The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome—A Comprehensive Review
Abstract
1. Introduction
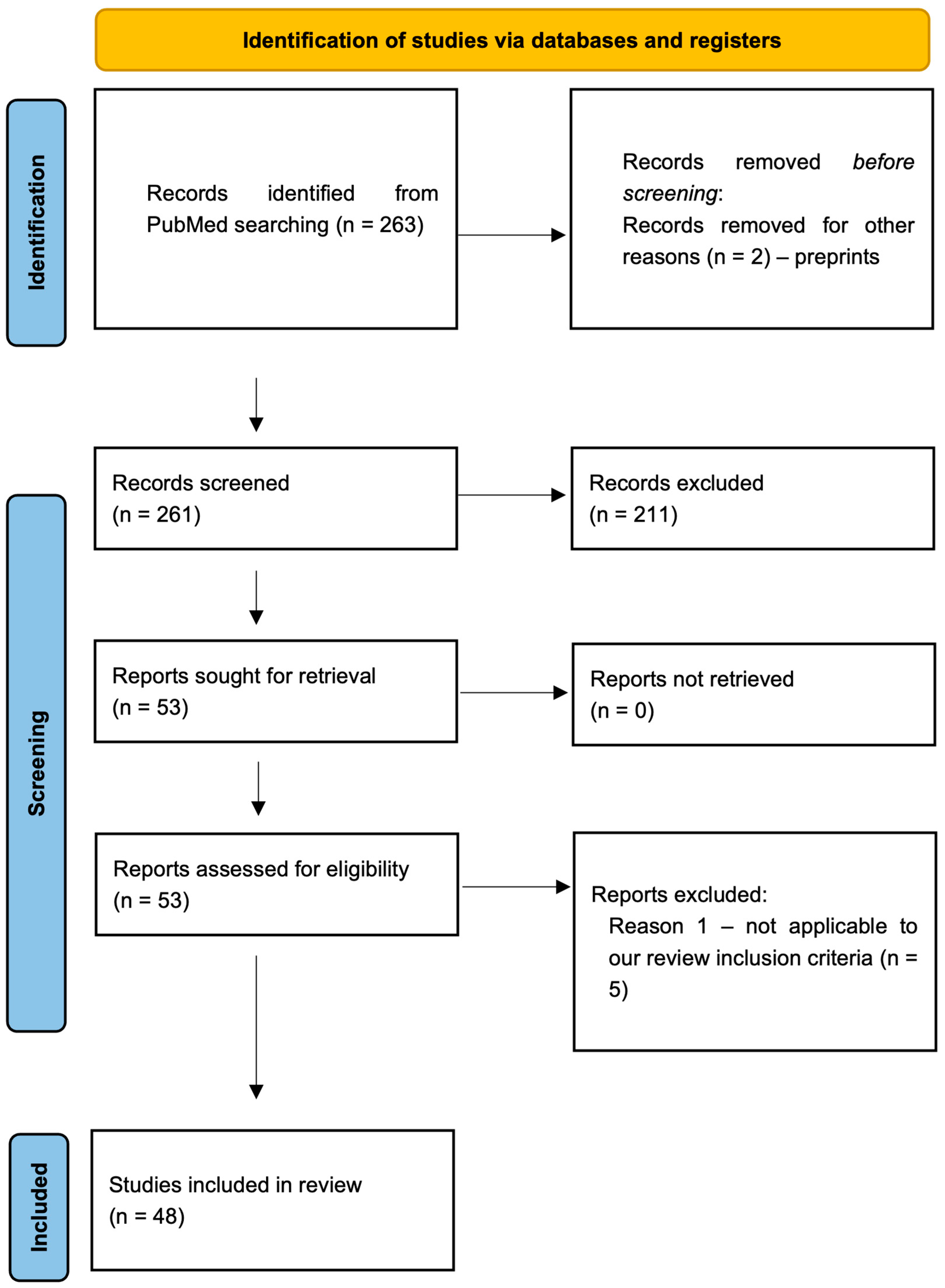
2. Methods
Literature Search Strategy
- Original research articles (clinical or basic science) on opioid use, including sequencing and analysis of the gastrointestinal microbiome;
- Studies presenting data on microbial composition, diversity, or function;
- Peer-reviewed articles, written in English.
3. Results
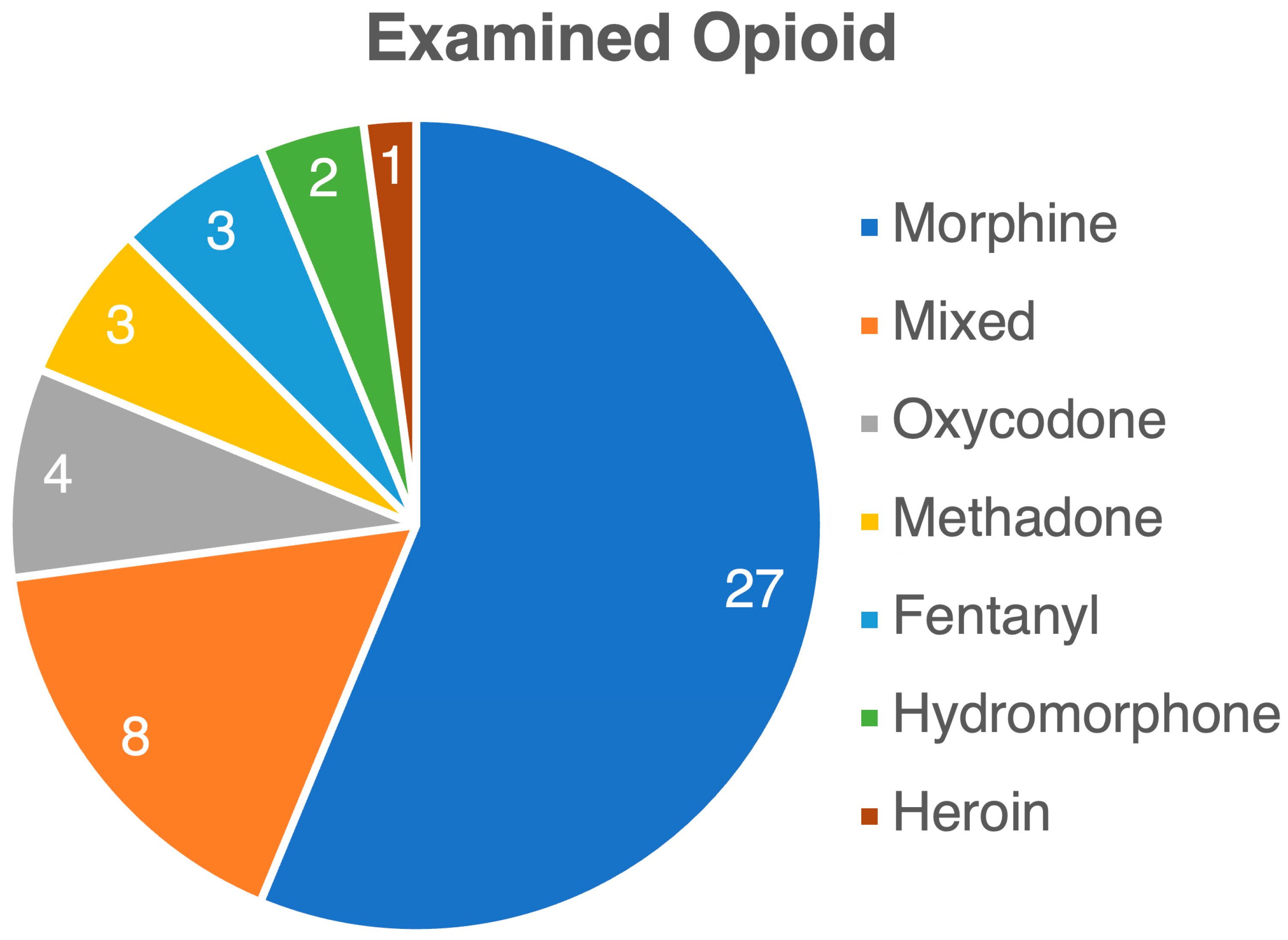
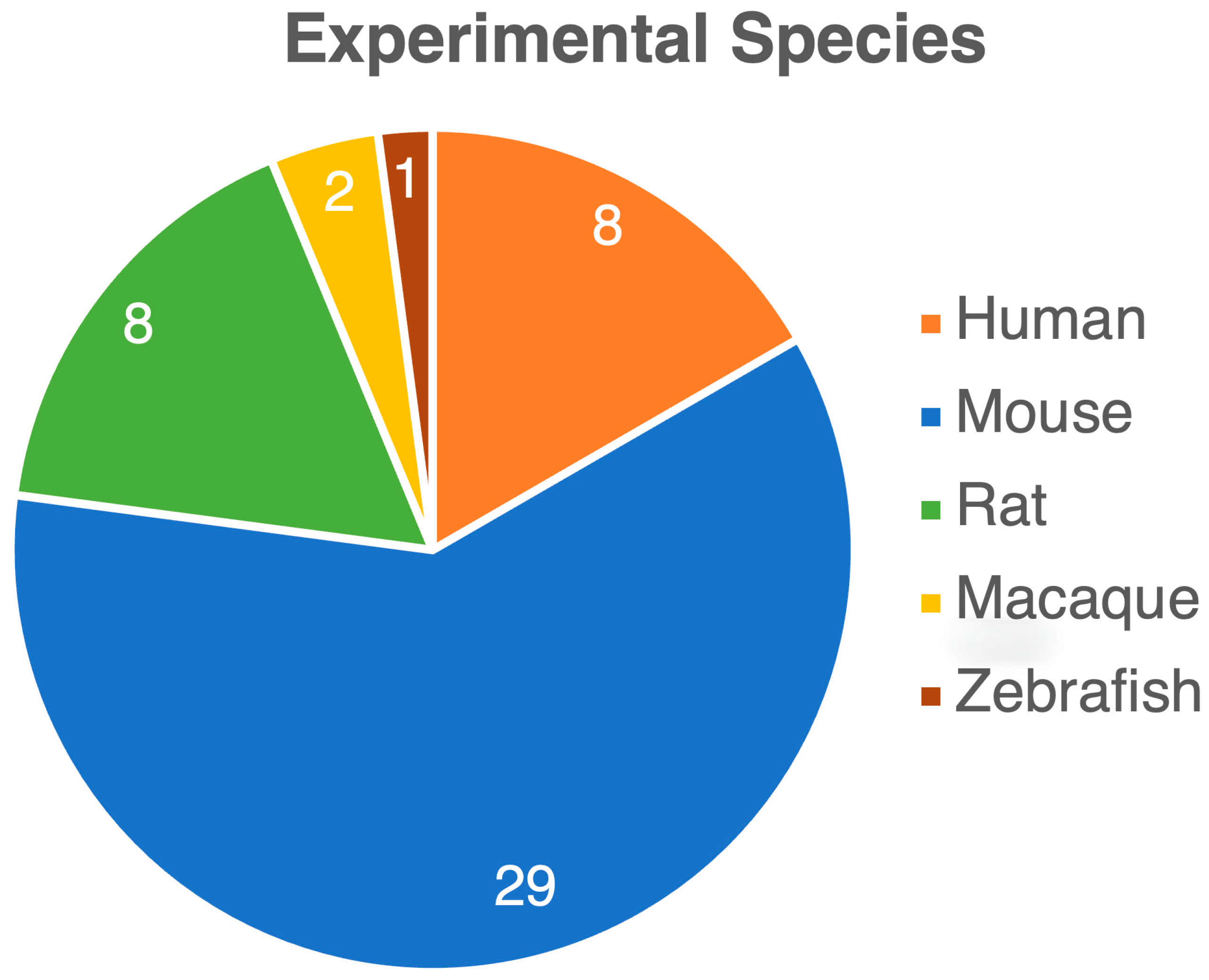
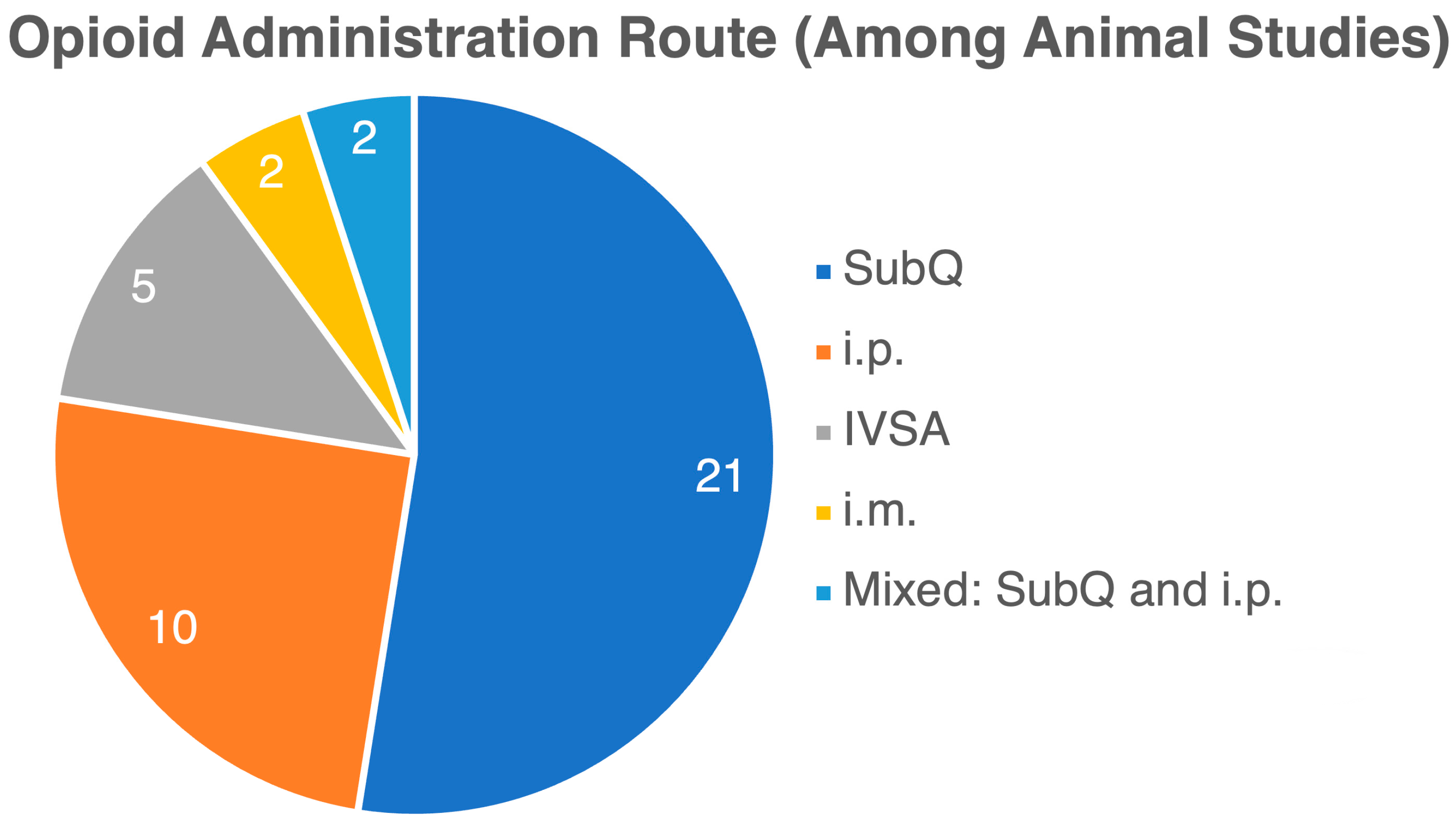
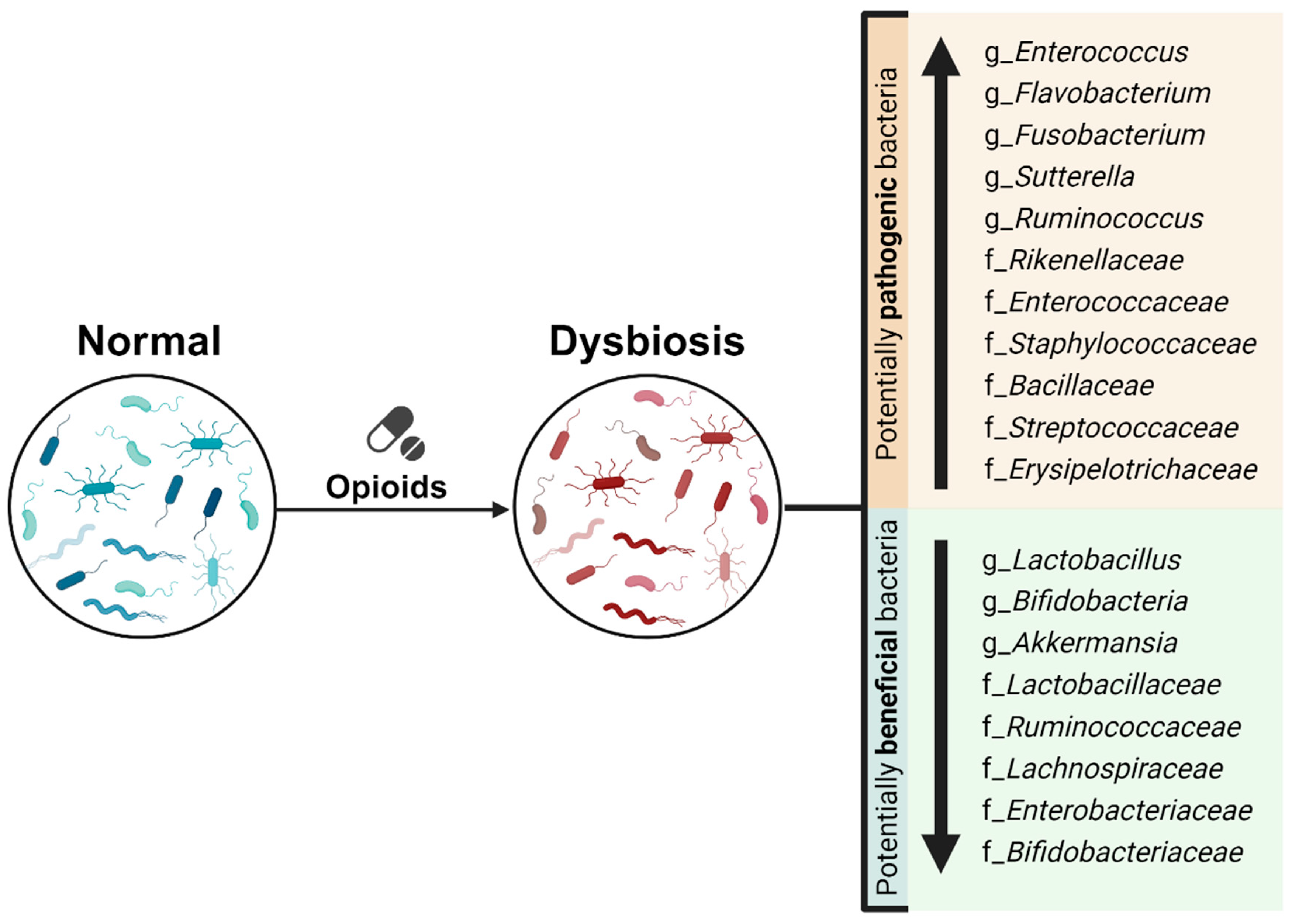
3.1. Opioid Use Impact on Gut Microbiome: Changes in Gut Microbiome Composition and Diversity
3.1.1. Opioid Use Impact on α-Diversity and β-Diversity
3.1.2. Opioid Use Impact on Microbial Taxonomic Composition
| No. | Author | Experimental Species | Examined Opioid | Treatment Protocol | Opioids Impact on Gut Microbiome Diversity | Opioids Impact on Gut Microbiome Composition | |
|---|---|---|---|---|---|---|---|
| Increased Abundance | Decreased Abundance | ||||||
| 1. | Meng et al., 2013 [18] | Mouse | Morphine | 75 mg pellet for 24 h SubQ | No data | No data | No data |
| 2. | Meng et al., 2015 [64] | Mouse | Morphine | 25 mg pellet for 3 days SubQ | Alpha diversity: no data Beta diversity: Morphine-treated CLP animals clustered distinctly from the Placebo-treated and Placebo-treated CLP animals | Genera: Staphylococcus; Enterococcus. Species: Staphylococcus sciuri, Staphylococcus cohnii, Staphylococcus aureus, Enterococcus durans, Enterococcus casseliflavus, Enterococcus faecium and Enterococcus faecalis | No data |
| 3. | Banerjee et al., 2016 [17] | Mouse | Morphine | 25 mg pellet for 2 days SubQ | Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between study groups | ↑ Diversity of Firmicutes Families (from phylum Firmicutes): Enterococcaceae, Staphylococcaceae, Bacillaceae, Streptococcaceae, Erysipelotrichaceae | ↓ Bile-deconjugating bacterial strains Phylum: Bacteroidetes; Reduced Bacteroidetes/Firmicutes ratio |
| 4. | Acharya et al., 2017 [63] | Human | Mixed: Oxycodone, morphine, hydromorphone, tramadol, methadone | Opioid-using patients were on therapy for a median of 5 months | Alpha diversity: no data Beta diversity: altered composition and distinct clustering between study groups (HE patients on opioids compared to HE patients not on opioids) | HE: Bifidobacterium (genus) | HE: Bacteroidaceae and Autochthonous taxa (Clostridiales XI (family), Ruminococcaceae (family)) |
| Non-HE: Peptostreptococcaceae (family) | Non-HE: Parasutterella (genus) | ||||||
| 5. | Kang et al., 2017 [67] | Mouse | Morphine | 75 mg pellet for 5 days SubQ | No data | Phylum: Proteobacteria (Enterobacteriales) | Phyla: Bacteroidetes (Bacteroidales) Firmicutes (Clostridiales, Lactobacilliales) |
| 6. | Xu et al., 2017 [60] | Human | Mixed: heroin, ice, ephedrine, heroin + ephedrine, and heroin + ice | - | Alpha diversity: higher in SUDs compared to the HCs, but no significant differences between the groups Beta diversity: altered composition and distinct clustering between study groups (SUDs vs. HCs) | Genera: Prevotella, Ruminococcus, Phascolarctobacterium, Alloprevotella, Megamonas, Roseburia, Clostridium XlVa | Genera: Bacteroides, Faecalibacterium, Alistipes, Gemmiger, Clostridium XI, Escherichia/Shigella, Dialister, Paraprevotella, Megasphaera, Haemophilus, Parabacteroides, Barnesiella, Blautia |
| 7. | Barengolts et al., 2018 [66] | Human | Mixed | The DSM-4 diagnostic criteria for Opioid Use Disorder | No data | Phylum: Actinobacteria, Order: Lactobacillales, Bifidobacteriales Genus: Bifidobacterium | Species: Prevotella copri |
| 8. | Lee et al., 2018 [43] | Mouse | Morphine | Intermittent: 10, 20, 30, 40 mg/kg every 12 h i.p. for 4 days | Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between study groups (intermittent or sustained morphine vs. controls) | ↑ Ruminococcus spp. | ↓ Lactobacillus spp. |
| 25 mg pellet for 4 days SubQ | ↑ Clostridium spp. ↑ Rikenellaceae spp. | - | |||||
| 9. | Mischel et al., 2018 [86] | Mouse | Morphine | 75 mg pellet SubQ | No data | No data | No data |
| 10. | Wang et al., 2018 [50] | Mouse | Morphine | 25 mg pellet for 3 days SubQ | Alpha diversity: reduced Beta diversity: altered composition and distinct clustering between study groups | Pathogenic genera: Flavobacterium, Enterococcus, Fusobacterium, Sutterella, Clostridium Enterococcus faecalis (species) | - |
| 11. | Hakimian et al., 2019 [87] | Mouse | Remifentanil, oxycodone | IVSA: 3 days 0.05 mg/kg/infusion of remifentanil; 10 days 0.25 mg/kg/infusion of oxycodone | No data on opioid vs. control groups | No data on opioid vs. control groups | No data on opioid vs. control groups |
| 12. | Komla et al., 2019 [88] | Mouse | Morphine | 25-, 50- (2 × 25), or 75-mg pellets for 3–5 days SubQ | No data | No data | No data |
| 13. | Meng et al., 2019 [45] | Mouse | Morphine | 75 mg pellet for 7 days SubQ | Alpha diversity: reduced Beta diversity: altered composition and distinct clustering between study groups | HIV + Morphine: Phyla: Firmicutes, Proteobacteria Genus: Enterococcus | HIV + Morphine: Phyla: Bacteroidetes, Actinobacteria, and Tenericutes; Families: Muribaculaceae, Lachnospiraceae, and Ruminococcaceae Genus: Lactobacillus |
| 14. | O’Sullivan et al., 2019 [68] | Rat | Morphine (withdrawal) | 2 × 75 mg pellets for 6 days SubQ | No data | Placebo vs. morphine groups—no statistical difference. | Placebo vs. morphine groups—no statistical difference. |
| Withdrawal: Phyla: Bacteroidetes, Verrucomicrobia Species: Bacteroides fragilis, B. vulgatus and B. thetaiotaomicron, Enterococcus faecalis, Enterococcus gallinarum | Withdrawal: Phyla: Firmicutes, Actinobacteria Genera: Butyricicoccus, Bifidobacterium Species: Butyricicoccus pullicaecorum, F. prausnitzii ↓ Firmicutes to Bacteroides ratio | ||||||
| 15. | Sindberg et al., 2019 [49] | Macaque | Morphine | 2à4 mg/kg every 8 h i.m. for 12 weeks | Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between pre and post morphine induction | - | Family: Leuconostocaceae Genera: Streptococcaceae streptococcus, Pasteurellaceae Aggregatibacter |
