Heterogeneous Expression of Arabidopsis Subclass II of SNF1-Related Kinase 2 Improves Drought Tolerance via Stomatal Regulation in Poplar
Abstract
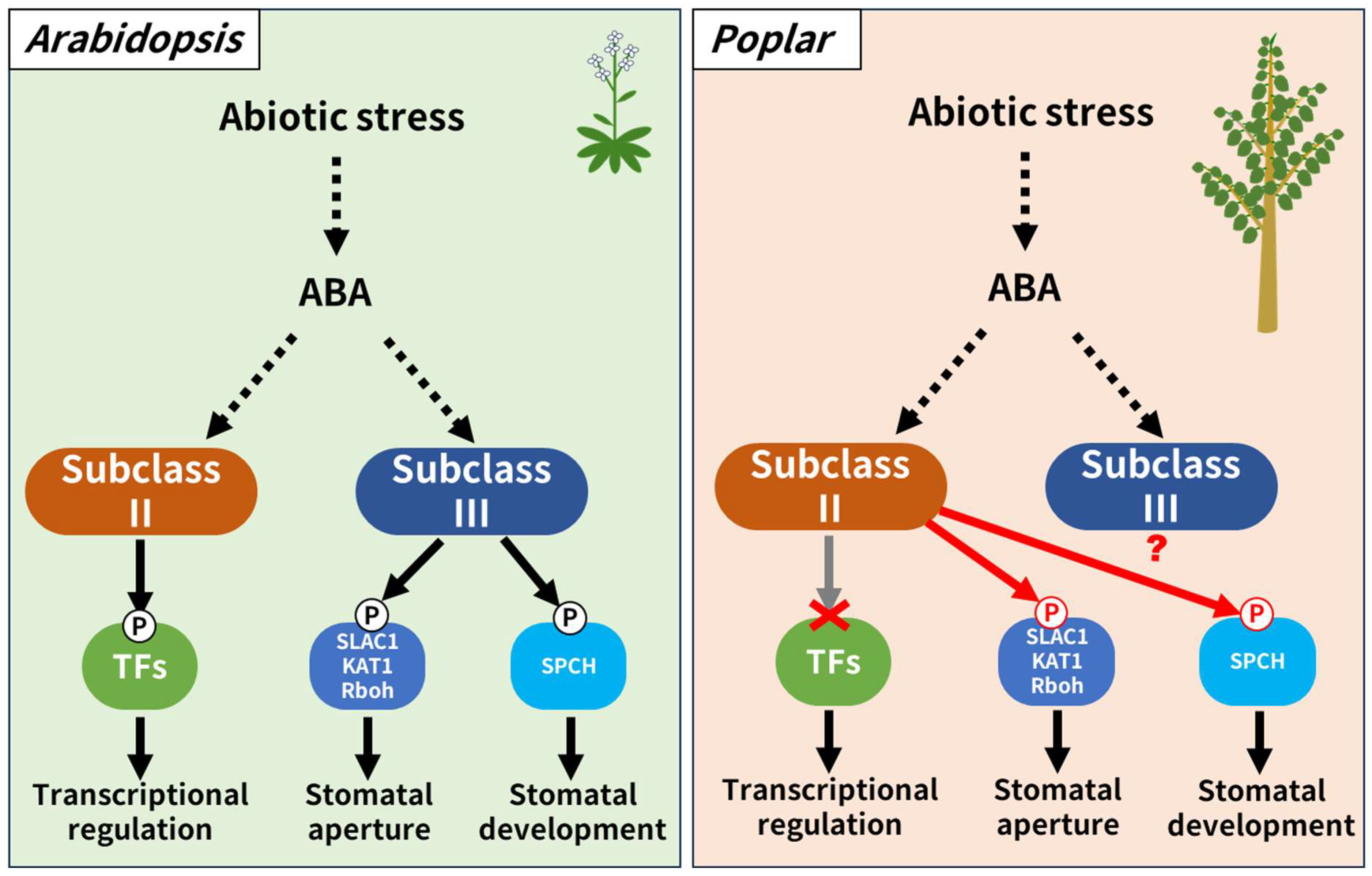
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation Conditions
2.2. DNA Gel Blot Analysis
2.3. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.4. Osmotic Stress Treatments
2.5. Measurement of Photosynthesis Quantum Yields (QYs)
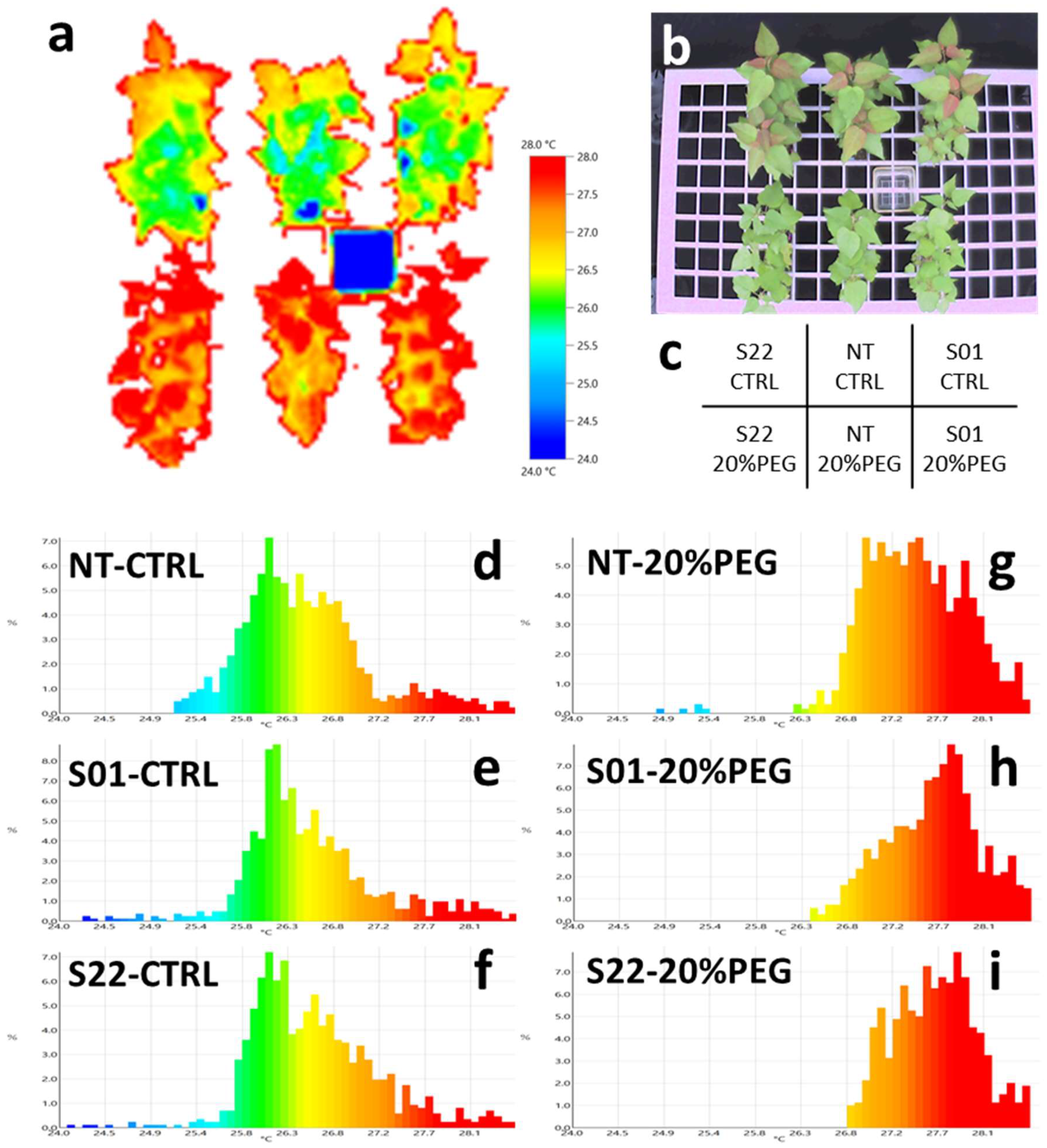
2.6. Thermography Analysis of Leaf Surface Temperature
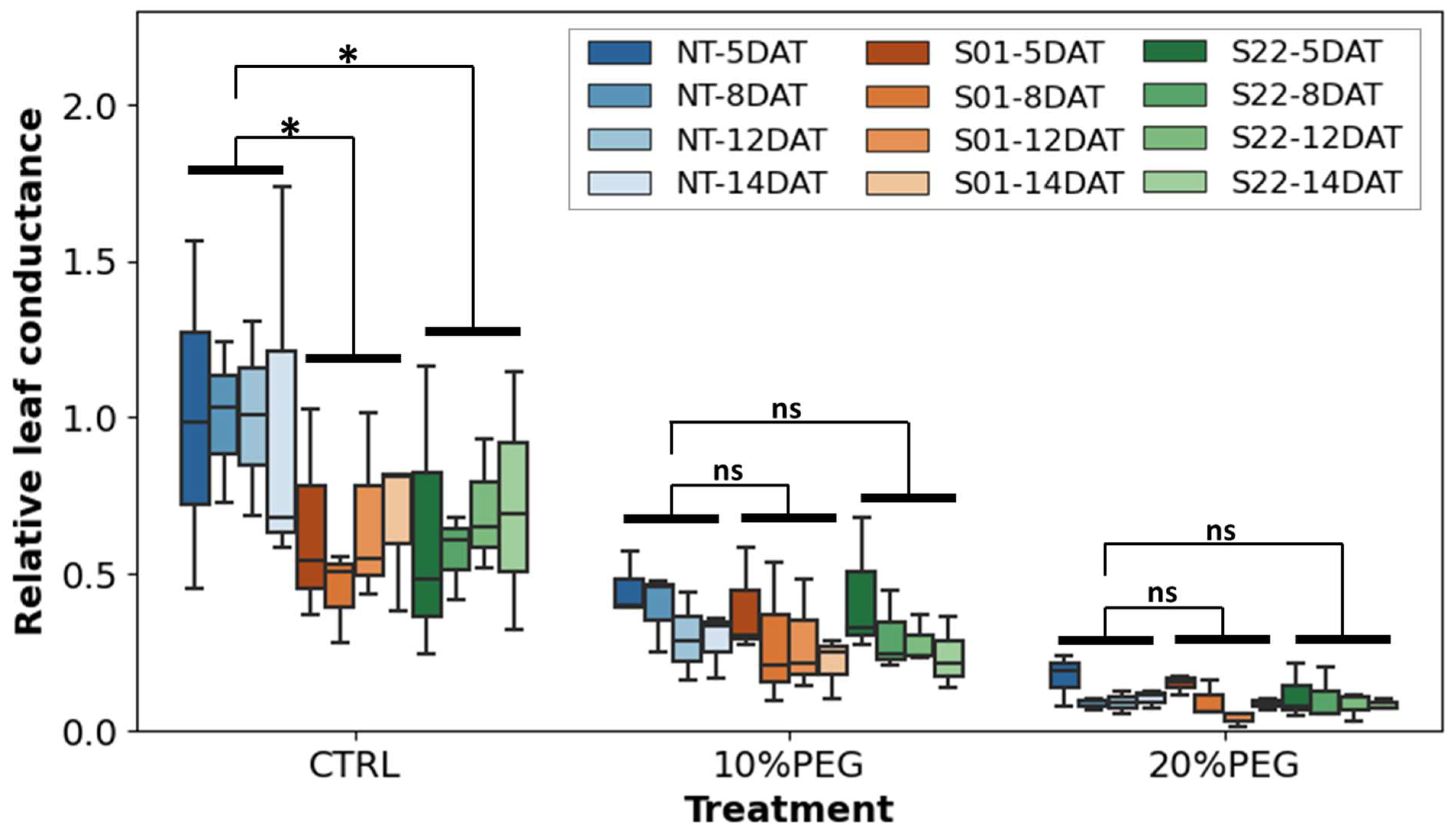
2.7. Leaf Conductance
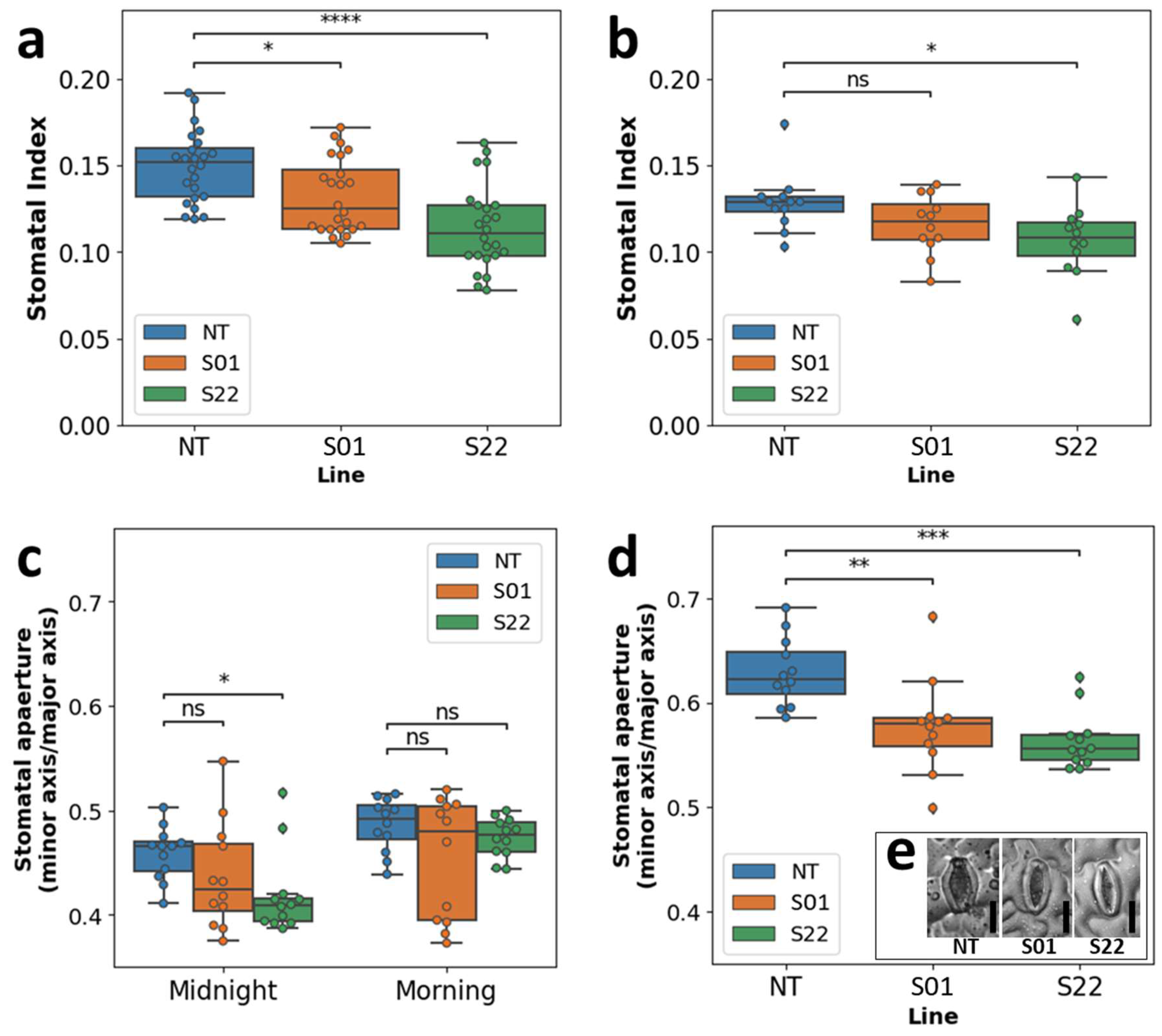
2.8. Analysis of Stomata from Leaf Imprints
2.9. Statistical Analysis
3. Results
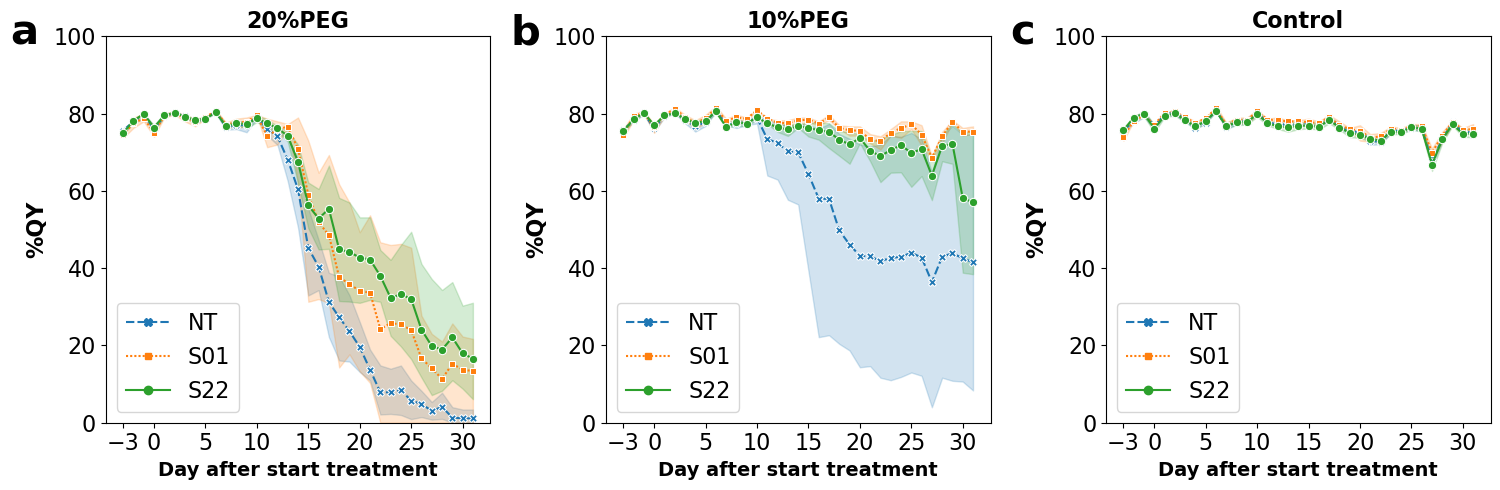
3.1. AtSnRK2.8 Transgenic Poplar Lines Showed Improved Tolerance to Moderate Osmotic Stress under Netted Greenhouse Conditions
3.2. AtSnRK2.8 Affects Leaf Conductance of Poplar in Both Stress and Normal Conditions
3.3. AtSnRK2.8 Might Affect Both Stomatal Index and Aperture in Poplar
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Gennaro, M.; Nervo, G.; Secchi, F.; Carra, A. Responses to Drought Stress in Poplar: What Do We Know and What Can We Learn? Life 2023, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Umezawa, T. The PP2C–SnRK2 Complex—The Central Regulation of an Abscisic Acid Signal Pathway. Plant Signal. Behav. 