Bidirectional Regulation of GABAA Reversal Potential in the Adult Brain: Physiological and Pathological Implications
Abstract
1. Introduction
2. GABAA Reversal Potential
2.1. GABAA Reversal Potential and Intracellular Chloride
2.2. Chloride/Potassium Cotransporters
3. Regulation of GABAA Reversal Potential in Physiological Conditions
3.1. Developmental Regulation
3.2. Cell Type and Spatial Heterogeneity of Chloride Transporters
3.3. Compartmental Distribution of Chloride Transporters in Neurons
3.3.1. Somatodendritic Compartment
3.3.2. Axonal Structures
3.4. Circadian Regulation of GABAA Reversal Potential
4. Regulation of Neuronal GABAA Reversal Potential in Disease
4.1. Depolarizing GABAA Current
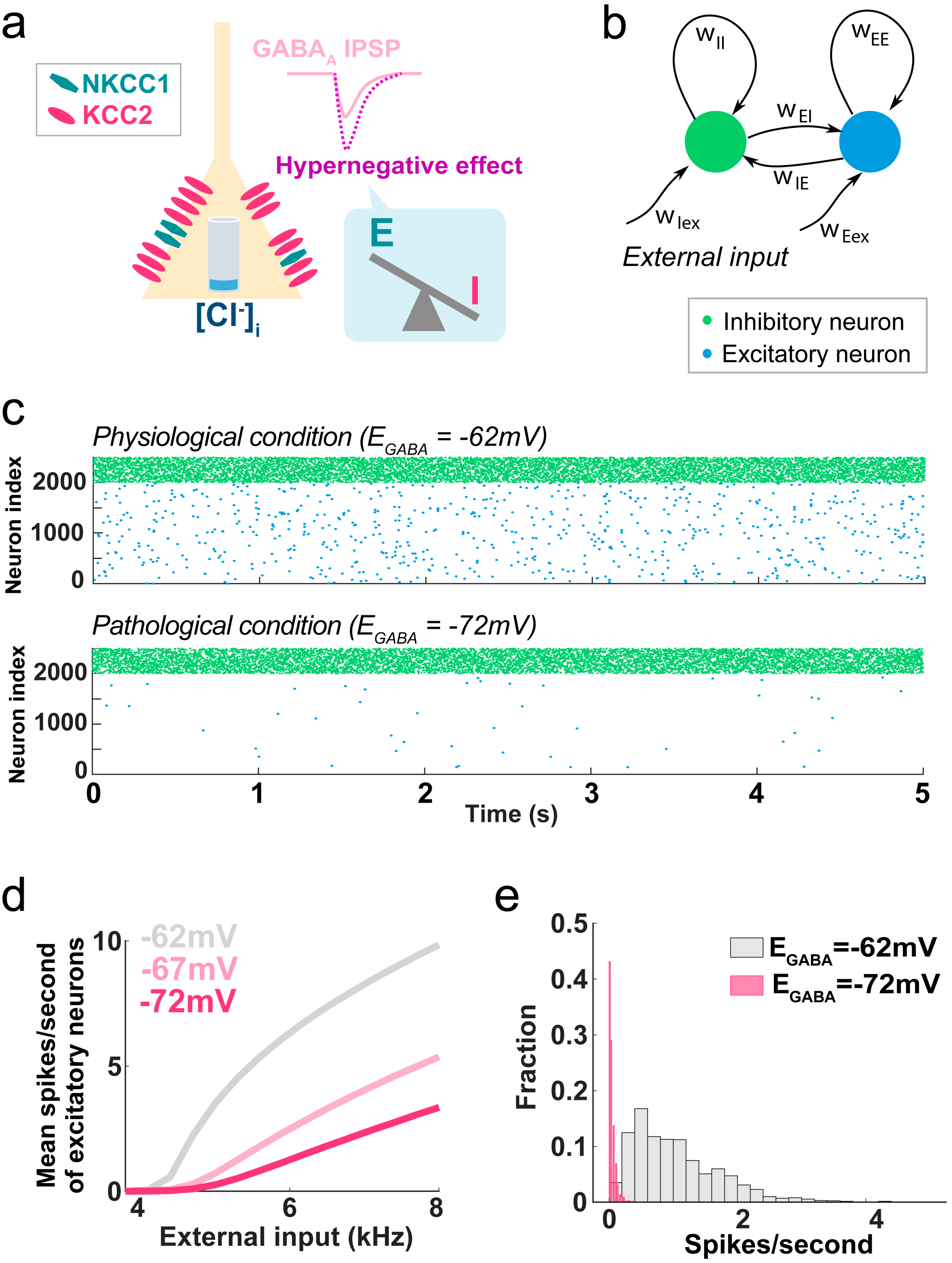
4.2. Hypernegative GABAA Current
4.3. GABAA Flexibility and Pathology
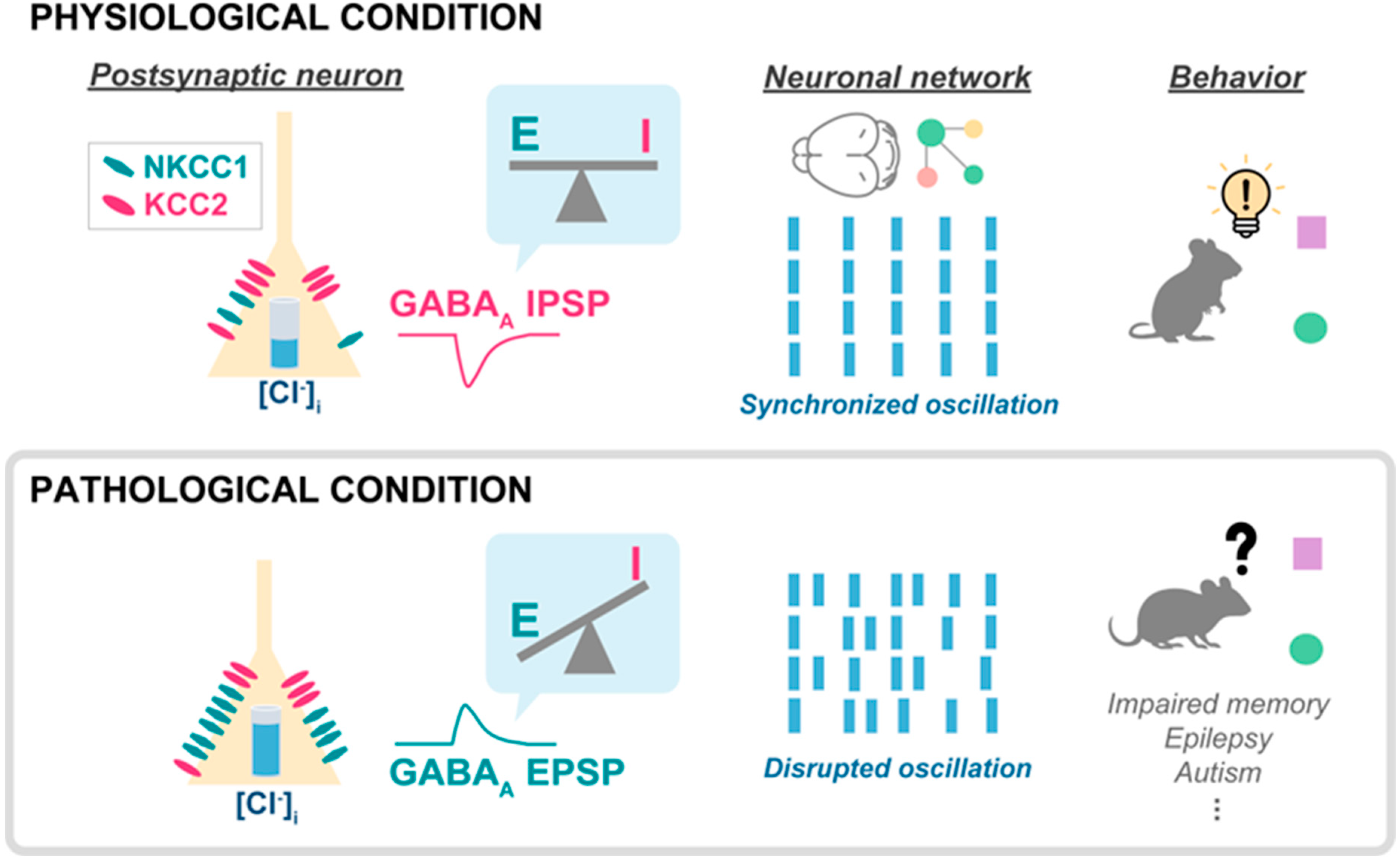
5. Network Effects and Clinical Implications of Flexible GABAA Reversal Potential
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atallah, B.V.; Scanziani, M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 2009, 62, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, N.; Peyrache, A.; Telenczuk, B.; Le Van Quyen, M.; Halgren, E.; Cash, S.S.; Hatsopoulos, N.G.; Destexhe, A. Dynamic Balance of Excitation and Inhibition in Human and Monkey Neocortex. Sci. Rep. 2016, 6, 23176. [Google Scholar] [CrossRef]
- Eichler, S.A.; Meier, J.C. E-I balance and human diseases—From molecules to networking. Front. Mol. Neurosci. 2008, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, J.A.; Froemke, R.C. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 2015, 86, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, J.S.; Scanziani, M. How inhibition shapes cortical activity. Neuron 2011, 72, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Li, Y.T.; Ma, W.P.; Pan, C.J.; Zhang, L.I.; Tao, H.W. Broad inhibition sharpens orientation selectivity by expanding input dynamic range in mouse simple cells. Neuron 2011, 71, 542–554. [Google Scholar] [CrossRef]
- Haider, B.; Hausser, M.; Carandini, M. Inhibition dominates sensory responses in the awake cortex. Nature 2013, 493, 97–100. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; Pletnikova, O.; O’Brien, R.; Yang, A.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Neurotransmitter Imbalance in the Brain and Alzheimer’s Disease Pathology. J. Alzheimers Dis. 2019, 72, 35–43. [Google Scholar] [CrossRef]