| 16. | Zhang et al., 2019 [65] | Mouse | Morphine | 8 days: constant dose of 15 mg/kg or escalating doses of (5, 10, 15, 20, 25, 30, 35, 40 mg/kg) morphine injection b.i.d intraperitoneally | Alpha diversity: no data Beta diversity: altered composition and distinct clustering between study groups (morphine-tolerant vs. saline-treated mice); no difference in TLR2KO and TLR4KO mice | Phyla: Actinobacteria and Firmicutes | Families: Bifidobacteriaceae and Lactobacillaceae; Genera: Bifidobacterium and Lactobacillus |
| 17. | Chen et al., 2020 [34] | Zebrafish | Morphine | On days 4, 6, and 8 injected with morphine (40 mg/kg) | Alpha diversity: decreased Beta diversity: altered composition and distinct clustering between study groups | Phylum: Fusobacteria ↑ Bacteroidetes/Firmicutes (B/F) ratio | Phylum: Actinobacteria |
| 18. | Gicquelais et al., 2020 [57] | Human | Mixed: Ag only (heroin or PO), AgAt (buprenorphine–naloxone and PO and naltrexone), At (naltrexone only), N (neither opioid agonist nor antagonist) | participants from the patient population attending a private, outpatient addiction treatment facility in Michigan | Alpha diversity: decreased (Ag vs. N) No significant changes (AgAt and At vs. N) Beta diversity: no distinct clustering between study groups | Ag vs. N: Genera: Unclassified Enterobacteriaceae, Lactobacillus, Clostridium cluster XIVa, Faecalicoccus, Anaerostipes, and Streptococcus No statistically significant differences between AgAt vs. N or At vs. N participants. | Ag vs. N: Genera: Unclassified Firmicutes, Bilophila, and Roseburia |
| 19. | Li et al., 2020 [58] | Human | Methadone; illicit drugs | MMT patients; current drug using (DU) participants with narcotic (heroin) and psychotropic (methamphetamine) drug use disorders; healthy controls; compulsory detention (CD) | Alpha diversity: no significant changes Beta diversity: significantly higher among MMT patients | MMT patients: Phyla: Cyanobacteria chloroplast and Actinobacteria. Genera: Lactobacillus, Streptococcus, Veillonella, Bifidobacterium, Intestinibacter, Fusicatenibacter; Streptococcus (genus) and Fusicatenibacter (genus) (MMT vs. CD and DU); Klebsiella (MMT vs. CD). | - |
| DU: Genera: Ruminococcus, Roseburia, Collinsella, and Succinivibrio | |||||||
| 20. | Sharma et al., 2020 [47] | Mouse | Hydromorphone | 7.5 mg/kg every 12 h × 7 d i.p. | Alpha diversity: decreased Beta diversity: altered composition and distinct clustering between study groups | Hydromorphone plus DSS-treated mice compared with control mice: Phyla: Proteobacteria, Verrucomicrobia Families: Bacteroidaceae, Porphyromonadaceae, Enterococcaceae, Enterobacteriaceae, Verrucomicrobiaceae, and Peptostreptococcaceae Genera: Bacteroides, Parabacteroides, Enterococcus, Turicibacter, Ruminococcus, Sutterella, Bilophila, and Akkermansia Species: Bacteroides acidifaciens, Ruminococcus gnavus, and Akkermansia muciniphila | Hydromorphone plus DSS-treated mice compared with control mice: Phylum: Firmicutes Families: Odoribacteraceae, Rikenellaceae, S24-7, Lactobacillaceae, Lachnospiraceae, and Ruminococcaceae Genera: Adlercreutzia, Odoribacter, AF12, Lactobacillus, and Anaerostipes Species: Mucispirillum schaedleri and Lactobacillus reuteri |
| 21. | Simpson et al., 2020 [48] | Rat | Oxycodone | 2 mg/kg every 12 h for 5 days SubQ | Alpha diversity: no significant changes Beta diversity: no data | Firmicutes (phylum) | Bacteroidetes (phylum) |
| 22. | Zhang et al., 2020 [51] | Rat | Morphine | 10 mg/kg i.p. (day 6, 8, 10, 12) | Alpha diversity: no significant changes Beta diversity: no data | M-Post-treatment vs. M-Baseline: Genera: Allobaculum, Parasutterella Families: Coriobacteriaceae and Peptococcaceae_1 | M-P vs. M-B: Genera: Alloprevotella, Desulfovibrio, Rikenella |
| MP vs. SalineP: - | MP vs. SalineP: Genera: Corynebacterium, and Clostridium_XlVa Families: Enterococcaceae, Staphylococcaceae, Streptococcaceae | ||||||
| 23. | Cruz-Lebrón et al., 2021 [56] | Human | Methadone | Methadone-treated individals | Alpha diversity: decreased Beta diversity: significantly decreased | Phylum: Actinobacteria; Family: Bifidobacteriaceae; Species: Bifidobacterium bifidum and Bifidobacterium longum | Phylum: VerrucomicrobiaI; Family: Akkermasiaceae; Species Akkermansia muciniphila |
| 24. | Grecco et al., 2021 [36] | Mouse | Methadone, oxycodone | Oxycodone 10 → 30 mg/kg twice daily for 9 days SubQ → methadone 10 mg/kg (mothers). These treatments continued throughout mating, pregnancy, and weaning (60 days) | Alpha diversity: increased in methadone-treated dams and PME offspring Beta diversity: distinct clustering by treatment for dams, but this clustering was lost in offspring | Dams: Famillies: Erysipelotrichaceae, Peptostreptococcaceae, Akkermansiaceae, Lactobacillaceae, Sutterellaceae, Eubacterium Coprostanoligenes Group, Anaerovoracaceae, Monoglobaceae, Eggerthellaceae | - |
| In offspring: Families: Rikenellaceae, Peptococcaceae, Saccharimonadaceae | |||||||
| In both dams and offspring: Families: Ruminococcaceae, Rikenellaceae, Bacteroidaceae, Erysipelotrichaceae, Acholeplasmataceae, Peptococcaceae | |||||||
| 25. | Hofford et al., 2021 [38] | Mouse | Morphine | 10 mL/kg twice daily for 7 days SubQ | Alpha diversity: no significant changes Beta diversity: near complete overlap between samples in H2O-Sal and H2O-Mor groups and relative proportions of bacterial phyla were similar between H2O-Sal and H2O-Mor | Morphine itself had minimal effects on microbiome composition | Phyla: Rokubacteria and Cyanobacteria However, these phyla are expressed at very low abundance in all tested groups (both <0.1%). |
| 26. | Thomaz et al., 2021 [54] | Mouse | Morphine | Twice daily for 2 days (day 1: 7.5 and 15 mg/kg; day 2: 30 and 30 mg/kg) i.p. | Alpha diversity: no significant changes Beta diversity: no distinct clustering between study groups | Phylum: Verrucomicrobia | Phylum: Firmicutes |
| 27. | Zhang et al., 2021 [52] | Mouse | Morphine | 10 mL/kg twice daily i.p. (on days 3, 5, 7, 9, 11 and 13) | Alpha diversity: increased richness but not diversity Beta diversity: altered composition and distinct clustering between study groups (different stages of morphine-induced CPP (acquisition, extinction, and reinstatement) and controls) | Phylum: Verrucomicrobia | |
| Acquisition stage: - | Acquisition stage: Genus: Bacteroides | ||||||