2010, 5, 160–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Crozet, P.; Margalha, L.; Confraria, A.; Rodrigues, A.; Martinho, C.; Adamo, M.; Elias, C.A.; Baena-González, E. Mechanisms of Regulation of SNF1/AMPK/SnRK1 Protein Kinases. Front. Plant Sci. 2014, 5, 190. [Google Scholar] [CrossRef]
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK Superfamily of Protein Kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef]
- Komatsu, K.; Takezawa, D.; Sakata, Y. Decoding ABA and Osmostress Signaling in Plants from an Evolutionary Point of View. Plant Cell Environ. 2020, 43, 2894–2911. [Google Scholar] [CrossRef]
- Kulik, A.; Wawer, I.; Krzywińska, E.; Bucholc, M.; Dobrowolska, G. SnRK2 Protein Kinases—Key Regulators of Plant Response to Abiotic Stresses. OMICS 2011, 15, 859–872. [Google Scholar] [CrossRef]
- Jamsheer, M.K.; Jindal, S.; Laxmi, A. Evolution of TOR–SnRK dynamics in green plants and its integration with phytohormone signaling networks. J. Exp. Bot. 2019, 70, 2239–2259. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, M.H.; Kim, J.-I.; Kim, S.Y. Arabidopsis Putative MAP Kinase Kinase Kinases Raf10 and Raf11 Are Positive Regulators of Seed Dormancy and ABA Response. Plant Cell Physiol. 2015, 56, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Int. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Fàbregas, N.; Yoshida, T.; Fernie, A.R. Role of Raf-like Kinases in SnRK2 Activation and Osmotic Stress Response in Plants. Nat. Commun. 2020, 11, 6184. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential Activation of the Rice Sucrose Nonfermenting1–Related Protein Kinase2 Family by Hyperosmotic Stress and Abscisic Acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef]
- Umezawa, T.; Yoshida, R.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. SRK2C, a SNF1-Related Protein Kinase 2, Improves Drought Tolerance by Controlling Stress-Responsive Gene Expression in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 17306–17311. [Google Scholar] [CrossRef]
- Fang, S.; Xue, J.; Tang, L. Biomass Production and Carbon Sequestration Potential in Poplar Plantations with Different Management Patterns. J. Environ. Manag. 2007, 85, 672–679. [Google Scholar] [CrossRef]
- Brunner, A.M.; Busov, V.B.; Strauss, S.H. Poplar Genome Sequence: Functional Genomics in an Ecologically Dominant Plant Species. Trends Plant Sci. 2004, 9, 49–56. [Google Scholar] [CrossRef]
- Jansson, S.; Douglas, C.J. Populus: A Model System for Plant Biology. Annu. Rev. Plant Biol. 2007, 58, 435–458. [Google Scholar] [CrossRef]
- Polle, A.; Douglas, C. The Molecular Physiology of Poplars: Paving the Way for Knowledge-Based Biomass Production. Plant Biol. 2010, 12, 239–241. [Google Scholar] [CrossRef]