- Yang, G.J.; Murray, J.D.; Wang, X.J.; Glahn, D.C.; Pearlson, G.D.; Repovs, G.; Krystal, J.H.; Anticevic, A. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc. Natl. Acad. Sci. USA 2016, 113, E219–E228. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, C.; Maziashvili, N.; Dugladze, T.; Gloveli, T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front. Mol. Neurosci. 2008, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Hare, E.B.; Hagerman, R.J. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr. Dis. Treat. 2014, 10, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, G.; Cho, R.Y.; Lewis, D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatry 2015, 77, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Bormann, J.; Hamill, O.P.; Sakmann, B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 1987, 385, 243–286. [Google Scholar] [CrossRef]
- Kim, H.R.; Long, M.; Sekerkova, G.; Maes, A.; Kennedy, A.; Martina, M. Hypernegative GABA(A) Reversal Potential in Pyramidal Cells Contributes to Medial Prefrontal Cortex Deactivation in a Mouse Model of Neuropathic Pain. J. Pain, 2023; ahead of print. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef]
- Fukuda, A.; Tanaka, M.; Yamada, Y.; Muramatsu, K.; Shimano, Y.; Nishino, H. Simultaneous optical imaging of intracellular Cl- in neurons in different layers of rat neocortical slices: Advantages and limitations. Neurosci. Res. 1998, 32, 363–371. [Google Scholar] [CrossRef]
- Kuner, T.; Augustine, G.J. A genetically encoded ratiometric indicator for chloride: Capturing chloride transients in cultured hippocampal neurons. Neuron 2000, 27, 447–459. [Google Scholar] [CrossRef]
- Kahle, K.T.; Delpire, E. Kinase-KCC2 coupling: Cl- rheostasis, disease susceptibility, therapeutic target. J. Neurophysiol. 2016, 115, 8–18. [Google Scholar] [CrossRef]
- Kurki, S.N.; Uvarov, P.; Pospelov, A.S.; Trontti, K.; Hubner, A.K.; Srinivasan, R.; Watanabe, M.; Hovatta, I.; Hubner, C.A.; Kaila, K.; et al. Expression patterns of NKCC1 in neurons and non-neuronal cells during cortico-hippocampal development. Cereb. Cortex 2023, 33, 5906–5923. [Google Scholar] [CrossRef]
- Markkanen, M.; Karhunen, T.; Llano, O.; Ludwig, A.; Rivera, C.; Uvarov, P.; Airaksinen, M.S. Distribution of neuronal KCC2a and KCC2b isoforms in mouse CNS. J. Comp. Neurol. 2014, 522, 1897–1914. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.Q.; Payne, J.A.; Copenhagen, D.R. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J. Neurosci. 2000, 20, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Hubner, C.A.; Stein, V.; Hermans-Borgmeyer, I.; Meyer, T.; Ballanyi, K.; Jentsch, T.J. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 2001, 30, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.A.; Orlando, B.J.; Zhang, J.; Latorraca, N.R.; Wang, A.; Hollingsworth, S.A.; Chen, D.H.; Dror, R.O.; Liao, M.; Feng, L. Structure and mechanism of the cation-chloride cotransporter NKCC1. Nature 2019, 572, 488–492. [Google Scholar] [CrossRef]
- Neumann, C.; Rosenbaek, L.L.; Flygaard, R.K.; Habeck, M.; Karlsen, J.L.; Wang, Y.; Lindorff-Larsen, K.; Gad, H.H.; Hartmann, R.; Lyons, J.A.; et al. Cryo-EM structure of the human NKCC1 transporter reveals mechanisms of ion coupling and specificity. EMBO J. 2022, 41, e110169. [Google Scholar] [CrossRef] [PubMed]