| Extinction stage: Genera: Bacteroides and Coprobacter | Extinction stage: Genera: Akkermansia, Saccharibacteria_genera_incertae_sedis, Eisenbergiella, and Ruminococcus | ||||||
| Phyla: Verrucomicrobia abundance increased in the acquisition of morphine CPP group compared to the control and decreased at the extinction stage compared to the acquisition stage, indicating an expansion response of Verrucomicrobia to morphine treatment. | Bacteroides was the genus that decreased after repeated morphine conditioning and had a recovery trend at the extinction stage. | ||||||
| 28. | Abu et al., 2022 [30] | Mouse | Hydromorphone | 10 mg/kg once daily i.p. Gestational day 11–13 (prenatal opioid exposure) | Four days post last hydromorphone (Dams): Alpha diversity: no significant changes | Four days post last hydromorphone (Dams): Phyla: Bacteoidetes and Proteobacteria; Genera: Bacteroides (from phylum Bacteoidetes) and Sutterella (from phylum Proteobacteria) | Four days post last hydromorphone (Dams): Phylum Firmicutes; Genera Adlercreutzia (from phylum Actinobacteria), and Anaerostipes (from phylum Firmicutes) |
| Birth: Alpha diversity: no significant changes Beta diversity: microbial composition of fecal samples from Prenatal opioid exposure (POE) mothers was significantly different from control mothers 4 dp last hydromorphone/saline treatment and at parturition | Birth: Phyla: Bacteoidetes and Proteobacteria; Genera: Bacteroides (from phylum Bacteoidetes) and Turicibacter (from phylum Firmicutes) | Birth: Phylum Firmicutes; Genera: Allobaculum (from phylum Firmicutes) and Roseburia (from phylum Firmicutes) | |||||
| Weaning: Alpha diversity: no significant changes | Weaning: Phylum Verrucomicrobia; Genera: Clostridium (from phylum Firmicutes) and Akkermansia (from phylum Verrucomicrobia) | Weaning: Phylum Firmicutes; Genera: Oscillospira (from phylum Firmicutes) and Roseburia (from phylum Firmicutes) | |||||
| 2 weeks: Alpha diversity: no significant changes Beta diversity: the microbial composition in POE offspring was significantly different from controls | 2 weeks: Phylum Firmicutes; Genera: Lactobacillus, Ruminococcus and Allobaculum (all genera from phylum Firmicutes) | 2 weeks: Phyla: Verrucomicrobia and Tenericutes; Genera: Akkermansia (from phylum Verrucomicrobia), Clostridium (from phylum Firmicutes), and Bifidobacterium (from phylum Actinobacteria) | |||||
| 3 weeks: Alpha diversity: no significant changes Beta diversity: the microbial composition in POE offspring was significantly different from controls | 3 weeks: Genera: Turicibacter (from phylum Firmicutes), Bacteroides (from phylum Bacteoidetes), Bifidobacterium (from phylum Actinobacteria), Allobaculum (from phylum Firmicutes) and Dehalobacterium (from phylum Firmicutes) | 3 weeks: Phyla: Verrucomicrobia and Proteobacteria; Genera: Akkermansia (from phylum Verrucomicrobia), Coprobacillus (from phylum Firmicutes), Dorea (from phylum Firmicutes) and Adlercreutzia (from phylum Actinobacteria) | |||||
| 5 weeks: Alpha diversity: increased Beta diversity: no significant difference | 5 weeks: Genera: Ruminococcus (from phylum Firmicutes) | 5 weeks: Genera: Lactobacillus (from phylum Firmicutes) and Staphylococcus (from phylum Firmicutes) | |||||
| Alpha diversity: no significant changes (stomach) Beta diversity: stomach samples from POE mice were significantly different from control mice | Offspring stomach: Genera: Staphylococcus (from phylum Firmicutes) and Lactobacillus (from phylum Firmicutes) | Offspring stomach: Genera: Akkermansia (from phylum Verrucomicrobia), Clostridium (from phylum Firmicutes) and an unknown genus from family S24-7 (from phylum Bacteoidetes) | |||||
| 29. | Antoine et al., 2022 [32] | Mouse | Morphine | post-natal day 7 ± 2 days for a duration of 7 ± 2 days total: 5 mg/kg/day once-a-day SubQ | Adolescence: Alpha diversity: increased Beta diversity: no significant difference | Adolescence: Phylum Firmicutes Increase in the Firmicutes/Bacteroidetes (F/B) ratio (female only); | Adolescence: Phyla: Bacteroidetes, Verrucomicrobia and Actinobacteria (female only); Genera: Lactobacillus (phylum Firmicutes), Turicibacter (phylum Firmicutes), Akkermansia (phylum Verrucomicrobiota) and Bifidobacterium (phylum Actinobacteria) |
| Adulthood: Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between study groups | Adulthood: Phylum Firmicutes (both male and female). The increase in the F/B ratio appeared later in life for male mice Genera: Allobaculum, Lactobacillus and Turicibacter | Adulthood: Phyla: Bacteroidetes and Verrucomicrobia Genus Akkermansia | |||||
| 30. | Blakeley-Ruiz et al., 2022 [33] | Mouse | Morphine | Subcutaneous osmotic pump: 10 mg/kg/day for 2 weeks | Alpha diversity: no significant changes Beta diversity: no data | - | Metaproteomic analysis: Proteins from species: Eubacterium sp. or Lachnospiraceae bacterium |
| 31. | Jalodia et al., 2022 [39] | Mouse | Morphine | 25 mg pellet for 24 h SubQ | Alpha diversity: increased Beta diversity: altered composition and distinct clustering between study groups | Genera: Staphylococcus, Enterococcus, and Bacteroides; | Reduced Firmicutes to Bacteroidetes ratio Lactobacillus genus |
| 32. | Ji et al., 2022 [55] | Mouse | Morphine | 10 mg/kg, once a day for 6 days i.p. | Alpha diversity: no significant changes Beta diversity: distinct clustering between study groups | Mor-dep vs. control: Genera: Coprobacter and Enterorhabdus | Mor-Dep vs. control: Genus: Anaerotruncus |
| Mor-nondep vs. control: Genus: Coprobacter | Mor-nondep vs. control: Genera: Eisenbergiella and Anaerotruncus | ||||||
| 33. | Johnson et al., 2022 [40] | Macaque | Morphine | Increased dose within 2 weeks to 6 mg/kg twice a day (i.m.) à 7 more weeks à infection with SIV | Alpha diversity: decreased Beta diversity: no data | Phylum Bacteroidetes; Family Prevotellaceae | Phylum Firmicutes; Family Ruminococcaceae |
| 34. | Lin et al., 2022 [85] | Human | Prescription opioids (e. g. morphine, oxycodone, codeine, fentanyl, pethidine, and tramadol) | A two-sample bi-directional Mendelian randomization using summary level Genome-wide association studies | No data | no clear evidence for any causal effect of POU on gut microbiota | no clear evidence for any causal effect of POU on gut microbiota |