- Song, X.; Ohtani, M.; Hori, C.; Takebayasi, A.; Hiroyama, R.; Rejab, N.A.; Suzuki, T.; Demura, T.; Yin, T.; Yu, X.; et al. Physical Interaction between SnRK2 and PP2C Is Conserved in Populus trichocarpa. Plant Biotechnol. 2015, 32, 337–341. [Google Scholar] [CrossRef][Green Version]
- Song, X.; Yu, X.; Hori, C.; Demura, T.; Ohtani, M.; Zhuge, Q. Heterologous Overexpression of Poplar SnRK2 Genes Enhanced Salt Stress Tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Takebayashi, A.; Demura, T.; Ohtani, M. Differential Expression of Poplar Sucrose Nonfermenting1-Related Protein Kinase 2 Genes in Response to Abiotic Stress and Abscisic Acid. J. Plant Res. 2017, 130, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Movahedi, A.; Xu, C.; Wang, P.; Sun, W.; Yin, T.; Zhuge, Q. Heterologous Overexpression of the Arabidopsis SnRK2.8 Gene Enhances Drought and Salt Tolerance in Populus × euramericana cv ‘Nanlin895’. Plant Biotechnol. Rep. 2019, 13, 245–261. [Google Scholar] [CrossRef]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 Regulator SLAC1 and Its Homologues Are Essential for Anion Homeostasis in Plant Cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gavya, S.L.; Zhou, Z.; Urano, D.; Lau, O.S. Abscisic Acid Regulates Stomatal Production by Imprinting a SnRK2 Kinase–Mediated Phosphocode on the Master Regulator SPEECHLESS. Sci. Adv. 2022, 8, eadd2063. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ohtani, M.; Kusano, M.; Nishikubo, N.; Uenoyama, M.; Umezawa, T.; Saito, K.; Shinozaki, K.; Demura, T. Enhancement of Abiotic Stress Tolerance in Poplar by Overexpression of Key Arabidopsis Stress Response Genes, AtSRK2C and AtGolS2. Mol. Breed. 2017, 37, 57. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Oguchi, T.; Matsunaga, E.; Kawaoka, A.; Watanabe, K.N.; Akira, K. Transcriptional enhancement of a bacterial choline oxidase A gene by an HSP terminator improves the glycine betaine production and salinity stress tolerance of Eucalyptus camaldulensis trees. Plant Biotechnol. 2018, 35, 215–224. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Pathoumthong, P.; Zhang, Z.; Roy, S.J.; El Habti, A. Rapid non-destructive method to phenotype stomatal traits. Plant Methods 2023, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Okayama, K.; Hirokawa, T. Determination of leaf color in rice plant, “Koshihikari” with a chlorophyll meter. Bull. Toyama Agric. Res. Cent. 1982, A-1, 9–16. [Google Scholar]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office for Disaster Risk Reduction. GAR Special Report on Drought 2021; United Nations Office for Disaster Risk Reduction (UNDRR): Geneva, Switzerland, 2021; ISBN 9789212320274. [Google Scholar]
