Habitat Suitability Assessment for Two Burrowing Rodents on the Island of Lesvos: A Niche-Based Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
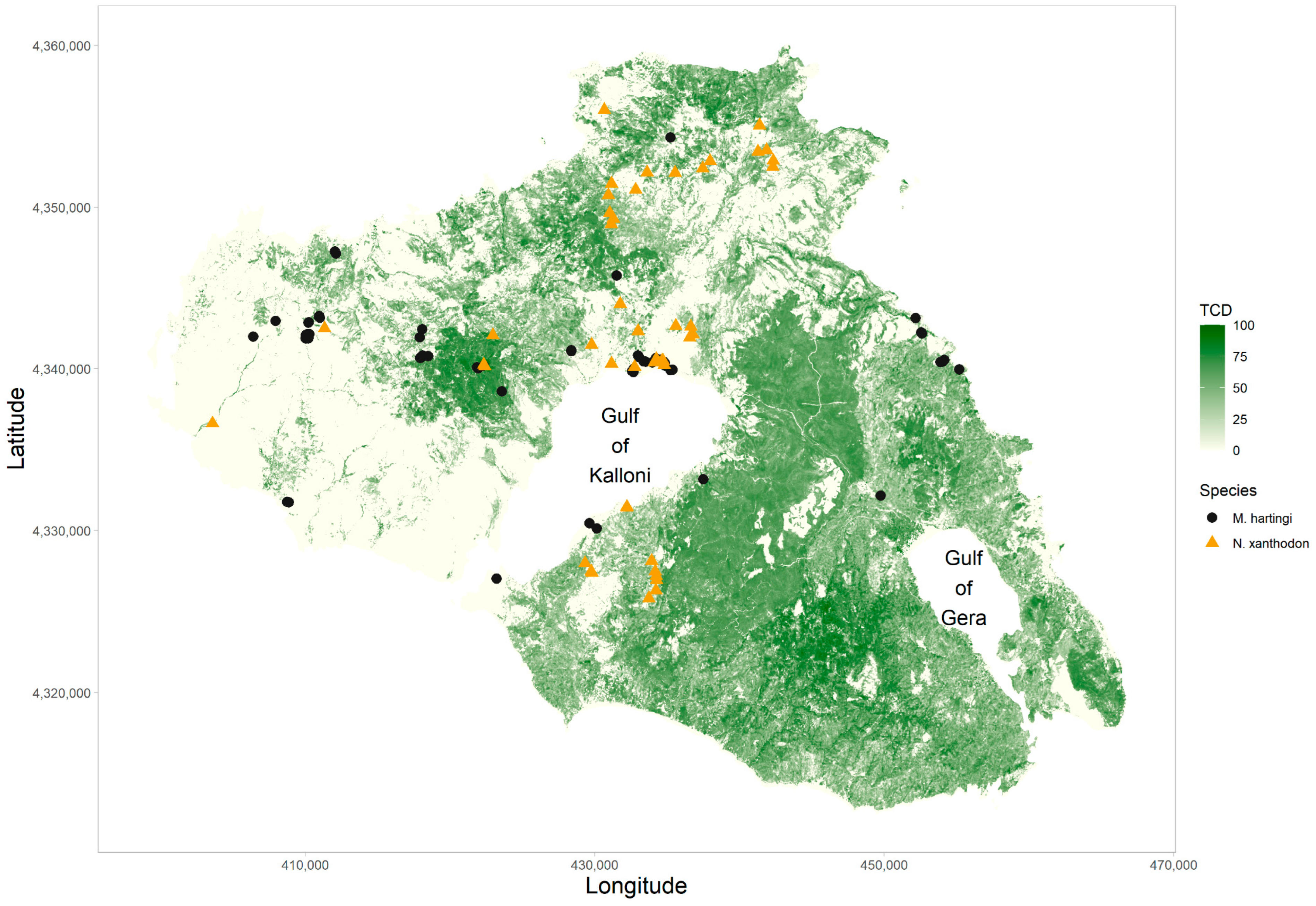
2.2. Field Collection Data
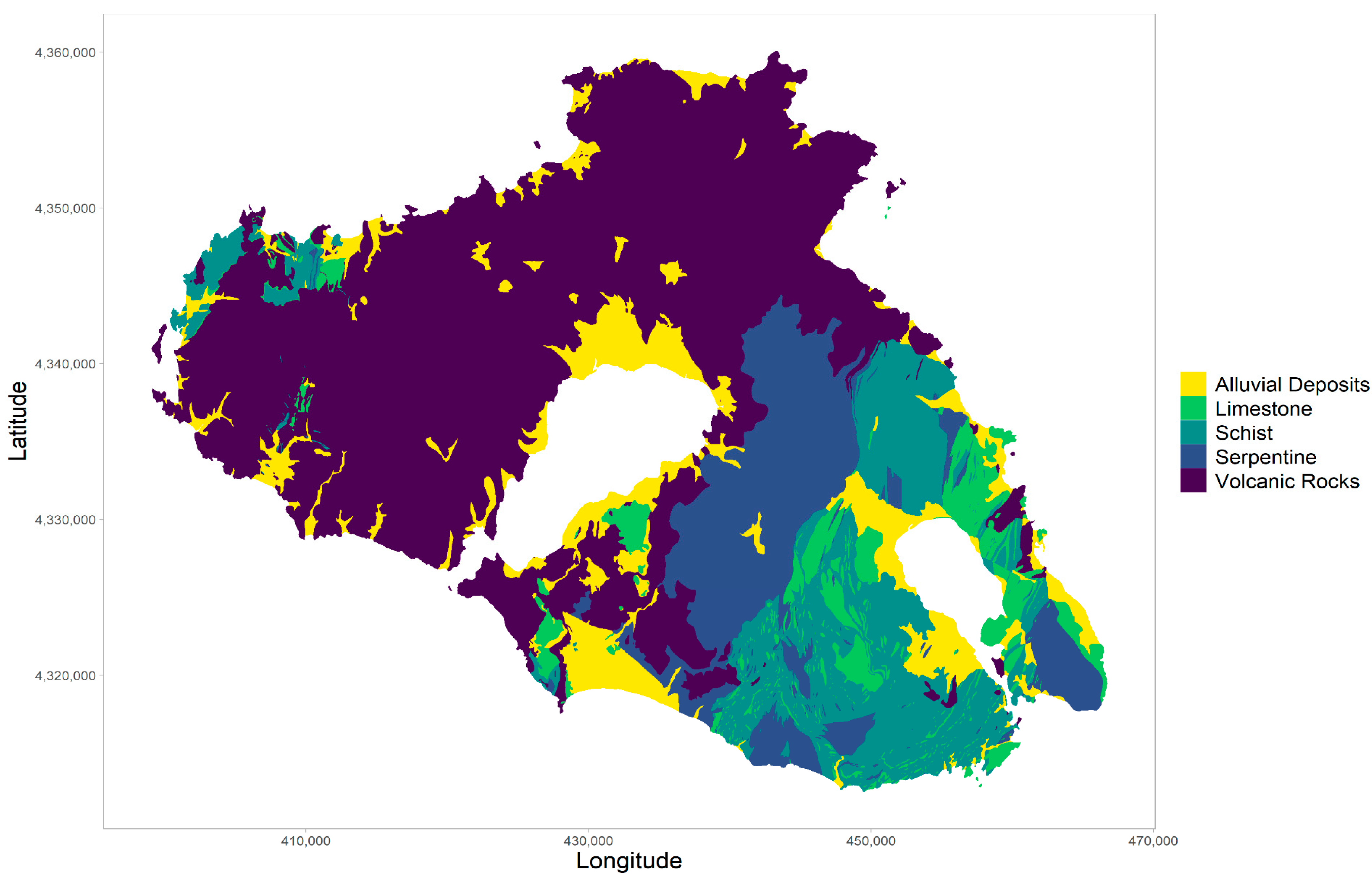
2.3. Environmental Predictors
2.4. Species Distribution Modelling (SDM) and Validation
3. Results
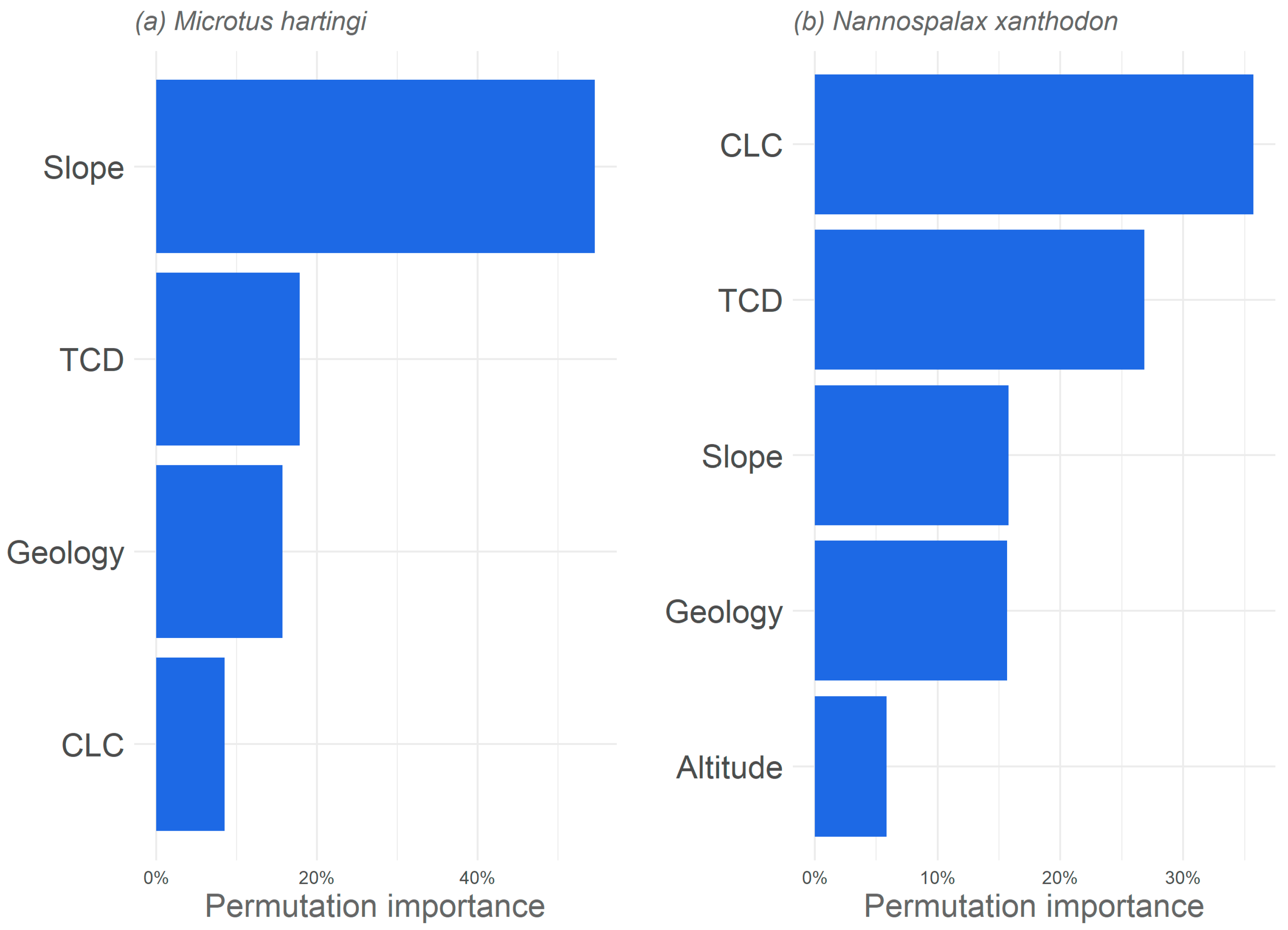
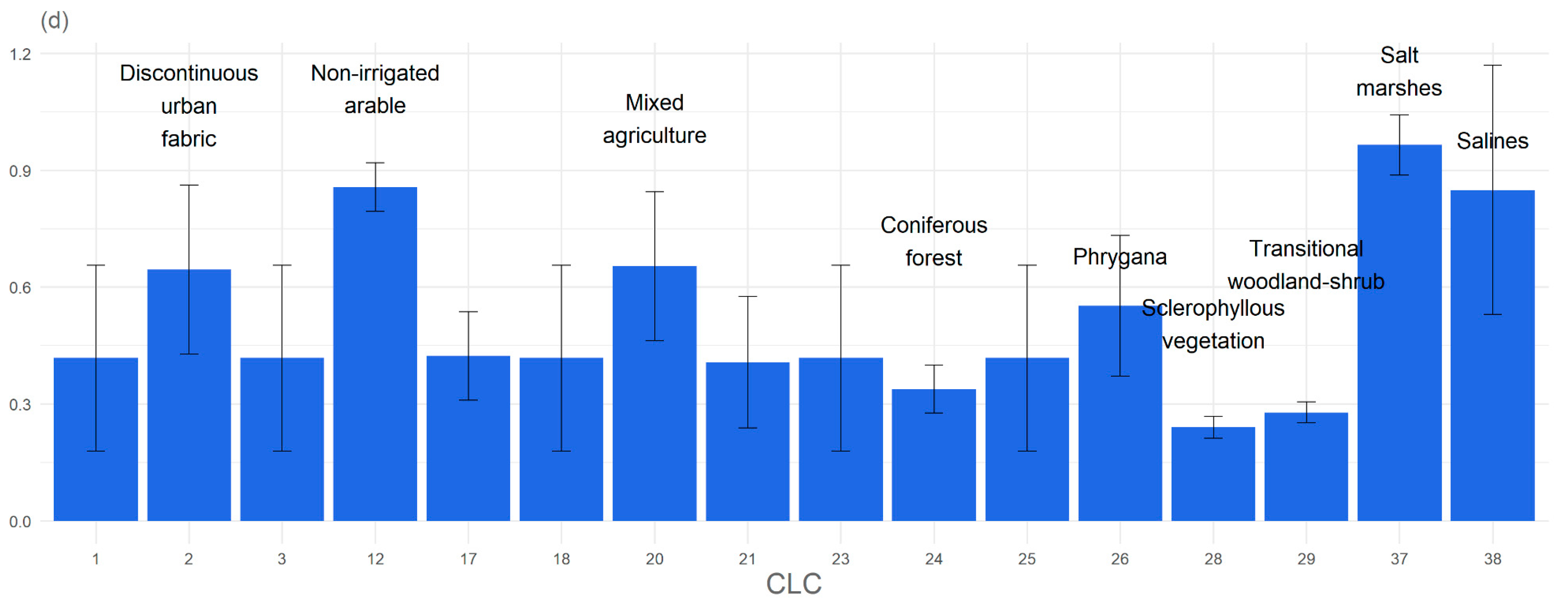
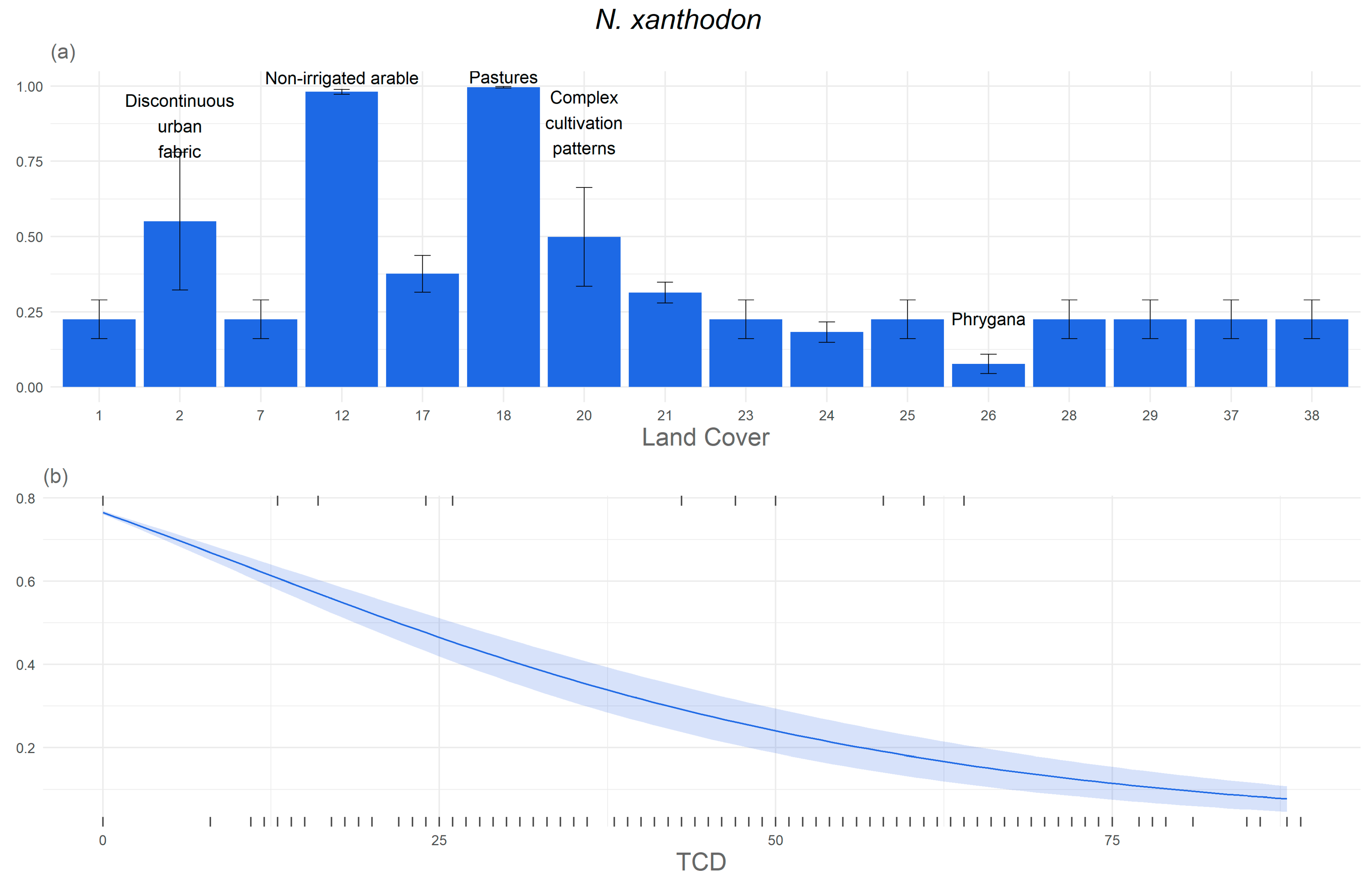
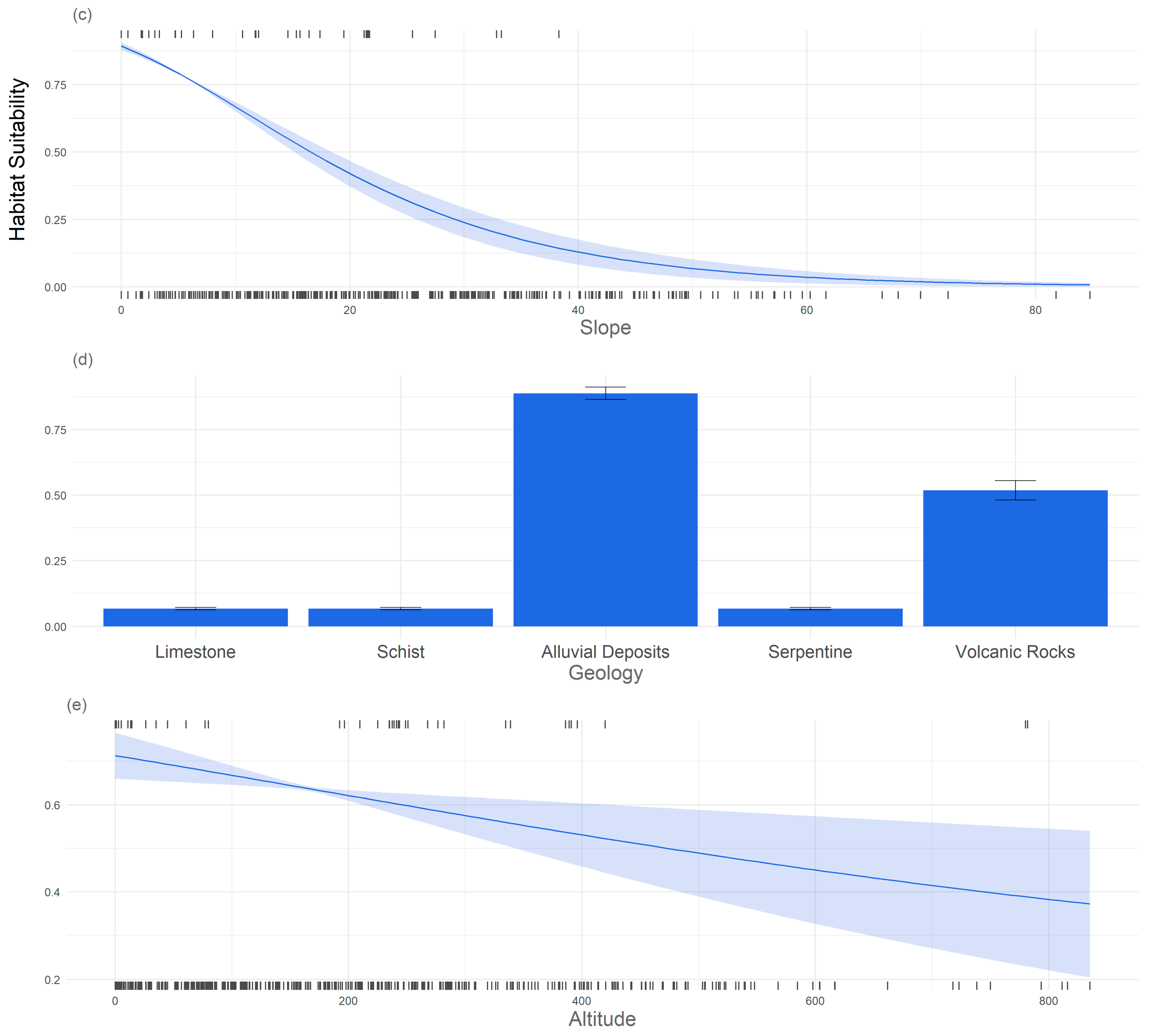
3.1. Significant Explanatory Variables and Model Performance
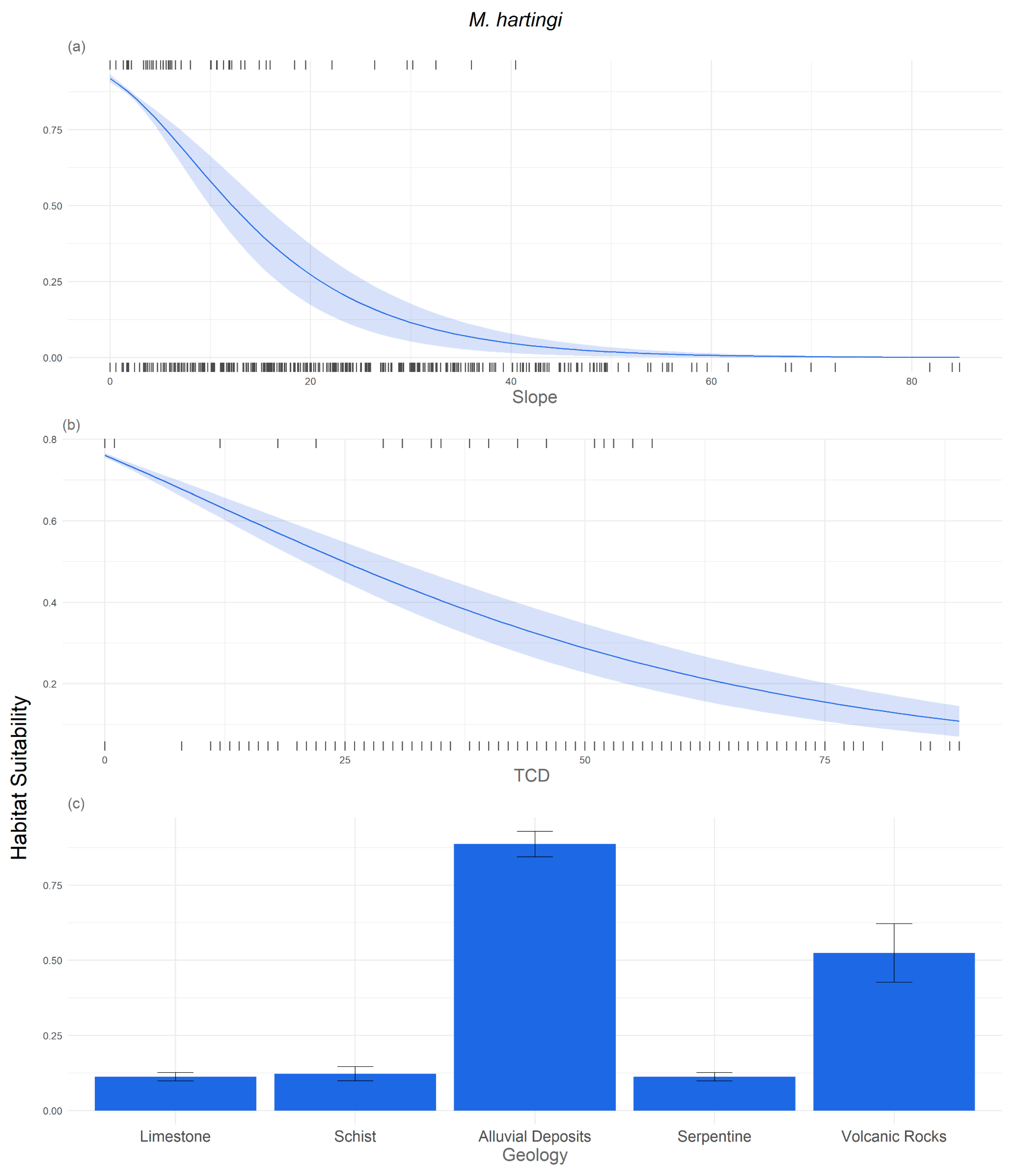