- Agez, M.; Schultz, P.; Medina, I.; Baker, D.J.; Burnham, M.P.; Cardarelli, R.A.; Conway, L.C.; Garnier, K.; Geschwindner, S.; Gunnarsson, A.; et al. Molecular architecture of potassium chloride co-transporter KCC2. Sci. Rep. 2017, 7, 16452. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chang, S.; Zhao, C.; Wang, F.; Liu, S.; Wang, J.; Delpire, E.; Ye, S.; Guo, J. Structures and an activation mechanism of human potassium-chloride cotransporters. Sci. Adv. 2020, 6, eabc5883. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Nothwang, H.G. NKCC1 and KCC2: Structural insights into phospho-regulation. Front. Mol. Neurosci. 2022, 15, 964488. [Google Scholar] [CrossRef]
- Han, B.; Bellemer, A.; Koelle, M.R. An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans. Genetics 2015, 199, 1159–1172. [Google Scholar] [CrossRef]
- Peerboom, C.; Wierenga, C.J. The postnatal GABA shift: A developmental perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef]
- Cherubini, E.; Gaiarsa, J.L.; Ben-Ari, Y. GABA: An excitatory transmitter in early postnatal life. Trends Neurosci. 1991, 14, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Leinekugel, X.; Khalilov, I.; McLean, H.; Caillard, O.; Gaiarsa, J.L.; Ben-Ari, Y.; Khazipov, R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv. Neurol. 1999, 79, 189–201. [Google Scholar] [PubMed]
- Ben-Ari, Y.; Cherubini, E.; Corradetti, R.; Gaiarsa, J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989, 416, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Verheugen, J.A.; Fricker, D.; Miles, R. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J. Neurosci. 1999, 19, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Dehorter, N.; Vinay, L.; Hammond, C.; Ben-Ari, Y. Timing of developmental sequences in different brain structures: Physiological and pathological implications. Eur. J. Neurosci. 2012, 35, 1846–1856. [Google Scholar] [CrossRef]
- Dzhala, V.I.; Talos, D.M.; Sdrulla, D.A.; Brumback, A.C.; Mathews, G.C.; Benke, T.A.; Delpire, E.; Jensen, F.E.; Staley, K.J. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005, 11, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Kaila, K. Two developmental switches in GABAergic signalling: The K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005, 562 Pt 1, 27–36. [Google Scholar] [CrossRef]
- Yamada, J.; Okabe, A.; Toyoda, H.; Kilb, W.; Luhmann, H.J.; Fukuda, A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 2004, 557 Pt 3, 829–841. [Google Scholar] [CrossRef]
- Su, G.; Kintner, D.B.; Flagella, M.; Shull, G.E.; Sun, D. Astrocytes from Na(+)-K(+)-Cl(−) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am. J. Physiol. Cell Physiol. 2002, 282, C1147–C1160. [Google Scholar] [CrossRef]