| 35. | Lyu et al., 2022 [44] | Mouse | Oxycodone | 5 mg/kg for 2 weeks i.p. prior to breeding and then throughout gestation | Female offspring (adulthood): Alpha diversity: no significant changes Beta diversity: no significant difference | Female offspring (adulthood): Phylum: Bacteroidetes, TM7 Class: Clostridia Genera: Butyricimonas spp., Anaeroplasma spp., Enterococcus spp. | Female offspring (adulthood): Genus: Clostridium spp. |
| Male offspring (adulthood): Alpha diversity: no significant changes Beta diversity: no significant difference | Male offspring (adulthood): Family: Coriobacteriaceae Class: Clostridia Genera: Roseburia spp., Sutterella spp. | Male offspring (adulthood): Phylum: Firmicutes Class: Bacilli Order: Lactobacillales Families: Peptococcaceade, Desulfovibionaceae Genera: Clostridium spp., Staphylococcus spp., Clostridium spp., Enterococcus spp., Turicibacter spp., Prevotella, Butyricicoccus | |||||
| 36. | Muchhala et al., 2022 [89] | Mouse | Morphine |
| No data | No data | No data |
| 37. | Ren and Lotfipour, 2022 [46] | Rat | Fentanyl | IVSA of fentanyl at 0, 1.25, or 2.5 μg/kg/infusion during daily 2-h sessions for 5 days at a FR1 schedule of reinforcement, 2 days at FR2, followed by 2 days on FR5, all with a 20-s timeout | Alpha diversity: increased in controls after fentanyl IVSA use vs. before fentanyl IVSA Beta diversity: no data | In antibiotic-treated animals after vs. before fentanyl IVSA: Phylum: Bacteroidetes | - |
| 38. | Ren and Lotfipour, 2022 [90] | Rat | Fentanyl | IVSA of fentanyl at 0, 1.25, or 2.5 μg/kg/infusion during daily 2-h sessions for 5 days at a FR1 schedule of reinforcement, 2 days at FR2, followed by 2 days on FR5, all with a 20-s timeout | Alpha diversity: increased after vs. before IVSA in males at 1.25 μg/kg/infusion decreased at Fentanyl IVSA at 1.25 vs. 0 μg/kg/infusion in females Beta diversity: fentanyl self-administration did not change beta-diversity | In males that self-administered fentanyl at 1.25 μg/kg/infusion: Phylum Verrucomicrobia; Genera Ruminococcus and Akkermansia | Firmicutes/Bacteroidetes ratios remained stable before and after fentanyl IVSA |
| In females that self-administered 1.25 μg/kg/infusion: Genus Prevotella | |||||||
| 39. | H. Wang et al., 2022 [59] | Human | Oxycodone | Retrospective Cohort Study (Patients with Moderate to Severe Cancer Pain) | Alpha diversity: no significant changes Beta-diversity: no significant difference in gut microbiota diversity among the Control, Opioid-S, and Opioid-T groups | Genera: Lactobacillus, Anaerostipes, Megamonas, Monoglobus, and Rikenellaceae_RC9_gut_group | At the phylum level, there were no significant differences |
| 40. | Ghosh et al., 2023 [53] | Mouse | Morphine | 25 mg pellet for 1 or 2 days SubQ | Alpha diversity: decreased Beta diversity: altered composition and distinct clustering between study groups | Phyla: Firmicutes and Verrucomicrobia Families: Enterococcaceae, Staphylococcaceae, Peptostreptococcoceae, Streptococcaceae, Erysipelotrichaceae, Pseudomonaceae, Akkermansiaceae, Coriobacteriaceae Genera: Staphylococcus, Enterococcus, Turicibacter, and Pseudomonas | Phyla: Bacteroidetes, actinobacteria, and tenericutes Families: Lactobacillaceae, Lachnospiraceae, Muribaculaceae, Ruminococcaceae, Burkholderiaceae, Eggerthelaceae, and Peptococcaceae Genus: Lactobacillus |
| 41. | Kolli et al., 2023 [42] | Mouse | Morphine | 25 mg pellet SubQ à antibiotics for 7 days | Alpha diversity: increased Beta diversity: altered composition and distinct clustering between study groups | Species: Parasutterella excrementihominis, Burkholderiales bacterium 1_1_47, Enterococcus faecalis, Staphylococus xylosus, Firmicutes bacterium M10–2, Bifidobacterium pseudolongum and Enterorhabdus caecimuris | Species Lactobacillus johnsonii |
| 42. | Truitt et al., 2023 [91] | Mouse | Morphine (withdrawal) | 75 mg pellet for 3 days SubQ | During withdrawal: Alpha diversity: decreased Beta diversity: distinct clustering between study groups | At 2 h post-pellet removal: Phylum: Verrucomicrobia | At 2 h post-pellet removal: Phylum: Firmicutes |
| - | At 12 h post-pellet removal: Phyla: Verrucomicrobia, Firmicutes, Actinobacteria | ||||||
| - | At 24 h post-pellet removal: Phylum: Actinobacteria | ||||||
| 43. | Abu et al., 2024 [31] | Mouse | Hydromorphone; methadone | Hydromorphone: 0.5 mg/kg, SubQ, b.i.d., pre-gestation day (PG) 1–3, 1.25 mg/kg, SubQ, b.i.d. PG 4–6, 2 mg/kg, SubQ, b.i.d. PG 7–9, 2.75 mg/kg, SubQ, b.i.d. PG 10–12, 3.5 mg/kg, SubQ, b.i.d. PG 13–14; transitioned to methadone (10 mg/kg, SubQ, b.i.d) until weaning of pups at 3 weeks of age | Mothers: Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between study groups (methadone-treated vs. control dams) | Mothers: Genera: Akkermansia and Bacteroides; aerobic, biofilm forming bacteria, and gram-negative bacteria | Mothers: Genus Turicibacter; gram-positive bacteria |
| Offspring: Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between study groups (prenatally methadone-exposed and control offspring) | Offspring: Phyla: Bacteroidota, Verrucomicrobiota; Genera: Akkermansia, Alistipes, Bacteroides, Butyricicoccus, Clostridium sensu stricto 1, and Lachnoclostridium gram negative, aerobic, biofilm-forming, and gram-positive bacteria | Offspring: Firmicutes (phylum); Genera: Lachnospiraceae A2, Anaeroplasma, Clostridium sp. ASF356, Bifidobacterium, Enterorhabdus, Erysipelatoclostridium, Family XIII UCG-001, Lachnospiraceae UCG-001, and Lactobacillus | |||||
| 44. | Crawford et al., 2024 [35] | Mouse | Morphine | 20 mg/kg once daily for 28 days SubQ | Alpha diversity: decreased Beta diversity: no data | - | SCFA-producing bacteria: Genus Bacteroides; Families: Lachnospiraceae, and Ruminococcaceae |
| 45. | Greenberg et al., 2024 [37] | Rat | Heroin | IVSA: 20 μg/kg/100 μL infusion over 3 s. A session lasted for 12 h, or terminated once 300 infusions was reached. The IVSA phase lasted for 15 sessions | Alpha diversity: no significant changes Beta diversity: altered composition and distinct clustering between study groups (heroin vs. saline-yoked groups) | heroin self-administration phase: Genus Bacteroides; Families: Lachnospiraceae, Muribaculaceae | heroin self-administration phase: Genus Alistipes; Families: Rikenellaceae |
