Diaphragmatic Ultrasonography in Sports Performance: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Source of Information
2.3. Data Collection
2.4. Methodological Quality Assessment
3. Results
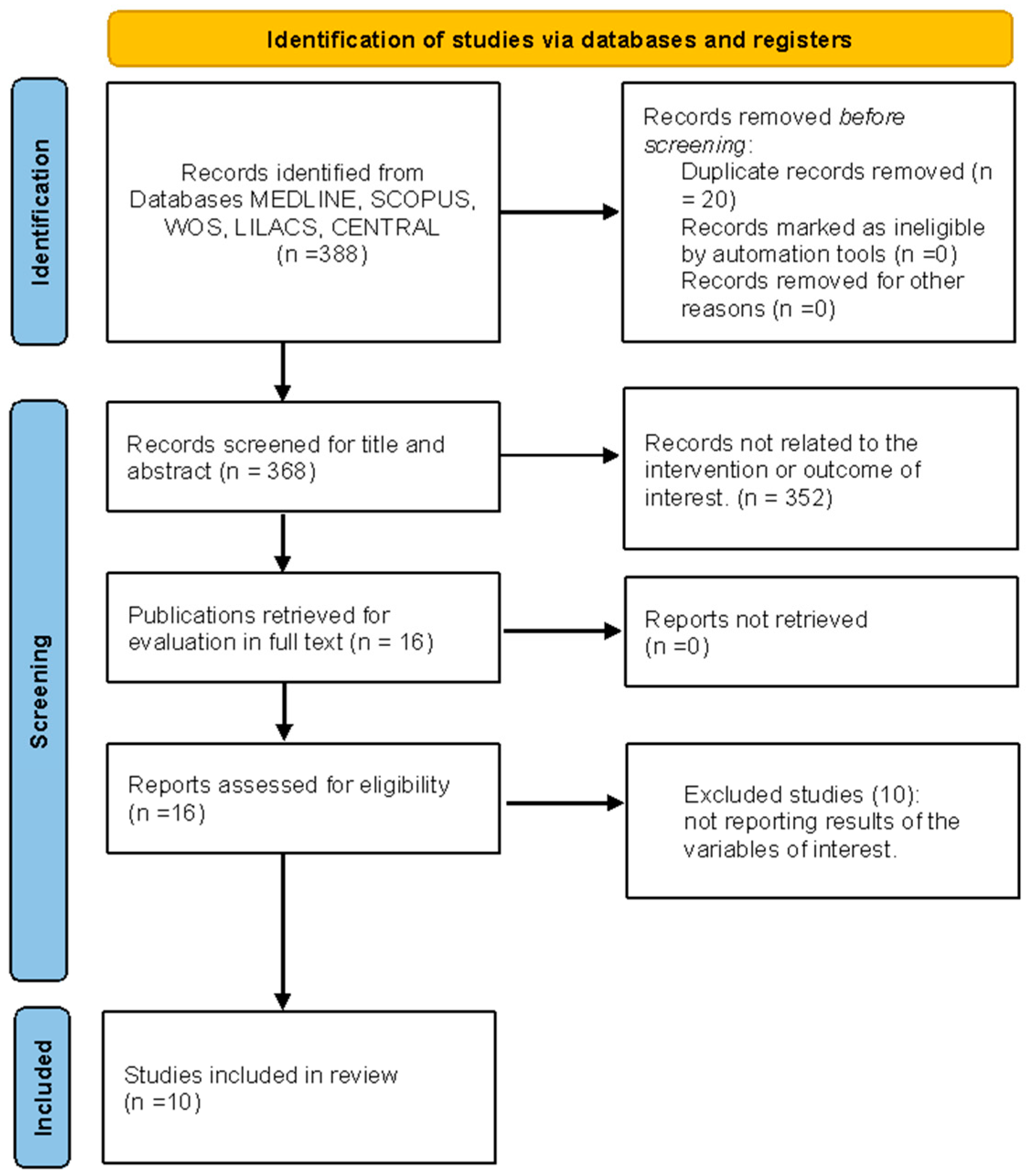
3.1. Study Selection
3.2. Characteristics of Excluded Studies
3.3. Characteristics of Included Studies
3.4. Risk of Bias and Methodological Quality Assessment
3.5. Results of the Association between Diaphragmatic Thickness/Excursion and Sports Performance
3.6. Results of the Association between Peak Respiratory Pressures and Sports Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hardy, T.I.M.A.; How, S.C.; Taylor, B.J. The Effect of Preexercise Expiratory Muscle Loading on Exercise Tolerance in Healthy Men. Med. Sci. Sports Exerc. 2021, 53, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Fulton, T.J.; Baranauskas, M.N.; Paris, H.L.; Koceja, D.M.; Mickleborough, T.D.; Chapman, R.F. Respiratory Muscle Fatigue Alters Cycling Performance and Locomotor Muscle Fatigue. Med. Sci. Sports Exerc. 2020, 52, 2380–2389. [Google Scholar] [CrossRef] [PubMed]
- Archiza, B.; Leahy, M.G.; Kipp, S.; Sheel, A.W. An integrative approach to the pulmonary physiology of exercise: When does biological sex matter? Eur. J. Appl. Physiol. 2021, 121, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- Illi, S.; Held, U.; Frank, I.; Spengler, C. Effect of Respiratory Muscle Training on Exercise Performance in Healthy Individuals A Systematic Review and Meta-Analysis. Sports Med. 2012, 42, 707–724. [Google Scholar] [CrossRef]
- Fabero-Garrido, R.; del Corral, T.; Angulo-Díaz-Parreño, S.; Plaza-Manzano, G.; Martín-Casas, P.; Cleland, J.; Fernandez-de-Las-Penas, C.; López-de-Uralde-Villanueva, I. Respiratory muscle training improves exercise tolerance and respiratory muscle function/structure post-stroke at short term: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101596. [Google Scholar] [CrossRef]
- Wüthrich, T.; Eberle, E.; Spengler, C. Locomotor and diaphragm muscle fatigue in endurance athletes performing time-trials of different durations. Eur. J. Appl. Physiol. 2014, 114, 1619–1633. [Google Scholar] [CrossRef]
- Fabrin, S.; Palinkas, M.; Fioco, E.M.; Gomes, G.; Regueiro, E.; da Silva, G.; Siéssere, S.; Verri, E.D.; Regalo, S.C.H. Functional assessment of respiratory muscles and lung capacity of CrossFit athletes. J. Exerc. Rehabil. 2023, 19, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Washino, S.; Mankyu, H.; Kanehisa, H.; Mayfield, D.L.; Cresswell, A.G.; Yoshitake, Y. Effects of inspiratory muscle strength and inspiratory resistance on neck inspiratory muscle activation during controlled inspirations. Exp. Physiol. 2019, 104, 556–567. [Google Scholar] [CrossRef]
- Dareh-deh, H.R.; Hadadnezhad, M.; Letafatkar, A.; Peolsson, A. Therapeutic routine with respiratory exercises improves posture, muscle activity, and respiratory pattern of patients with neck pain: A randomized controlled trial. Sci. Rep. 2022, 12, 4149. [Google Scholar] [CrossRef]
