxf. Engl. 1999, 14, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Muresan, D.; Ona, D.; Cruciat, G.; Rotar, I.; Stamatian, F. Recurrent intrahepatic cholestasis of pregnancy. A case report. J. Gastrointest. Liver Dis. 2008, 17, 323–325. [Google Scholar]
- Hubschmann, A.G.; Orzechowski, K.M.; Berghella, V. Severe First Trimester Recurrent Intrahepatic Cholestasis of Pregnancy: A Case Report and Literature Review. AJP Rep. 2016, 6, e38–e41. [Google Scholar] [CrossRef]
- Yun, F.; Fu, L.; Xu, D.; Qu, F.; Wang, F. Severe intrahepatic cholestasis of pregnancy due to a Sertoli-Leydig cell tumour in a woman with polycystic ovary syndrome: A case report. BMC Pregnancy Childbirth 2022, 22, 807. [Google Scholar] [CrossRef]
- Wånggren, K.; Sparre, L.S.; Wramsby, H. Severe jaundice in early IVF pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 228–229. [Google Scholar] [CrossRef]
- Kamimura, K.; Abe, H.; Kamimura, N.; Yamaguchi, M.; Mamizu, M.; Ogi, K.; Takahashi, Y.; Mizuno, K.I.; Kamimura, H.; Kobayashi, Y.; et al. Successful management of severe intrahepatic cholestasis of pregnancy: Report of a first Japanese case. BMC Gastroenterol. 2014, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Smolarczyk, R.; Grymowicz, M.; Sienko, J.; Czajkowski, K. Successful perinatal outcome in an early onset intrahepatic cholestasis of pregnancy with extremely high serum hepatic function tests. Gynecol. Endocrinol. 2009, 25, 475–476. [Google Scholar] [CrossRef]
- Ajala, T.; Rafi, J.; Wray, R.; Whitehead, M.W.; Zaidi, J. There may be a link between intrahepatic cholestasis of pregnancy and familial combined hyperlipidaemia: A case report. Cases J. 2009, 2, 8679. [Google Scholar] [CrossRef]
- Zamah, A.M.; El-Sayed, Y.Y.; Milki, A.A. Two cases of cholestasis in the first trimester of pregnancy after ovarian hyperstimulation. Fertil. Steril. 2008, 90, 1202.e7–1202.e10. [Google Scholar] [CrossRef]
- Salame, A.A.; Jaffal, M.J.; Mouanness, M.A.; Nasser Eddin, A.R.; Ghulmiyyah, L.M. Unexplained First Trimester Intrahepatic Cholestasis of Pregnancy: A Case Report and Literature Review. Case Rep. Obstet. Gynecol. 2019, 2019, 4980610. [Google Scholar] [CrossRef]
- Brites, D.; Rodrigues, C.M.; da Conceição Cardoso, M.; Graça, L.M. Unusual case of severe cholestasis of pregnancy with early onset, improved by ursodeoxycholic acid administration. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 76, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Robles, A.; Gunnala, V.; Chung, P.; Rosenwaks, Z. Unilateral pleural effusion as the sole clinical presentation of severe ovarian hyperstimulation syndrome: A systematic review. Gynecol. Endocrinol. 2018, 34, 92–99. [Google Scholar] [CrossRef]
- Alemdaroğlu, S.; Yılmaz Baran, Ş.; Durdağ, G.D.; Yuksel Şimşek, S.; Yetkinel, S.; Alkaş Yağınç, D.; Kalaycı, H.; Şimşek, E. Intrahepatic cholestasis of pregnancy: Are in vitro fertilization pregnancies at risk? J. Matern.-Fetal Neonatal Med. 2021, 34, 2548–2553. [Google Scholar] [CrossRef]