| extinction phase: Genera: Ruminoclostridium 6, Ruminiclostridium 5; Family Muribaculaceae | extinction phase: Genera: Mucisprillum, Ruminiclostridium 5; Family Lachnospiraceae | ||||||
| 46. | Hofford et al., 2024 [92] | Rat | Fentanyl | IVSA 2.5 μg/kg/infusion: daily 3-h sessions for 10 days at a FR1 schedule of reinforcement; then 2 days at FR2, 2 days at FR3, and 2 days on FR5 (increasing FR) or 6 days of FR1, then 2 d of PR and 2 days of FR1; then 20 d of home cage abstinence | Alpha diversity: no significant changes (opioids vs. saline) Beta diversity: no significant changes (opioids vs. saline) | - | Abundance of genera Ruminococcus, Butyricicoccus, Lachnospiracae_unclassified, and Anaerotignum negatively correlated with fentanyl intake during the last 2 d of fentanyl increasing FR or maintenance |
| 47. | Inan et al., 2024 [61] | Rat | Oxycodone | Increasing doses for 12 days twice a day SubQ: days 1–4, 1 mg/kg; days 5–8, 2 mg/kg; days 9–12, 3 mg/kg | Alpha diversity: no significant changes Beta diversity: no data | - | - |
| 48. | Kesh et al., 2024 [41] | Mouse | Morphine, oxycodone | Morphine for 5 weeks at escalating doses (10, 20, 30, 40, 50 mg/kg, i.p., b.i.d); Oxycodone for 5 weeks with at escalating doses of (5, 15, 25, 35, 45 mg/kg, i.p., b.i.d.) | Alpha diversity: increased in those from CP, Morphine, and CP + morphine mice with 11-week CP vs. controls No significant changes between mice treated with CP, Morphine, and CP + morphine Beta diversity: altered composition and distinct clustering between study groups (control mice vs. CP mice, morphine, or CP + morphine treatment groups; CP + morphine mice vs. CP-only mice) | CP Morphine vs. CP: Species: Adlercreutzia caecimuris, species from Anaerotruncus, Adlercreutzia muris | CP Morphine vs. CP: Species: Lactobacillus johnsonii, an unidentified species from Lactobacillus, and Ducaniella muris |
| Similar α-diversity results were observed in oxycodone-treated CP animals in 11-week samples. Similar β-diversity results were observed in oxycodone-treated CP animals in 11-week samples. | CP Oxy vs. CP: Species: Adlercreutzia muri, unidentified species from Enterorhabdus, Adlercreutzia mucosicola | CP Oxy vs. CP: - | |||||
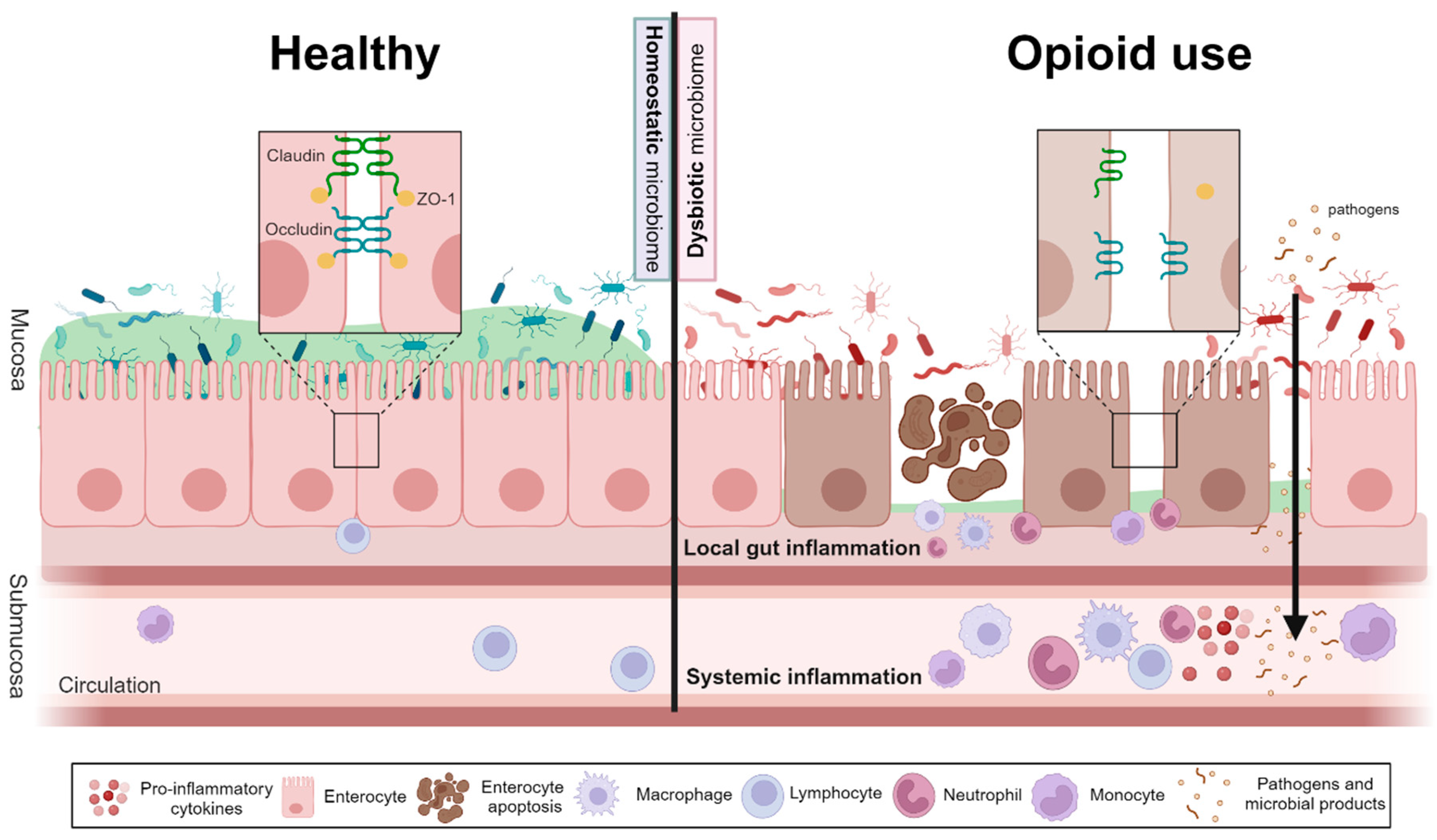
3.2. The Impact of Opioid Use on Gut Barrier Function, Inflammation, and Short Chain Fatty Acid Levels
3.2.1. Opioid Use Increases Gut Permeability and Gastrointestinal Inflammation
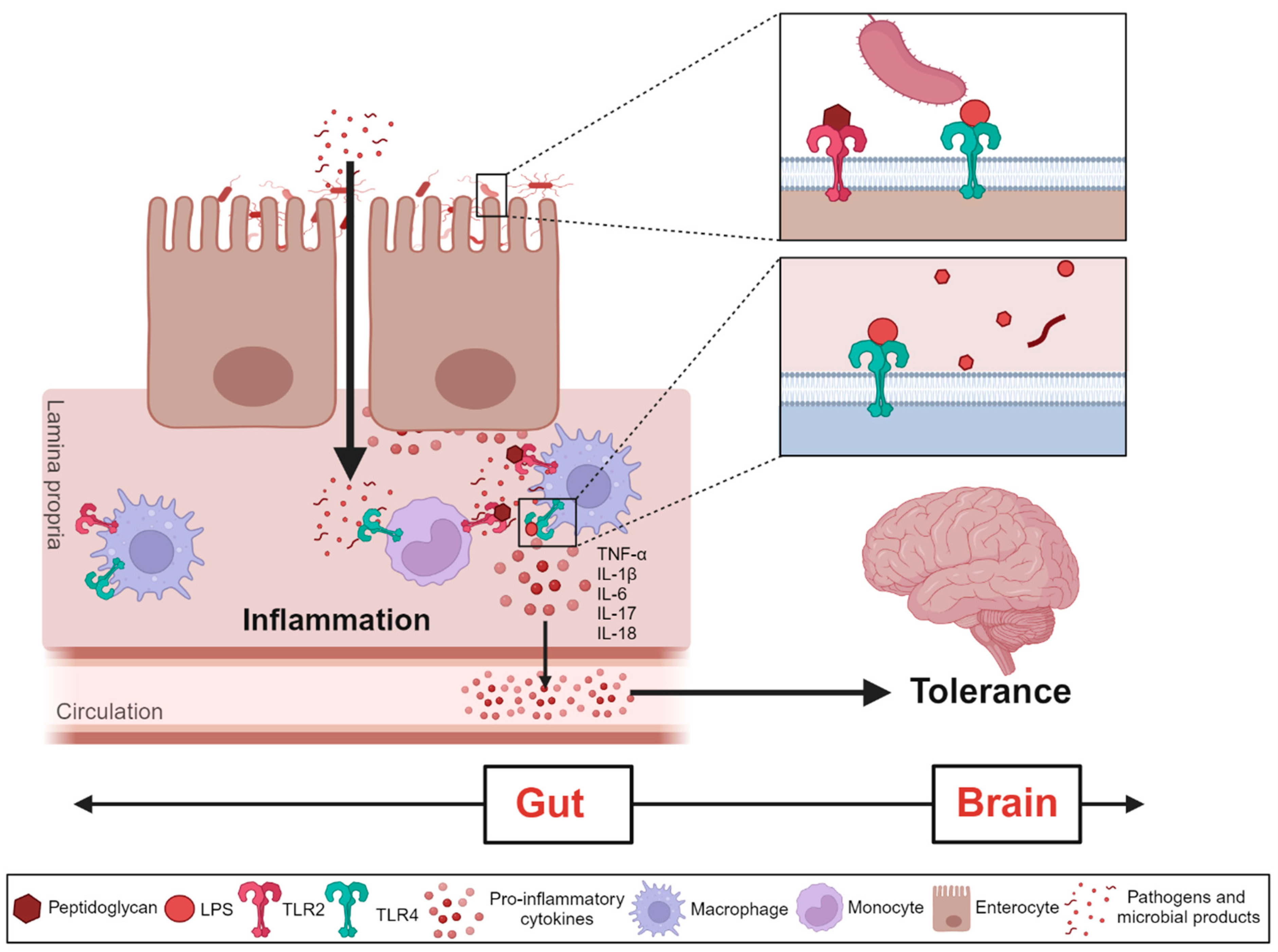
3.2.2. Opioid Use Is Associated with Systemic Inflammation
3.2.3. Opioid Use Results in Reduced Short Chain Fatty Acid Levels
4. Gut Microbiota Impact on Opioid Use Disorder
4.1. Associations between Gut Microbiota and Antinociceptive Tolerance to Opioids
4.2. Gut Microbiome Induces Changes in the Brain during Different Stages of Opioid Use
4.3. Changes in Microbiome Alter Behavioral Response to Opioids
5. Gut Microbiota Modulation as a Treatment Option for Opioid Use Disorder
5.1. Short-Chain Fatty Acids (SCFAs) for Treatment of Opioid Use Disorder
5.2. Probiotics for Treatment of Opioid Use Disorder
5.3. Fecal Microbiota Transplantation for Treatment of Opioid Use Disorder
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Strang, J.; Volkow, N.D.; Degenhardt, L.; Hickman, M.; Johnson, K.; Koob, G.F.; Marshall, B.D.L.; Tyndall, M.; Walsh, S.L. Opioid use disorder. Nat. Rev. Dis. Primer 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Samet, J.H. Opioid Use Disorder. Ann. Intern. Med. 2022, 175, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Barrio, G.; Bravo, M.J.; Indave, B.I.; Degenhardt, L.; Wiessing, L.; Ferri, M.; Pastor-Barriuso, R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ 2017, 357, j1550. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Li, L.; Min, J.; Huang, D.; Urada, D.; Liu, L.; Hser, Y.-I.; Nosyk, B. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–2010. Addict. Abingdon Engl. 2015, 110, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009, CD002209. [Google Scholar] [CrossRef]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014, CD002207. [Google Scholar] [CrossRef]
- Centre for Addiction and Mental Health. Opioid Agonist Therapy: A Synthesis of Canadian Guidelines for Treating Opioid Use Disorder; Centre for Addiction and Mental Health: Toronto, ON, Canada, 2021. [Google Scholar]
- Gowing, L.; Farrell, M.F.; Bornemann, R.; Sullivan, L.E.; Ali, R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst. Rev. 2011, CD004145. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Cousins, G.; Durand, L.; Barry, J.; Boland, F. Retention of patients in opioid substitution treatment: A systematic review. PLoS ONE 2020, 15, e0232086. [Google Scholar] [CrossRef]
- Skosnik, P.D.; Cortes-Briones, J.A. Targeting the ecology within: The role of the gut-brain axis and human microbiota in drug addiction. Med. Hypotheses 2016, 93, 77–80. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.W.; Pan, Y.X. Mu opioids and their receptors: Evolution of a concept. Pharmacol. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Patierno, S.; Selmer, I.S.; Kirchgessner, A. The opioid system in the gastrointestinal tract. Neurogastroenterol. Motil. 2004, 16 (Suppl. S2), 3–16. [Google Scholar] [CrossRef]
- Eisenstein, T.K. Opioids and the immune system: What is their mechanism of action? Br. J. Pharmacol. 2011, 164, 1826–1828. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; Sternini, C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 363–378. [Google Scholar] [PubMed]
- Banerjee, S.; Sindberg, G.; Wang, F.; Meng, J.; Sharma, U.; Zhang, L.; Dauer, P.; Chen, C.; Dalluge, J.; Johnson, T.; et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016, 9, 1418–1428. [Google Scholar] [CrossRef]