- Kim, W.; Iizumi, T.; Nishimori, M. Global Patterns of Crop Production Losses Associated with Droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Richardson, J. Ecology and Physiology of Poplars and Willows. In Poplars and Willows: Trees for Society and the Environment; CABI: Wallingford, UK, 2014; p. 111. [Google Scholar]
- Shikakura, Y.; Oguchi, T.; Yu, X.; Ohtani, M.; Demura, T.; Kikuchi, A.; Watanabe, K.N. Transgenic Poplar Trees Overexpressing AtGolS2, a Stress-Responsive Galactinol Synthase Gene Derived from Arabidopsis thaliana, Improved Drought Tolerance in a Confined Field. Transgenic Res. 2022, 31, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Acharya, B.R.; Jeon, B.W.; Zhang, W.; Assmann, S.M. Open Stomata 1 (OST1) Is Limiting in Abscisic Acid Responses of Arabidopsis Guard Cells. New Phytol. 2013, 200, 1049–1063. [Google Scholar] [CrossRef]
- Lou, D.; Wang, H.; Liang, G.; Yu, D. OsSAPK2 Confers Abscisic Acid Sensitivity and Tolerance to Drought Stress in Rice. Front. Plant Sci. 2017, 8, 993. [Google Scholar] [CrossRef]
- Tian, S.; Mao, X.; Zhang, H.; Chen, S.; Zhai, C.; Yang, S.; Jing, R. Cloning and Characterization of TaSnRK2.3, a Novel SnRK2 Gene in Common Wheat. J. Exp. Bot. 2013, 64, 2063–2080. [Google Scholar] [CrossRef]