- Marin, L.; Vitagliano, A.; Capobianco, G.; Dessole, F.; Ambrosini, G.; Andrisani, A. Which is the optimal timing for starting chemoprotection with gonadotropin-releasing hormone agonists after oocyte cryopreservation? Reflections on a critical case of ovarian hyperstimulation syndrome. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101815. [Google Scholar] [CrossRef]
- Conrad, K.P.; Baker, V.L. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R69–R72. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Reyes, H.; Arrese, M.; Figueroa, D.; Lorca, B.; Andresen, M.; Segovia, N.; Molina, C.; Arce, S. Intrahepatic cholestasis of pregnancy in twin pregnancies. J. Hepatol. 1989, 9, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; Briz, O.; Serrano, M.A.; Monte, M.J.; Marin, J.J.G. Potential role of trans-inhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J. Hepatol. 2006, 44, 1150–1157. [Google Scholar] [CrossRef]
- Abu-Hayyeh, S.; Martinez-Becerra, P.; Sheikh Abdul Kadir, S.H.; Selden, C.; Romero, M.R.; Rees, M.; Marschall, H.U.; Marin, J.J.; Williamson, C. Inhibition of Na+-Taurocholate Co-transporting Polypeptide-mediated Bile Acid Transport by Cholestatic Sulfated Progesterone Metabolites. J. Biol. Chem. 2010, 285, 16504–16512. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hayyeh, S.; Papacleovoulou, G.; Lövgren-Sandblom, A.; Tahir, M.; Oduwole, O.; Jamaludin, N.A.; Ravat, S.; Nikolova, V.; Chambers, J.; Selden, C.; et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology 2013, 57, 716–726. [Google Scholar] [CrossRef]
- Feng, C.; Li, W.J.; He, R.H.; Sun, X.W.; Wang, G.; Wang, L.Q. Impacts of different methods of conception on the perinatal outcome of intrahepatic cholestasis of pregnancy in twin pregnancies. Sci. Rep. 2018, 8, 3985. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xie, R.; Wang, M.; Sun, Y. Patients with IVF complicated by moderate-to-critical OHSS experience increased thrombosis, GDM and neonatal NICU admission but slightly shorter gestation compared with matched IVF counterparts: A retrospective Chinese cohort study. Reprod. Biol. Endocrinol. 2021, 19, 8. [Google Scholar] [CrossRef]
- Romito, I.; Gulino, F.A.; Laganà, A.S.; Vitale, S.G.; Tuscano, A.; Leanza, G.; Musmeci, G.; Leanza, V.; Rapisarda, A.M.C.; Palumbo, M.A. Renal and Hepatic Functions after A Week of Controlled Ovarian Hyperstimulation during In Vitro Fertilization Cycles. Int. J. Fertil. Steril. 2017, 11, 15–19. [Google Scholar] [CrossRef]
- Rahim, M.N.; Theocharidou, E.; Yen Lau, K.G.; Ahmed, R.; Marattukalam, F.; Long, L.; Cannon, M.D.; Heneghan, M.A. Safety and efficacy of in vitro fertilisation in patients with chronic liver disease and liver transplantation recipients. J. Hepatol. 2021, 74, 1407–1415. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Lang, T.; Meier, Y.; Zodan-Marin, T.; Jung, D.; Breymann, C.; Zimmermann, R.; Kenngott, S.; Beuers, U.; Reichel, C.; et al. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics 2004, 14, 91–102. [Google Scholar] [CrossRef]