3.2. Response of the Study Species to Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kryštufek, B.; Vohralík, V. Mammals of Turkey and Cyprus, Rodentia II: Cricetinae. Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae; Knjižnica Annales Majora: Koper, Slovenia, 2009; ISBN 978-961-6732-11-6. [Google Scholar]
- Bukhnikashvili, A.; Kandaurov, A. Threatened and Insufficiently Studied Species (Insectivora, Rodentia); Institute of Zoology of Georgia: Tbilisi, Georgia, 1998. [Google Scholar]
- Šidlovskij, M. Key to the Rodents of Transcaucasia; Opredelitelgryzunov Zakavkazja; Akademija nauk Gruzinsko SSR Institut Zoologii: Tbilisi, Georgia, 1976. (In Russian) [Google Scholar]
- Masseti, M. Atlas of Terrestrial Mammals of the Ionian and Aegean Islands; Masseti, M., Ed.; Walter de Gruyter: Berlin, Germany, 2012; ISBN 978-3-11-025457-0. [Google Scholar]
- Sözen, M. A biological investigation on turkish spalax guldenstaedt, 1770 (Mammalia: Rodentia). Gazi Univ. J. Sci. 2005, 18, 167–181. [Google Scholar]
- Mitchell-Jones, A.J.; Mitchell, J.; Amori, G.; Bogdanowicz, W.; Spitzenberger, F.; Krystufek, B.; Vohralík, V.; Thissen, J.; Reijnders, P.; Ziman, J.; et al. The Atlas of European Mammals; T & AD Poyser: London, UK, 1999; Volume 3. [Google Scholar]
- IUCN. Nannospalax xanthodon: Arslan, A., Gazzard, A., Matur, F. & Sozen, M.: The IUCN Red List of Threatened Species 2023; E.T14327A22276510; IUCN: Gland, Switzerland, 2023. [Google Scholar]