- Kanaka, C.; Ohno, K.; Okabe, A.; Kuriyama, K.; Itoh, T.; Fukuda, A.; Sato, K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 2001, 104, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, T. The Role of Astrocytes in the Modulation of K(+)-Cl(−)-Cotransporter-2 Function. Int. J. Mol. Sci. 2020, 21, 9539. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Ishibashi, M.; Sinha, A.S.; Watanabe, M.; Kato, D.; Horiuchi, H.; Wake, H.; Fukuda, A. Astrocytic NKCC1 inhibits seizures by buffering Cl(−) and antagonizing neuronal NKCC1 at GABAergic synapses. Epilepsia 2023, 64, 3389–3403. [Google Scholar] [CrossRef]
- Fellin, T.; Pascual, O.; Gobbo, S.; Pozzan, T.; Haydon, P.G.; Carmignoto, G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 2004, 43, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Covelo, A.; Araque, A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 2018, 7, e32237. [Google Scholar] [CrossRef] [PubMed]
- Huda, R.; Chang, Z.; Do, J.; McCrimmon, D.R.; Martina, M. Activation of astrocytic PAR1 receptors in the rat nucleus of the solitary tract regulates breathing through modulation of presynaptic TRPV1. J. Physiol. 2018, 596, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Kintner, D.B.; Luo, J.; Gerdts, J.; Ballard, A.J.; Shull, G.E.; Sun, D. Role of Na+-K+-Cl− cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am. J. Physiol. Cell Physiol. 2007, 292, C1113–C1122. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Verkhratsky, A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia 2016, 64, 1611–1627. [Google Scholar] [CrossRef]
- Kahle, K.T.; Simard, J.M.; Staley, K.J.; Nahed, B.V.; Jones, P.S.; Sun, D. Molecular mechanisms of ischemic cerebral edema: Role of electroneutral ion transport. Physiology 2009, 24, 257–265. [Google Scholar] [CrossRef]
- Kim, H.R.; Rajagopal, L.; Meltzer, H.Y.; Martina, M. Depolarizing GABA(A) current in the prefrontal cortex is linked with cognitive impairment in a mouse model relevant for schizophrenia. Sci. Adv. 2021, 7, eaba5032. [Google Scholar] [CrossRef]
- Del Turco, D.; Paul, M.H.; Schlaudraff, J.; Muellerleile, J.; Bozic, F.; Vuksic, M.; Jedlicka, P.; Deller, T. Layer-specific changes of KCC2 and NKCC1 in the mouse dentate gyrus after entorhinal denervation. Front. Mol. Neurosci. 2023, 16, 1118746. [Google Scholar] [CrossRef] [PubMed]
- Otsu, Y.; Donneger, F.; Schwartz, E.J.; Poncer, J.C. Cation-chloride cotransporters and the polarity of GABA signalling in mouse hippocampal parvalbumin interneurons. J. Physiol. 2020, 598, 1865–1880. [Google Scholar] [CrossRef] [PubMed]
- Pol, E.; Come, E.; Merlaud, Z.; Gouhier, J.; Russeau, M.; Scotto-Lomassese, S.; Moutkine, I.; Marques, X.; Levi, S. NKCC1 and KCC2 Chloride Transporters Have Different Membrane Dynamics on the Surface of Hippocampal Neurons. Cells 2023, 12, 2363. [Google Scholar] [CrossRef] [PubMed]