- Anzivino, C.; Odoardi, M.R.; Meschiari, E.; Baldelli, E.; Facchinetti, F.; Neri, I.; Ruggiero, G.; Zampino, R.; Bertolotti, M.; Loria, P.; et al. ABCB4 and ABCB11 mutations in intrahepatic cholestasis of pregnancy in an Italian population. Dig. Liver Dis. 2013, 45, 226–232. [Google Scholar] [CrossRef]
- Sepúlveda, W.H.; González, C.; Cruz, M.A.; Rudolph, M.I. Vasoconstrictive effect of bile acids on isolated human placental chorionic veins. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 42, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.; Gorelik, J.; Eaton, B.M.; Lab, M.; de Swiet, M.; Korchev, Y. The bile acid taurocholate impairs rat cardiomyocyte function: A proposed mechanism for intra-uterine fetal death in obstetric cholestasis. Clin. Sci. 2001, 100, 363–369. [Google Scholar] [CrossRef]
- Labbe, C.; Delesalle, C.; Creveuil, C.; Dreyfus, M. [Early and later intrahepatic cholestasis of pregnancy (ICP): Study of adverse pregnancy outcomes]. Gynecol. Obstet. Fertil. Senol. 2018, 46, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Glantz, A.; Marschall, H.U.; Mattsson, L.A. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology 2004, 40, 467–474. [Google Scholar] [CrossRef]
- Jin, J.; Pan, S.L.; Huang, L.P.; Yu, Y.H.; Zhong, M.; Zhang, G.W. Risk factors for adverse fetal outcomes among women with early- versus late-onset intrahepatic cholestasis of pregnancy. Int. J. Gynaecol. Obstet. 2015, 128, 236–240. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Salerno, S.; Li, Y.; Zhou, L.; Zeng, X.; Li, H. Prediction of intrahepatic cholestasis of pregnancy in the first 20 weeks of pregnancy. J. Matern.-Fetal Neonatal Med. 2022, 35, 6329–6335. [Google Scholar] [CrossRef]
- Lee, R.H.; Kwok, K.M.; Ingles, S.; Wilson, M.L.; Mullin, P.; Incerpi, M.; Pathak, B.; Goodwin, T.M. Pregnancy Outcomes during an Era of Aggressive Management for Intrahepatic Cholestasis of Pregnancy. Am. J. Perinatol. 2008, 25, 341–345. [Google Scholar] [CrossRef]
| Early-Onset ICP | p | 1st Trimester ICP | p | |||
|---|---|---|---|---|---|---|
| Spontaneous (n = 19) | IVF (n = 15) | Spontaneous (n = 10) | IVF (n = 13) | |||
| Age, years | 28.2 ± 4.4 | 30.9 ± 3.2 | 0.048 | 29 ± 4.8 | 31.1 ± 3.4 | 0.233 |
| Ethnicity | 0.015 | 0.059 | ||||
| Caucasian | 15 (78.9) | 5 (33.3) | 8 (80) | 4 (30.8) | ||
| Asian | 1 (5.3) | 5 (33.3) | 0 | 4 (30.8) | ||
| Hispanic | 2 (10.5) | 0 | 1 (10) | 0 | ||
| Middle Eastern | 1 (5.3) | 3 (20) | 1 (10) | 3 (23.1) | ||
| Indian | 0 | 2 (13.3) | 0 | 2 (15.3) | ||
| Gravidity | 2 (1–6) | 1 (0–3) | 0.019 | 2 (1–6) | 1 (0–3) | 0.022 |
| Parity | 1 (0–5) | 0 (0–1) | 0.006 | 1 (0–5) | 0 (0–1) | 0.006 |
| IVF indication | Na | Na | ||||
| Primary infertility | 8 (53.3) | 7 (53.8) | ||||