- Shimozawa, Y.; Kurihara, T.; Kusagawa, Y.; Hori, M.; Numasawa, S.; Sugiyama, T.; Tanaka, T.; Suga, T.; Terada, R.S.; Isaka, T.; et al. Point Prevalence of the Biomechanical Dimension of Dysfunctional Breathing Patterns among Competitive Athletes. J. Strength Cond. Res. 2023, 37, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Marugán-Rubio, D.; Chicharro, J.L.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; Vicente-Campos, D.; Molina-Hernández, N.; Calvo-Lobo, C. Effectiveness of Ultrasonography Visual Biofeedback of the Diaphragm in Conjunction with Inspiratory Muscle Training on Muscle Thickness, Respiratory Pressures, Pain, Disability, Quality of Life and Pulmonary Function in Athletes with Non-Specific Low Bac. J. Clin. Med. 2022, 11, 4318. [Google Scholar] [CrossRef]
- Sikora, M.; Mikołajczyk, R.; Łakomy, O.; Karpiński, J.; Żebrowska, A.; Kostorz-Nosal, S.; Jastrzębski, D. Influence of the breathing pattern on the pulmonary function of endurance-trained athletes. Sci. Rep. 2024, 14, 1113. [Google Scholar] [CrossRef] [PubMed]
- Archiza, B.; Welch, J.F.; Geary, C.M.; Allen, G.P.; Borghi-Silva, A.; Sheel, A.W. Temporal characteristics of exercise-induced diaphragmatic fatigue. J. Appl. Physiol. 2018, 124, 906–914. [Google Scholar] [CrossRef]
- Santana, P.; Cardenas, L.; de Albuquerque, A.; de Carvalho, C.; Caruso, P. Diaphragmatic ultrasound: A review of its methodological aspects and clinical uses. J. Bras. Pneumol. 2020, 46, e20200064. [Google Scholar] [CrossRef] [PubMed]
- Kharat, A.; Plojoux, J. Diaphragmatic assessment by ultrasonography. Rev. Med. Suisse 2021, 17, 1962–1966. [Google Scholar] [PubMed]
- Hackett, D. Lung Function and Respiratory Muscle Adaptations of Endurance-and Strength-Trained Males. Sports 2020, 8, 160. [Google Scholar] [CrossRef]
- Tuinman, P.R.; Jonkman, A.H.; Dres, M.; Shi, Z.H.; Goligher, E.C.; Goffi, A.; de Korte, C.; Demoule, A.; Heunks, L. Respiratory muscle ultrasonography: Methodology, basic and advanced principles and clinical applications in ICU and ED patients—A narrative review. Intensive Care Med. 2020, 46, 594–605. [Google Scholar] [CrossRef]
- Vetrugno, L.; Guadagnin, G.M.; Barbariol, F.; Langiano, N.; Zangrillo, A.; Bove, T. Ultrasound Imaging for Diaphragm Dysfunction: A Narrative Literature Review. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2525–2536. [Google Scholar] [CrossRef]
- Lesniak, B.P.; Loveland, D.; Jose, J.; Selley, R.; Jacobson, J.A.; Bedi, A. Use of ultrasonography as a diagnostic and therapeutic tool in sports medicine. Arthrosc.-J. Arthrosc. Relat. Surg. 2014, 30, 260–270. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary. Mil. Med. Res. 2002, 7, 1–11. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.S.; Soloaga, A.O.; Rezende, L.C.; Zanatto, S.F.; Silva, V.M.D.; Coelho-Ravagnani, C.D.F. Effects of COVID-19 on diaphragm thickness and physical performance of athletes. Fisioter. Mov. 2023, 36, e36129. [Google Scholar] [CrossRef]
- Ichiba, T.; Okuda, K.; Miyagawa, T.; Kataoka, M.; Yahagi, K. Relationship between pulmonary function, throw distance, and psychological competitive ability of elite highly trained Japanese boccia players via correlation analysis. Heliyon 2020, 6, e03581. [Google Scholar] [CrossRef] [PubMed]