- Lou, D.; Lu, S.; Chen, Z.; Lin, Y.; Yu, D.; Yang, X. Molecular Characterization Reveals That OsSAPK3 Improves Drought Tolerance and Grain Yield in Rice. BMC Plant Biol. 2023, 23, 53. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvat, B.; Shikakura, Y.; Ohtani, M.; Demura, T.; Kikuchi, A.; Watanabe, K.N.; Oguchi, T. Heterogeneous Expression of Arabidopsis Subclass II of SNF1-Related Kinase 2 Improves Drought Tolerance via Stomatal Regulation in Poplar. Life 2024, 14, 161. https://doi.org/10.3390/life14010161
Horvat B, Shikakura Y, Ohtani M, Demura T, Kikuchi A, Watanabe KN, Oguchi T. Heterogeneous Expression of Arabidopsis Subclass II of SNF1-Related Kinase 2 Improves Drought Tolerance via Stomatal Regulation in Poplar. Life. 2024; 14(1):161. https://doi.org/10.3390/life14010161
Chicago/Turabian StyleHorvat, Borislav, Yuhei Shikakura, Misato Ohtani, Taku Demura, Akira Kikuchi, Kazuo N. Watanabe, and Taichi Oguchi. 2024. "Heterogeneous Expression of Arabidopsis Subclass II of SNF1-Related Kinase 2 Improves Drought Tolerance via Stomatal Regulation in Poplar" Life 14, no. 1: 161. https://doi.org/10.3390/life14010161
APA StyleHorvat, B., Shikakura, Y., Ohtani, M., Demura, T., Kikuchi, A., Watanabe, K. N., & Oguchi, T. (2024). Heterogeneous Expression of Arabidopsis Subclass II of SNF1-Related Kinase 2 Improves Drought Tolerance via Stomatal Regulation in Poplar. Life, 14(1), 161. https://doi.org/10.3390/life14010161