- Baldi, R.; Varga, C.; Tamas, G. Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur. J. Neurosci. 2010, 32, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Wicki-Stordeur, L.E.; Groten, C.J.; LeDue, J.M.; Kahle, K.T.; MacVicar, B.A. KCC2 drives chloride microdomain formation in dendritic blebbing. Cell Rep. 2022, 41, 111556. [Google Scholar] [CrossRef] [PubMed]
- Al Awabdh, S.; Donneger, F.; Goutierre, M.; Seveno, M.; Vigy, O.; Weinzettl, P.; Russeau, M.; Moutkine, I.; Levi, S.; Marin, P.; et al. Gephyrin Interacts with the K-Cl Cotransporter KCC2 to Regulate Its Surface Expression and Function in Cortical Neurons. J. Neurosci. 2022, 42, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Gulyas, A.I.; Sik, A.; Payne, J.A.; Kaila, K.; Freund, T.F. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 2001, 13, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Chamma, I.; Heubl, M.; Chevy, Q.; Renner, M.; Moutkine, I.; Eugene, E.; Poncer, J.C.; Levi, S. Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J. Neurosci. 2013, 33, 15488–15503. [Google Scholar] [CrossRef]
- Chamma, I.; Chevy, Q.; Poncer, J.C.; Levi, S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell Neurosci. 2012, 6, 5. [Google Scholar] [CrossRef]
- Woodin, M.A.; Ganguly, K.; Poo, M.M. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron 2003, 39, 807–820. [Google Scholar] [CrossRef]
- Khirug, S.; Yamada, J.; Afzalov, R.; Voipio, J.; Khiroug, L.; Kaila, K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J. Neurosci. 2008, 28, 4635–4639. [Google Scholar] [CrossRef] [PubMed]
- Szabadics, J.; Varga, C.; Molnar, G.; Olah, S.; Barzo, P.; Tamas, G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 2006, 311, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Rinetti-Vargas, G.; Phamluong, K.; Ron, D.; Bender, K.J. Periadolescent Maturation of GABAergic Hyperpolarization at the Axon Initial Segment. Cell Rep. 2017, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pracucci, E.; Graham, R.T.; Alberio, L.; Nardi, G.; Cozzolino, O.; Pillai, V.; Pasquini, G.; Saieva, L.; Walsh, D.; Landi, S.; et al. Daily rhythm in cortical chloride homeostasis underpins functional changes in visual cortex excitability. Nat. Commun. 2023, 14, 7108. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, E.; Di Cristo, G.; Avoli, M. Dysregulation of GABAergic Signaling in Neurodevelomental Disorders: Targeting Cation-Chloride Co-transporters to Re-establish a Proper E/I Balance. Front. Cell Neurosci. 2021, 15, 813441. [Google Scholar] [CrossRef] [PubMed]