- Pałac, M.; Sikora, D.; Wolny, T.; Linek, P. Relationship between respiratory muscles ultrasound parameters and running tests performance in adolescent football players. A pilot study. PeerJ 2023, 11, e15214. [Google Scholar] [CrossRef]
- Erail, S.; Bostanci, Ö.; Polat, A.V. Ultrasound Assessment of Diaphragm Thickness in Athletes. Int. J. Morphol. 2022, 40, 376–383. [Google Scholar] [CrossRef]
- West, C.R.; Taylor, B.J.; Campbell, I.G.; Romer, L.M. Effects of inspiratory muscle training on exercise responses in Paralympic athletes with cervical spinal cord injury. Scand. J. Med. Sci. Sports 2014, 24, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.I.; Venables, H.K.; Liu, H.; De-Witt, J.T.; Brown, M.R.; Faghy, M.A. Ventilatory muscle strength, diaphragm thickness and pulmonary function in world-class powerlifters. Eur. J. Appl. Physiol. 2013, 113, 2849–2855. [Google Scholar] [CrossRef]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of Respiratory Muscle Training on Performance in Athletes. J. Strength Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef]
- Sales, A.; Fregonezi, G.d.F.; Ramsook, A.; Guenette, J.; Lima, I.; Reid, W. Respiratory muscle endurance after training in athletes and non-athletes: A systematic review and meta-analysis. Phys. Ther. Sport 2016, 17, 76–86. [Google Scholar] [CrossRef]
- Karsten, M.; Ribeiro, G.S.; Esquivel, M.S.; Matte, D.L. The effects of inspiratory muscle training with linear workload devices on the sports performance and cardiopulmonary function of athletes: A systematic review and meta-analysis. Phys. Ther. Sport 2018, 34, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Moya, W.; Buitrago, R.; Alonso, J. Revisión sobre el entrenamiento de los músculos respiratorios en jugadores de fútbol. Kronos 2021, 20, 1–11. [Google Scholar]
- Holtzhausen, S.; Unger, M.; Lupton-Smith, A.; Hanekom, S. An investigation into the use of ultrasound as a surrogate measure of diaphragm function. Heart Lung 2018, 47, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Polla, B.; D’Antona, G.; Bottinelli, R.; Reggiani, C. Respiratory muscle fibres: Specialisation and plasticity. Thorax 2004, 59, 808–817. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Armstrong, N.; Barker, A.R.; McManus, A.M. Muscle metabolism changes with age and maturation: How do they relate to youth sport performance? Br. J. Sports Med. 2015, 49, 860–864. [Google Scholar] [CrossRef]
- Bergeron, M.F.; Mountjoy, M.; Armstrong, N.; Chia, M.; Côté, J.; Emery, C.A.; Faigenbaum, A.; Hall, G.; Kriemler, S.; Léglise, M.; et al. International Olympic Committee consensus statement on youth athletic development. Br. J. Sports Med. 2015, 49, 843–851. [Google Scholar] [CrossRef]
- Storey, A.; Smith, H.K. Unique aspects of competitive weightlifting: Performance, training and physiology. Sports Med. 2012, 42, 769–790. [Google Scholar] [CrossRef]
- Fry, A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef]