- Meng, J.; Yu, H.; Ma, J.; Wang, J.; Banerjee, S.; Charboneau, R.; Barke, R.A.; Roy, S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS ONE 2013, 8, e54040. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Rey, F.E.; Denu, J.M. Chemical signaling between gut microbiota and host chromatin: What is your gut really saying? J. Biol. Chem. 2017, 292, 8582–8593. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Safadi, J.M.; Quinton, A.M.G.; Lennox, B.R.; Burnet, P.W.J.; Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: A novel trans-diagnostic construct? A systematic review and meta-analysis. Mol. Psychiatry 2022, 27, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Sil, S.; Kumar, M.; Buch, S. Substance use, microbiome and psychiatric disorders. Pharmacol. Biochem. Behav. 2022, 219, 173432. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, H.; Fan, X.; Wu, X.; Zhou, R.; Lei, Y.; Xue, D.; Yang, F.; Xu, Y.; Wang, K. The Gut Microbiota-Brain Axis: Potential Mechanism of Drug Addiction. Curr. Top. Med. Chem. 2023, 23, 1782–1792. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, C.; Chen, L.; Zhang, M.; Luo, W. Potential roles of the gut microbiota in the manifestations of drug use disorders. Front. Psychiatry 2022, 13, 1046804. [Google Scholar] [CrossRef]
- Lucerne, K.E.; Kiraly, D.D. The role of gut-immune-brain signaling in substance use disorders. Int. Rev. Neurobiol. 2021, 157, 311–370. [Google Scholar] [CrossRef]
- Thomas, K.R.; Watt, J.; Wu, C.M.J.; Akinrinoye, A.; Amjad, S.; Colvin, L.; Cowe, R.; Duncan, S.H.; Russell, W.R.; Forget, P. Pain and Opioid-Induced Gut Microbial Dysbiosis. Biomedicines 2022, 10, 1815. [Google Scholar] [CrossRef]
- Abu, Y.; Tao, J.; Dutta, R.; Yan, Y.; Vitari, N.; Kolli, U.; Roy, S. Brief Hydromorphone Exposure During Pregnancy Sufficient to Induce Maternal and Neonatal Microbial Dysbiosis. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2022, 17, 367–375. [Google Scholar] [CrossRef]
- Abu, Y.F.; Singh, S.; Tao, J.; Chupikova, I.; Singh, P.; Meng, J.; Roy, S. Opioid-induced dysbiosis of maternal gut microbiota during gestation alters offspring gut microbiota and pain sensitivity. Gut Microbes 2024, 16, 2292224. [Google Scholar] [CrossRef]
- Antoine, D.; Singh, P.K.; Tao, J.; Roy, S. Neonatal Morphine Results in Long-Lasting Alterations to the Gut Microbiome in Adolescence and Adulthood in a Murine Model. Pharmaceutics 2022, 14, 1879. [Google Scholar] [CrossRef] [PubMed]
- Blakeley-Ruiz, J.A.; McClintock, C.S.; Shrestha, H.K.; Poudel, S.; Yang, Z.K.; Giannone, R.J.; Choo, J.J.; Podar, M.; Baghdoyan, H.A.; Lydic, R.; et al. Morphine and high-fat diet differentially alter the gut microbiota composition and metabolic function in lean versus obese mice. ISME Commun. 2022, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhijie, C.; Yuting, Z.; Shilin, X.; Qichun, Z.; Jinying, O.; Chaohua, L.; Jing, L.; Zhixian, M. Antibiotic-Driven Gut Microbiome Disorder Alters the Effects of Sinomenine on Morphine-Dependent Zebrafish. Front. Microbiol. 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Liu, S.; Tao, R.; Kramer, P.; Bender, S.; Tao, F. Ketogenic diet mitigates opioid-induced hyperalgesia by restoring short-chain fatty acids-producing bacteria in the gut. Pain 2024, 165, e106–e114. [Google Scholar] [CrossRef] [PubMed]
- Grecco, G.G.; Gao, Y.; Gao, H.; Liu, Y.; Atwood, B.K. Prenatal opioid administration induces shared alterations to the maternal and offspring gut microbiome: A preliminary analysis. Drug Alcohol Depend. 2021, 227, 108914. [Google Scholar] [CrossRef]
- Greenberg, J.M.; Winters, A.D.; Zagorac, B.; Kracht, D.J.; Francescutti, D.M.; Cannella, N.; Ciccocioppo, R.; Woods, L.C.S.; Mackle, J.; Hardiman, G.T.; et al. Long access heroin self-administration significantly alters gut microbiome composition and structure. Front. Psychiatry 2024, 15, 1369783. [Google Scholar] [CrossRef]
- Hofford, R.S.; Mervosh, N.L.; Euston, T.J.; Meckel, K.R.; Orr, A.T.; Kiraly, D.D. Alterations in microbiome composition and metabolic byproducts drive behavioral and transcriptional responses to morphine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2021, 46, 2062–2072. [Google Scholar] [CrossRef]
- Jalodia, R.; Kolli, U.; Braniff, R.G.; Tao, J.; Abu, Y.F.; Chupikova, I.; Moidunny, S.; Ramakrishnan, S.; Roy, S. Morphine mediated neutrophil infiltration in intestinal tissue play essential role in histological damage and microbial dysbiosis. Gut Microbes 2022, 14, 2143225. [Google Scholar] [CrossRef]
- Johnson, S.D.; Fox, H.S.; Buch, S.; Byrareddy, S.N. Chronic Opioid Administration is Associated with Prevotella-dominated Dysbiosis in SIVmac251 Infected, cART-treated Macaques. J. Neuroimmune Pharmacol. 2022, 17, 3–14. [Google Scholar] [CrossRef]
- Kesh, K.; Tao, J.; Ghosh, N.; Jalodia, R.; Singh, S.; Dawra, R.; Roy, S. Prescription opioids induced microbial dysbiosis worsens severity of chronic pancreatitis and drives pain hypersensitivity. Gut Microbes 2024, 16, 2310291. [Google Scholar] [CrossRef]
- Kolli, U.; Jalodia, R.; Moidunny, S.; Singh, P.K.; Ban, Y.; Tao, J.; Cantu, G.N.; Valdes, E.; Ramakrishnan, S.; Roy, S. Multi-omics analysis revealing the interplay between gut microbiome and the host following opioid use. Gut Microbes 2023, 15, 2246184. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Vuong, H.E.; Nusbaum, D.J.; Hsiao, E.Y.; Evans, C.J.; Taylor, A.M.W. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Schmidt, R.R.; Martin, R.E.; Green, M.T.; Kinkade, J.A.; Mao, J.; Bivens, N.J.; Joshi, T.; Rosenfeld, C.S. Long-Term Effects of Developmental Exposure to Oxycodone on Gut Microbiota and Relationship to Adult Behaviors and Metabolism. mSystems 2022, 7, e0033622. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Banerjee, S.; Zhang, L.; Sindberg, G.; Moidunny, S.; Li, B.; Robbins, D.J.; Girotra, M.; Segura, B.; Ramakrishnan, S.; et al. Opioids Impair Intestinal Epithelial Repair in HIV-Infected Humanized Mice. Front. Immunol. 2019, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Lotfipour, S. Antibiotic Knockdown of Gut Bacteria Sex-Dependently Enhances Intravenous Fentanyl Self-Administration in Adult Sprague Dawley Rats. Int. J. Mol. Sci. 2022, 24, 409. [Google Scholar] [CrossRef]
- Sharma, U.; Olson, R.K.; Erhart, F.N.; Zhang, L.; Meng, J.; Segura, B.; Banerjee, S.; Sharma, M.; Saluja, A.K.; Ramakrishnan, S.; et al. Prescription Opioids induce Gut Dysbiosis and Exacerbate Colitis in a Murine Model of Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 801–817. [Google Scholar] [CrossRef]