- Savardi, A.; Borgogno, M.; De Vivo, M.; Cancedda, L. Pharmacological tools to target NKCC1 in brain disorders. Trends Pharmacol. Sci. 2021, 42, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Navarro, V.; Clemenceau, S.; Baulac, M.; Miles, R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 2002, 298, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Deidda, G.; Parrini, M.; Naskar, S.; Bozarth, I.F.; Contestabile, A.; Cancedda, L. Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat. Med. 2015, 21, 318–326. [Google Scholar] [CrossRef]
- MacKenzie, G.; Maguire, J. Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res. 2015, 109, 13–27. [Google Scholar] [CrossRef]
- Arion, D.; Lewis, D.A. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch. Gen. Psychiatry 2011, 68, 21–31. [Google Scholar] [CrossRef]
- Jentsch, J.D.; Roth, R.H. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999, 20, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Steeds, H.; Carhart-Harris, R.L.; Stone, J.M. Drug models of schizophrenia. Ther. Adv. Psychopharmacol. 2015, 5, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Merner, N.D.; Mercado, A.; Khanna, A.R.; Hodgkinson, A.; Bruat, V.; Awadalla, P.; Gamba, G.; Rouleau, G.A.; Kahle, K.T. Gain-of-function missense variant in SLC12A2, encoding the bumetanide-sensitive NKCC1 cotransporter, identified in human schizophrenia. J. Psychiatr. Res. 2016, 77, 22–26. [Google Scholar] [CrossRef]
- Chen, S.R.; Zhu, L.; Chen, H.; Wen, L.; Laumet, G.; Pan, H.L. Increased spinal cord Na(+)-K(+)-2Cl(−) cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J. Biol. Chem. 2014, 289, 31111–31120. [Google Scholar] [CrossRef] [PubMed]
- Ferando, I.; Faas, G.C.; Mody, I. Diminished KCC2 confounds synapse specificity of LTP during senescence. Nat. Neurosci. 2016, 19, 1197–1200. [Google Scholar] [CrossRef]
- Lam, P.; Vinnakota, C.; Guzmán, B.C.F.; Newland, J.; Peppercorn, K.; Tate, W.P.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Beta-Amyloid (Aβ) Increases the Expression of NKCC1 in the Mouse Hippocampus. Molecules 2022, 27, 2440. [Google Scholar] [CrossRef]
- Keramidis, I.; McAllister, B.B.; Bourbonnais, J.; Wang, F.; Isabel, D.; Rezaei, E.; Sansonetti, R.; Degagne, P.; Hamel, J.P.; Nazari, M.; et al. Restoring neuronal chloride extrusion reverses cognitive decline linked to Alzheimer’s disease mutations. Brain 2023, 146, 4903–4915. [Google Scholar] [CrossRef]
- Rong, J.; Yang, Y.; Liang, M.; Zhong, H.; Li, Y.; Zhu, Y.; Sha, S.; Chen, L.; Zhou, R. Neonatal inflammation increases hippocampal KCC2 expression through methylation-mediated TGF-beta1 downregulation leading to impaired hippocampal cognitive function and synaptic plasticity in adult mice. J. Neuroinflamm. 2023, 20, 15. [Google Scholar] [CrossRef]
- Orlov, S.N. NKCC1 as an epigenetically regulated transporter involved in blood pressure elevation with age. Am. J. Hypertens. 2011, 24, 1264. [Google Scholar] [CrossRef][Green Version]