| Secondary infertility | 3 (20) | 3 (23.1) | ||||
| Tubal factor | 3 (20) | 2 (15.4) | ||||
| Male factor | 1 (6.7) | 1 (7.7) | ||||
| ET | 2 (1–3) | Na | 2 (1–3) | Na | ||
| Missing data | 3 | 3 | ||||
| OHSS | 10 (66.7) | Na | 10 (66.7) | Na | ||
| Mild | 1 (10) | 1 (10) | ||||
| Moderate | 4 (40) | 4 (40) | ||||
| Severe | 5 (50) | 5 (50) | ||||
| History | 14 (73.7) | 2 (13.3) | <0.001 | 7 (70) | 2 (15.4) | 0.008 |
| ICP in previous pregnancy | 8 (42.5) | 0 | 4 (40) | 0 | ||
| Family history of ICP | 3 (15.7) | 0 | 1 (10) | 0 | ||
| Contraception-induced cholestasis | 3 (15.7) | 1 (6.7) | 3 (30) | 1 (7.7) | ||
| Miscarriage | 2 (10.5) | 0 | 0 | 0 | ||
| Stillbirth | 2 (10.5) | 0 | 1 (10) | 0 | ||
| Cholelithiasis or cholecystectomy | 4 (21.1) | 0 | 3 (30) | 0 | ||
| Viral hepatitis | 2 (10.5) | 0 | 1 (10) | 0 | ||
| Familial hyperlipidemia or cholestasis | 1 (5.3) | 1 (6.7) | 0 | 1 (7.7) | ||
| Jaundice | 6 (31.6) | 3 (21.4) | 0.518 | 3 (30) | 3 (23.1) | 0.793 |
| ICP onset, GW | 15.2 ± 8.0 | 8.1 ± 6.7 | 0.003 | 8.7 ± 2.7 | 5.8 ± 3.0 | 0.029 |
| ICP recurrence a | 11 (61.1) | 5 (45.5) | 0.089 | 5 (55.5) | 3 (33.3) | 0.230 |
| Peak LFT | ||||||
| ALT, U/L | 119 (35–2415) | 275 (85–3372) | 0.270 | 159 (95–1496) | 314 (85–3372) | 0.650 |
| Missing data | 4 | 1 | 3 | 1 | ||
| AST, U/L | 123 (48–2748) | 255 (1156) | 0.240 | 123 (86–1117) | 225 (109–1156) | 0.672 |
| Missing data | 4 | 2 | 3 | 1 | ||
| T-Br, μmol/L | 84.6 (31–170) | 22.3 (6.8–161) | 0.010 | 98.3 (47.8–114) | 23.1 (6.8–161) | 0.073 |
| Missing data | 9 | 6 | 6 | 5 | ||
| TBA, μmol/L | 141.1 (12–462) | 137.2 (18.3–308) | 0.869 | 242 (27–462) | 137.2 (18.3–308) | 0.113 |
| Missing data | 2 | 4 | 1 | 4 | ||
| ICP treatment | 0.439 | 0.412 | ||||
| UDCA | 14 (73.7) | 8 (53.3) | 9 (90) | 6 (46.2) | ||
| Antihistamines | 8 (42.1) | 5 (33.3) | 4 (40) | 4 (30.8) | ||
| Corticosteroids | 5 (26.3) | 1 (6.7) | 1 (10) | 1 (7.7) | ||
| Cholestyramine | 2 (10.5) | 1 (6.7) | 1 (10) | 1 (7.7) | ||
| Hypnotics | 2 (10.5) | 0 | 1 (10) | 0 | ||
| Plasmapheresis | 2 (10.5) | 0 | 1 (10) | 0 | ||
| SAMe | 1 (5.3) | 3 (20) | 1 (10) | 1 (7.7) | ||
| Pregnancy outcomes | ||||||
| Delivery | 18 (94.7) | 11 (73.3) | 0.080 | 9 (90) | 9 (75) | 0.231 |
| Miscarriage | 0 | 4 (26.7) | 0.017 | 0 | 4 (25) | 0.054 |
| Artificial abortion | 1 (5.3) | 0 | 0.367 | 1 (10) | 0 | 0.599 |
| GA at delivery, GW a | 35 (29–38) | 36 (30–39) | 0.769 | 35 (30–37) | 37 (34–39) | 0.868 |
| Term delivery | 4 (22.2) | 4 (40) | 0.439 | 1 (11.1) | 4 (50) | 0.412 |
| Preterm delivery | 14 (77.8) | 6 (60) | 0.035 | 8 (88.9) | 4 (50) | 0.053 |
| Singleton pregnancy | 17 (94.4) | 7 (63.6) | 9 (100) | 6 (66.7) | 0.365 | |
| Twin pregnancy | 1 (5.6) | 4 (36.4) | 0 | 3 (33.3) | 0.103 | |
| Livebirth | 18 | 16 | 8 | 13 | 0.232 | |
| Stillbirth | 1 | 0 | 1 | 0 | 0.244 | |
| Delivery mode a | 0.219 | 0.123 | ||||
| Spontaneous vaginal delivery | 4 (22.2) | 4 (36.4) | 0 | 3 (37.5) | ||