- Simpson, S.; Kimbrough, A.; Boomhower, B.; McLellan, R.; Hughes, M.; Shankar, K.; de Guglielmo, G.; George, O. Depletion of the Microbiome Alters the Recruitment of Neuronal Ensembles of Oxycodone Intoxication and Withdrawal. eNeuro 2020, 7, ENEURO.0312-19.2020. [Google Scholar] [CrossRef]
- Sindberg, G.M.; Callen, S.E.; Banerjee, S.; Meng, J.; Hale, V.L.; Hegde, R.; Cheney, P.D.; Villinger, F.; Roy, S.; Buch, S. Morphine Potentiates Dysbiotic Microbial and Metabolic Shifts in Acute SIV Infection. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2019, 14, 200–214. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Yang, C.; Chen, T.; Wang, Z.; Li, J.; Qin, F.; Deng, Q.; Zhang, X. Sensitivity to Morphine Reward Associates With Gut Dysbiosis in Rats With Morphine-Induced Conditioned Place Preference. Front. Psychiatry 2020, 11, 631. [Google Scholar] [CrossRef]
- Zhang, J.; Deji, C.; Fan, J.; Chang, L.; Miao, X.; Xiao, Y.; Zhu, Y.; Li, S. Differential alteration in gut microbiome profiles during acquisition, extinction and reinstatement of morphine-induced CPP. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110058. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Kesh, K.; Singh, P.K.; Sharma, U.; Chupikova, I.; Ramakrishnan, S.; Roy, S. Morphine use induces gastric microbial dysbiosis driving gastric inflammation through TLR2 signalling which is attenuated by proton pump inhibition. Br. J. Pharmacol. 2023, 180, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, A.C.; Iyer, V.; Woodward, T.J.; Hohmann, A.G. Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp. Neurol. 2021, 343, 113787. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yan, N.; Zhang, Z.; Li, B.; Xue, R.; Dang, Y. Characterized profiles of gut microbiota in morphine abstinence-induced depressive-like behavior. Neurosci. Lett. 2022, 788, 136857. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lebrón, A.; Johnson, R.; Mazahery, C.; Troyer, Z.; Joussef-Piña, S.; Quiñones-Mateu, M.E.; Strauch, C.M.; Hazen, S.L.; Levine, A.D. Chronic Opioid Use Modulates Human Enteric Microbiota and Intestinal Barrier Integrity. Gut Microbes 2021, 13, 1946368. [Google Scholar] [CrossRef]
- Gicquelais, R.E.; Bohnert, A.S.B.; Thomas, L.; Foxman, B. Opioid agonist and antagonist use and the gut microbiota: Associations among people in addiction treatment. Sci. Rep. 2020, 10, 19471. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.; Liu, K.; Long, D.; Liu, D.; Jing, Z.; Huang, X. Differences in Gut Microbial Diversity are Driven by Drug Use and Drug Cessation by Either Compulsory Detention or Methadone Maintenance Treatment. Microorganisms 2020, 8, 411. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Chen, X.; Hu, H.; Li, S.; Zhang, Y.; Shi, C. Clinical Observation of the Effects of Oral Opioid on Inflammatory Cytokines and Gut Microbiota in Patients with Moderate to Severe Cancer Pain: A Retrospective Cohort Study. Pain Ther. 2022, 11, 667–681. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Z.; Wang, H.; Shen, Z.; Guo, Y.; Gao, Y.; Chen, X.; Wu, Q.; Li, X.; Wang, K. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci. Rep. 2017, 7, 3628. [Google Scholar] [CrossRef]
- Inan, S.; Meissler, J.J.; Bessho, S.; Wiah, S.; Tukel, C.; Eisenstein, T.K.; Rawls, S.M. Blocking IL-17A prevents oxycodone-induced depression-like effects and elevation of IL-6 levels in the ventral tegmental area and reduces oxycodone-derived physical dependence in rats. Brain. Behav. Immun. 2024, 117, 100–111. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Dewey, W.L. Gastrointestinal motility, dysbiosis and opioid-induced tolerance: Is there a link? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Betrapally, N.S.; Gillevet, P.M.; Sterling, R.K.; Akbarali, H.; White, M.B.; Ganapathy, D.; Fagan, A.; Sikaroodi, M.; Bajaj, J.S. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 319–331. [Google Scholar] [CrossRef]
- Meng, J.; Banerjee, S.; Li, D.; Sindberg, G.M.; Wang, F.; Ma, J.; Roy, S. Opioid Exacerbation of Gram-positive sepsis, induced by Gut Microbial Modulation, is Rescued by IL-17A Neutralization. Sci. Rep. 2015, 5, 10918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, J.; Ban, Y.; Jalodia, R.; Chupikova, I.; Fernandez, I.; Brito, N.; Sharma, U.; Abreu, M.T.; Ramakrishnan, S.; et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 13523–13532. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E.; Green, S.J.; Eisenberg, Y.; Akbar, A.; Reddivari, B.; Layden, B.T.; Dugas, L.; Chlipala, G. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS ONE 2018, 13, e0194171. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Dewey, W.L.; Akbarali, H.I. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep. 2017, 7, 42658. [Google Scholar] [CrossRef]
- O’Sullivan, S.J.; Malahias, E.; Park, J.; Srivastava, A.; Reyes, B.A.S.; Gorky, J.; Vadigepalli, R.; Van Bockstaele, E.J.; Schwaber, J.S. Single-Cell Glia and Neuron Gene Expression in the Central Amygdala in Opioid Withdrawal Suggests Inflammation With Correlated Gut Dysbiosis. Front. Neurosci. 2019, 13, 665. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef]
- Bahar-Tokman, H.; Demirci, M.; Keskin, F.E.; Cagatay, P.; Taner, Z.; Ozturk-Bakar, Y.; Ozyazar, M.; Kiraz, N.; Kocazeybek, B.S. Firmicutes/Bacteroidetes Ratio in the Gut Microbiota and IL-1β, IL-6, IL-8, TLR2, TLR4, TLR5 Gene Expressions in Type 2 Diabetes. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef]
- Takezawa, K.; Fujita, K.; Matsushita, M.; Motooka, D.; Hatano, K.; Banno, E.; Shimizu, N.; Takao, T.; Takada, S.; Okada, K.; et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. The Prostate 2021, 81, 1287–1293. [Google Scholar] [CrossRef]
- Petakh, P.; Oksenych, V.; Kamyshnyi, A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomed. Pharmacother. 2023, 163, 114892. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, A.; van Nispen, J.; Armstrong, A.; Song, E.; Voigt, M.; Murali, V.; Krebs, J.; Manithody, C.; Denton, C.; Ericsson, A.C.; et al. Lower systemic inflammation is associated with gut firmicutes dominance and reduced liver injury in a novel ambulatory model of parenteral nutrition. Ann. Med. 2022, 54, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Sartor, R.B. The intestinal microbiota in inflammatory bowel diseases. Nestle Nutr. Inst. Workshop Ser. 2014, 79, 29–39. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lin, J.; Qiu, J.; Wei, F.; Bai, X.; Ma, W.; Zeng, J.; Lin, D. Gut microbiota alterations may increase the risk of prescription opioid use, but not vice versa: A two-sample bi-directional Mendelian randomization study. Front. Microbiol. 2022, 13, 994170. [Google Scholar] [CrossRef]