- Raghavachari, S.; Kahana, M.J.; Rizzuto, D.S.; Caplan, J.B.; Kirschen, M.P.; Bourgeois, B.; Madsen, J.R.; Lisman, J.E. Gating of human theta oscillations by a working memory task. J. Neurosci. 2001, 21, 3175–3183. [Google Scholar] [CrossRef]
- Lee, H.; Simpson, G.V.; Logothetis, N.K.; Rainer, G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 2005, 45, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mulert, C.; Kirsch, V.; Pascual-Marqui, R.; McCarley, R.W.; Spencer, K.M. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int. J. Psychophysiol. 2011, 79, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Gonzalez, M.; Gener, T.; Nebot, P.; Vilademunt, M.; Dierssen, M.; Puig, M.V. Prefrontal-hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability. Proc. Natl. Acad. Sci. USA 2020, 117, 11788–11798. [Google Scholar] [CrossRef] [PubMed]
- Fricker, D.; Miles, R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron 2000, 28, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Cardin, J.A. Inhibitory Interneurons Regulate Temporal Precision and Correlations in Cortical Circuits. Trends Neurosci. 2018, 41, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.R.; Gao, W.J. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front. Neural Circuits 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R.; Cowan, J.D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 1972, 12, 1–24. [Google Scholar] [CrossRef]
- Loscher, W.; Kaila, K. CNS pharmacology of NKCC1 inhibitors. Neuropharmacology 2022, 205, 108910. [Google Scholar] [CrossRef]
- Kahle, K.T.; Deeb, T.Z.; Puskarjov, M.; Silayeva, L.; Liang, B.; Kaila, K.; Moss, S.J. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 2013, 36, 726–737. [Google Scholar] [CrossRef]
- Porcher, C.; Medina, I.; Gaiarsa, J.L. Mechanism of BDNF Modulation in GABAergic Synaptic Transmission in Healthy and Disease Brains. Front. Cell Neurosci. 2018, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.R.; Martina, M. Bidirectional Regulation of GABAA Reversal Potential in the Adult Brain: Physiological and Pathological Implications. Life 2024, 14, 143. https://doi.org/10.3390/life14010143
Kim HR, Martina M. Bidirectional Regulation of GABAA Reversal Potential in the Adult Brain: Physiological and Pathological Implications. Life. 2024; 14(1):143. https://doi.org/10.3390/life14010143
Chicago/Turabian StyleKim, Haram R., and Marco Martina. 2024. "Bidirectional Regulation of GABAA Reversal Potential in the Adult Brain: Physiological and Pathological Implications" Life 14, no. 1: 143. https://doi.org/10.3390/life14010143
APA StyleKim, H. R., & Martina, M. (2024). Bidirectional Regulation of GABAA Reversal Potential in the Adult Brain: Physiological and Pathological Implications. Life, 14(1), 143. https://doi.org/10.3390/life14010143