- Kryštufek, B.; Vohralík, V. Mammals of Turkey and Cyprus Rodentia I: Sciuridae, Dipodidae, Gliridae, Arvicolinae; Knjižnica Annales Majora: Koper, Slovenia, 2005; ISBN 961-6033-60-3. [Google Scholar]
- Kryštufek, B. Microtus guentheri (Danford & Alston, 1880). In The Atlas of European Mammals; Mitchell-Jones, A.J., Amori, G., Bogdanowicz, W., Kryštufek, B., Reijnders, P.J.H., Spitzenberger, F., Stubbe, M., Thissen, J.B.M., Vohralík, V., Zima, J., Eds.; Poyser Natural History: London, UK, 1880; pp. 238–239. [Google Scholar]
- Stamatopoulos, C.; Ondrias, I. First Record of the Levant Vole Microtus Guentheri Danford and Alston,1880 in Lesbos Island, Greece. Säugetierkundliche Mitteilungen 1995, 36, 53–59. [Google Scholar]
- Thanou, E.; Paragamian, K.; Lymberakis, P. Social but Lonely: Species Delimitation of Social Voles and the Evolutionary History of the Only Microtus Species Living in Africa. J. Zool. Syst. Evol. Res. 2020, 58, 475–498. [Google Scholar] [CrossRef]
- Ondrias, J.C. Contribution to the Knowledge of Microtus Guentheri Hartingi from Thebes. Mammalia 1965, 29, 489–506. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Koufaki, T.; Sazeides, C.I.; Spyroglou, G.; Theodorou, K. Potential Impacts of Climate Change on the Habitat Suitability of the Dominant Tree Species in Greece. Plants 2022, 11, 1616. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Mudereri, B.T.; Abdel-Rahman, E.M.; Dube, T.; Landmann, T.; Khan, Z.; Kimathi, E.; Owino, R.; Niassy, S. Multi-Source Spatial Data-Based Invasion Risk Modeling of Striga (Striga asiatica) in Zimbabwe. GISci. Remote Sens. 2020, 57, 553–571. [Google Scholar] [CrossRef]
- Nolan, V.; Kaky, E.D.; Alatawi, A.S.; Gilbert, F. Mapping the Indian Crested Porcupine across Iraq: The Benefits of Species Distribution Modelling When Species Data Are Scarce. Mamm. Biol. 2022, 102, 1851–1866. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kamatsos, E.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Estimating Productivity, Detecting Biotic Disturbances, and Assessing the Health State of Traditional Olive Groves, Using Nondestructive Phenotypic Techniques. Sustainability 2021, 14, 391. [Google Scholar] [CrossRef]
- Palaiologou, P.; Kalabokidis, K.; Ager, A.A.; Day, M.A. Development of Comprehensive Fuel Management Strategies for Reducing Wildfire Risk in Greece. Forests 2020, 11, 789. [Google Scholar] [CrossRef]
- HNMS. Climatic Data for Selected Stations in Greece, Hellenic National Meteorological Service. Available online: http://emy.gr/emy/el/climatology/climatology_city?perifereia=North%20Aegean&poli=Mytilini (accessed on 12 June 2024).