- Mischel, R.A.; Dewey, W.L.; Akbarali, H.I. Tolerance to Morphine-Induced Inhibition of TTX-R Sodium Channels in Dorsal Root Ganglia Neurons Is Modulated by Gut-Derived Mediators. iScience 2018, 2, 193–209. [Google Scholar] [CrossRef]
- Hakimian, J.K.; Dong, T.S.; Barahona, J.A.; Lagishetty, V.; Tiwari, S.; Azani, D.; Barrera, M.; Lee, S.; Severino, A.L.; Mittal, N.; et al. Dietary Supplementation with Omega-3 Polyunsaturated Fatty Acids Reduces Opioid-Seeking Behaviors and Alters the Gut Microbiome. Nutrients 2019, 11, 1900. [Google Scholar] [CrossRef]
- Komla, E.; Stevens, D.L.; Zheng, Y.; Zhang, Y.; Dewey, W.L.; Akbarali, H.I. Experimental Colitis Enhances the Rate of Antinociceptive Tolerance to Morphine via Peripheral Opioid Receptors. J. Pharmacol. Exp. Ther. 2019, 370, 504–513. [Google Scholar] [CrossRef]
- Muchhala, K.H.; Koseli, E.; Gade, A.R.; Woods, K.; Minai, S.; Kang, M.; McQuiston, A.R.; Dewey, W.L.; Akbarali, H.I. Chronic Morphine Induces IL-18 in Ileum Myenteric Plexus Neurons Through Mu-opioid Receptor Activation in Cholinergic and VIPergic Neurons. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2022, 17, 111–130. [Google Scholar] [CrossRef]
- Ren, M.; Lotfipour, S. Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota. Microorganisms 2022, 10, 1127. [Google Scholar] [CrossRef]
- Truitt, B.; Venigalla, G.; Singh, P.; Singh, S.; Tao, J.; Chupikova, I.; Roy, S. The gut microbiome contributes to somatic morphine withdrawal behavior and implicates a TLR2 mediated mechanism. Gut Microbes 2023, 15, 2242610. [Google Scholar] [CrossRef]
- Hofford, R.S.; Meckel, K.R.; Wiser, E.J.; Wang, W.; Sens, J.P.; Kim, M.; Godino, A.; Lam, T.T.; Kiraly, D.D. Microbiome Depletion Increases Fentanyl Self-Administration and Alters the Striatal Proteome Through Short-Chain Fatty Acids. eNeuro 2024, 11, ENEURO.0388-23.2023. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.V.; Sadler, R.K.; Llovera, G.; Singh, V.; Roth, S.; Heindl, S.; Sebastian Monasor, L.; Verhoeven, A.; Peters, F.; Parhizkar, S.; et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. eLife 2021, 10, e59826. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar] [CrossRef]
- Lai, S.; Wang, J.; Wang, B.; Wang, R.; Li, G.; Jia, Y.; Chen, T.; Chen, Y. Alterations in gut microbiota affect behavioral and inflammatory responses to methamphetamine in mice. Psychopharmacology 2022, 239, 1–16. [Google Scholar] [CrossRef]
- Volkow, N.D.; Jones, E.B.; Einstein, E.B.; Wargo, E.M. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA Psychiatry 2019, 76, 208–216. [Google Scholar] [CrossRef]
- Corder, G.; Tawfik, V.L.; Wang, D.; Sypek, E.I.; Low, S.A.; Dickinson, J.R.; Sotoudeh, C.; Clark, J.D.; Barres, B.A.; Bohlen, C.J.; et al. Loss of μ-opioid receptor signaling in nociceptors, and not spinal microglia, abrogates morphine tolerance without disrupting analgesic efficacy. Nat. Med. 2017, 23, 164–173. [Google Scholar] [CrossRef]
- Cahill, C.M.; Walwyn, W.; Taylor, A.M.W.; Pradhan, A.A.A.; Evans, C.J. Allostatic Mechanisms of Opioid Tolerance Beyond Desensitization and Downregulation. Trends Pharmacol. Sci. 2016, 37, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 2002, 159, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [CrossRef]
- Merighi, S.; Gessi, S.; Varani, K.; Fazzi, D.; Stefanelli, A.; Borea, P.A. Morphine mediates a proinflammatory phenotype via μ-opioid receptor–PKCɛ–Akt–ERK1/2 signaling pathway in activated microglial cells. Biochem. Pharmacol. 2013, 86, 487–496. [Google Scholar] [CrossRef]
- Santoni, A.; Mercadante, S.; Arcuri, E. Chronic cancer and non-cancer pain and opioid-induced hyperalgesia share common mechanisms: Neuroinflammation and central sensitization. Minerva Anestesiol. 2021, 87, 210–222. [Google Scholar] [CrossRef]
- Shen, C.-H.; Tsai, R.-Y.; Shih, M.-S.; Lin, S.-L.; Tai, Y.-H.; Chien, C.-C.; Wong, C.-S. Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesth. Analg. 2011, 112, 454–459. [Google Scholar] [CrossRef]
- Thomas, J.; Hutchinson, M.R. Exploring neuroinflammation as a potential avenue to improve the clinical efficacy of opioids. Expert Rev. Neurother. 2012, 12, 1311–1324. [Google Scholar] [CrossRef]
- Meckel, K.R.; Simpson, S.S.; Godino, A.; Peck, E.G.; Sens, J.P.; Leonard, M.Z.; George, O.; Calipari, E.S.; Hofford, R.S.; Kiraly, D.D. Microbial short-chain fatty acids regulate drug seeking and transcriptional control in a model of cocaine seeking. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2024, 49, 386–395. [Google Scholar] [CrossRef]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. Taiwan Yi Zhi 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Maxwell, J.R.; Newville, J.C.; Yellowhair, T.R.; Kitase, Y.; Madurai, N.; Ramachandra, S.; Bakhireva, L.N.; Northington, F.J.; Gerner, G.; et al. Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain. Behav. Immun. 2020, 84, 45–58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkus, A.; Baltrūnienė, V.; Baušienė, J.; Baltrūnas, T.; Barkienė, L.; Kazlauskaitė, P.; Baušys, A. The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome—A Comprehensive Review. Life 2024, 14, 1227. https://doi.org/10.3390/life14101227
Barkus A, Baltrūnienė V, Baušienė J, Baltrūnas T, Barkienė L, Kazlauskaitė P, Baušys A. The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome—A Comprehensive Review. Life. 2024; 14(10):1227. https://doi.org/10.3390/life14101227
Chicago/Turabian StyleBarkus, Artūras, Vaida Baltrūnienė, Justė Baušienė, Tomas Baltrūnas, Lina Barkienė, Paulina Kazlauskaitė, and Augustinas Baušys. 2024. "The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome—A Comprehensive Review" Life 14, no. 10: 1227. https://doi.org/10.3390/life14101227
APA StyleBarkus, A., Baltrūnienė, V., Baušienė, J., Baltrūnas, T., Barkienė, L., Kazlauskaitė, P., & Baušys, A. (2024). The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome—A Comprehensive Review. Life, 14(10), 1227. https://doi.org/10.3390/life14101227