| Induced vaginal delivery | 8 (44.4) | 2 (18.2) | 7 (77.8) | 2 (25.0) | ||
| Planned Caesarean section | 3 (16.7) | 1 (9.1) | 1 (11.1) | 1 (12.5) | ||
| Urgent Caesarean section | 3 (16.7) | 3 (27.3) | 1 (11.1) | 2 (25.0) | ||
| Obstetrical complications | n = 19 | n = 12 | n = 9 | n = 8 | ||
| PPROM | 9 (47.4) | 3 (25) | 0.097 | 5 (55.5) | 1 (12.5) | 0.022 |
| LFT aberration | 2 (10.5) | 3 (25) | 0.439 | 1 (11.1) | 3 (37.5) | 0.412 |
| Fetal distress | 1 (5.3) | 1 (8.3) | 0.863 | 0 | 1 (12.5) | 0.370 |
| MAF | 2 (10.5) | 1 (8.3) | 0.694 | 1 (11.1) | 0 | 0.244 |
| Presentation | 2 (10.5) | 1 (8.3) | 0.195 | 1 (11.1) | 0 | 0.244 |
| Maternal comorbidities | 2 (10.5) | 0 | 0.195 | 0 | 0 | Na |
| Chorioamnionitis | 1 (5.3) | 0 | 0.367 | 1 (11.1) | 0 | 0.244 |
| Oligohydramnios | 0 | 1 (8.3) | 0.253 | 0 | 1 (12.5) | 0.370 |
| Doppler aberrations | 0 | 1 (8.3) | 0.253 | 0 | 1 (12.5) | 0.370 |
| Preeclampsia | 0 | 1 (8.3) | 0.253 | 0 | 1 (12.5) | 0.370 |
| Newborns with perinatal complication | 6 (33.3) | 1 (6.25) | 4 (50) | 0 | ||
| Perinatal complications | n = 7 | n = 3 | 0.148 | n = 6 | n = 0 | 0.021 |
| Respiratory distress | 4 (57.1) | 1 (33.3) | 0.240 | 3 (50) | 0 | 0.034 |
| Neonatal jaundice | 1 (14.3) | 1 (33.3) | 0.863 | 1 (16.7) | 0 | 0.244 |
| Neonatal infection | 1 (14.3) | 1 (33.3) | Na | 1 (16.7) | 0 | Na |
| Intestinal stenosis | 1 (14.3) | 0 | Na | 1 (16.7) | 0 | Na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumančić, S.; Mikuš, M.; Palčić, Z.; Habek, D.; Tešanović, M.; Mimica, M.D.; Marušić, J. Severe Early-Onset Intrahepatic Cholestasis of Pregnancy Following Ovarian Hyperstimulation Syndrome with Pulmonary Presentation after In Vitro Fertilization: Case Report and Systematic Review of Case Reports. Life 2024, 14, 129. https://doi.org/10.3390/life14010129
Dumančić S, Mikuš M, Palčić Z, Habek D, Tešanović M, Mimica MD, Marušić J. Severe Early-Onset Intrahepatic Cholestasis of Pregnancy Following Ovarian Hyperstimulation Syndrome with Pulmonary Presentation after In Vitro Fertilization: Case Report and Systematic Review of Case Reports. Life. 2024; 14(1):129. https://doi.org/10.3390/life14010129
Chicago/Turabian StyleDumančić, Stipe, Mislav Mikuš, Zdenka Palčić, Dubravko Habek, Mara Tešanović, Marko Dražen Mimica, and Jelena Marušić. 2024. "Severe Early-Onset Intrahepatic Cholestasis of Pregnancy Following Ovarian Hyperstimulation Syndrome with Pulmonary Presentation after In Vitro Fertilization: Case Report and Systematic Review of Case Reports" Life 14, no. 1: 129. https://doi.org/10.3390/life14010129
APA StyleDumančić, S., Mikuš, M., Palčić, Z., Habek, D., Tešanović, M., Mimica, M. D., & Marušić, J. (2024). Severe Early-Onset Intrahepatic Cholestasis of Pregnancy Following Ovarian Hyperstimulation Syndrome with Pulmonary Presentation after In Vitro Fertilization: Case Report and Systematic Review of Case Reports. Life, 14(1), 129. https://doi.org/10.3390/life14010129