- European Environment Agency. CORINE Land Cover 2018 (Raster 100 m); Europe, 6-Yearly-Version 2020_20u1 May 2020; European Environment Agency: Copenhagen, Denmark, 2019. [Google Scholar]
- European Environment Agency. Tree Cover Density 2018 (Raster 100 m); Europe, 3-Yearly, Sep. 2020; European Environment Agency: Copenhagen, Denmark, 2020. [Google Scholar]
- Kontos, T.D.; Komilis, D.P.; Halvadakis, C.P. Siting MSW landfills on Lesvos Island with a GIS-based methodology. Waste Manag. Res. 2003, 21, 262–277. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common Mistakes in Ecological Niche Models. Int. J. Geogr. Inf. Sci. 2021, 35, 213–226. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. blockCV: An r Package for Generating Spatially or Environmentally Separated Folds for k-Fold Cross-Validation of Species Distribution Models. Br. Ecol. Soc. 2019, 2019. 10, 225–232. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the Predictive Performance of Habitat Models Developed Using Logistic Regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Bontzorlos, V.; Vlachopoulos, K.; Xenos, A. Distribution of Four Vole Species through the Barn Owl Tyto alba Diet Spectrum: Pattern Responses to Environmental Gradients in Intensive Agroecosystems of Central Greece. Life 2023, 13, 105. [Google Scholar] [CrossRef]
- Diamantopoulos, J.; Pirintsos, S.A.; Margaris, N.S.; Stamou, G.P. Variation in Greek phrygana vegetation in relation to soil and climate. J. Veg. Sci. 1994, 5, 355–360. [Google Scholar] [CrossRef]
- Lövy, M.; Šklíba, J.; Hrouzková, E.; Dvořáková, V.; Nevo, E.; Šumbera, R. Habitat and Burrow System Characteristics of the Blind Mole Rat Spalax galili in an Area of Supposed Sympatric Speciation. PLoS ONE 2015, 10, e0133157. [Google Scholar] [CrossRef] [PubMed]
- Kryštufek, B.; Zorenko, T.; Bontzorlos, V.; Mahmoudi, A.; Atanasov, N.; Ivajnšič, D. Incipient Road to extinction of a keystone herbivore in south-eastern Europe: Harting’s vole (Microtus hartingi) under climate change. Clim. Chang. 2018, 149, 443–456. [Google Scholar] [CrossRef]
- Delmelle, P.; Opfergelt, S.; Cornelis, J.-T.; Ping, C.-L. Chapter 72-Volcanic Soils. In The Encyclopedia of Volcanoes, 2nd ed.; Sigurdsson, H., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 1253–1264. ISBN 978-0-12-385938-9. [Google Scholar]
- Boettinger, J.L. Alluvium and Alluvial Soils. In Encyclopedia of Soils in the Environment, 2nd ed.; Goss, M.J., Oliver, M., Eds.; Academic Press: Oxford, UK, 2023; pp. 417–423. ISBN 978-0-323-95133-3. [Google Scholar]
- Kierczak, J.; Pietranik, A.; Pędziwiatr, A. Ultramafic Geoecosystems as a Natural Source of Ni, Cr, and Co to the Environment: A Review. Sci. Total Environ. 2021, 755, 142620. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannetos, S.P.; Theodorou, K.; Zevgolis, Y.G.; Galinou, E.; Akriotis, T. Habitat Suitability Assessment for Two Burrowing Rodents on the Island of Lesvos: A Niche-Based Approach. Life 2024, 14, 1231. https://doi.org/10.3390/life14101231
Zannetos SP, Theodorou K, Zevgolis YG, Galinou E, Akriotis T. Habitat Suitability Assessment for Two Burrowing Rodents on the Island of Lesvos: A Niche-Based Approach. Life. 2024; 14(10):1231. https://doi.org/10.3390/life14101231
Chicago/Turabian StyleZannetos, Stylianos P., Konstantinos Theodorou, Yiannis G. Zevgolis, Eleni Galinou, and Triantaphyllos Akriotis. 2024. "Habitat Suitability Assessment for Two Burrowing Rodents on the Island of Lesvos: A Niche-Based Approach" Life 14, no. 10: 1231. https://doi.org/10.3390/life14101231
APA StyleZannetos, S. P., Theodorou, K., Zevgolis, Y. G., Galinou, E., & Akriotis, T. (2024). Habitat Suitability Assessment for Two Burrowing Rodents on the Island of Lesvos: A Niche-Based Approach. Life, 14(10), 1231. https://doi.org/10.3390/life14101231