- Campos, G.E.R.; Luecke, T.J.; Wendeln, H.K.; Toma, K.; Hagerman, F.C.; Murray, T.F.; Ragg, K.E.; Ratamess, N.A.; Kraemer, W.J.; Staron, R.S. Muscular adaptations in response to three different resistance-training regimens: Specificity of repetition maximum training zones. Eur. J. Appl. Physiol. 2002, 88, 50–60. [Google Scholar] [CrossRef]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C.; de Oliveira, C.R.; Ricci, P.A.; Amaral, A.C.D.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of inspiratory muscle training in professional women football players: A randomized sham-controlled trial. J. Sports Sci. 2017, 36, 771–780. [Google Scholar] [CrossRef]
- Cavalcante Silva, R.; Hall, E.; Maior, A. Inspiratory muscle training improves performance of a repeated sprints ability test in professional soccer players. J. Bodyw. Mov. Ther. 2019, 23, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Hartz, C.S.; Sindorf, M.A.G.; Lopes, C.R.; Batista, J.; Moreno, M.A. Effect of Inspiratory Muscle Training on Performance of Handball Athletes. J. Hum. Kinet. 2018, 63, 43–51. [Google Scholar] [CrossRef]
- Riganas, C.; Papadopoulou, Z.; Margaritelis, N.V.; Christoulas, K.; Vrabas, I.S. Inspiratory muscle training effects on oxygen saturation and performance in hypoxemic rowers: Effect of sex. J. Sports Sci. 2019, 37, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Vašíčková, J.; Neumannová, K.; Svozil, Z. The effect of respiratory muscle training on fin-swimmers’ performance. J. Sports Sci. Med. 2017, 16, 521–526. [Google Scholar] [PubMed]
- Gee, C.M.; Williams, A.M.; Sheel, A.W.; Eves, N.D.; West, C.R. Respiratory muscle training in athletes with cervical spinal cord injury: Effects on cardiopulmonary function and exercise capacity. J. Physiol. 2019, 597, 3673–3685. [Google Scholar] [CrossRef]
- Hardy, T.A.; Chadwick, M.R.; Davies, M.J. Mechanisms of improved exercise capacity following respiratory muscle training in athletes with cervical spinal cord injury. J. Physiol. 2019, 597, 5531–5532. [Google Scholar] [CrossRef]
- Naik, L.Y.S.; Sondekoppam, R.V.; Jenkin Tsui, J.; Tsui, B.C.H. An ultrasound-guided ABCDE approach with a sniff test to evaluate diaphragmatic function without acoustic windows. Can. J. Anesth. 2016, 63, 1199–1200. [Google Scholar] [CrossRef]
- Da Conceicao, D.; Perlas, A.; Girón Arango, L.; Wild, K.; Li, Q.; Huszti, E.; Chowdhury, J.; Chan, V. Validation of a novel point-of-care ultrasound method to assess diaphragmatic excursion. Reg. Anesth. Pain Med. 2023, 10, 11–36. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Yang, D.; Gao, F.; Du, L.; Yang, M. Ultrasonographic evaluation of diaphragm thickness and excursion in patients with cervical spinal cord injury. J. Spinal Cord. Med. 2021, 44, 742–747. [Google Scholar] [CrossRef]
- Berlowitz, D.J.; Wadsworth, B.; Ross, J. Respiratory problems and management in people with spinal cord injury. Breathe 2016, 12, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Fouladi, S.; Ramouz, A.; Shadvar, K.; Ostadi, Z.; Soleimanpour, H. Diaphragm ultrasound to predict weaning outcome: Systematic review and meta-analysis. Anaesthesiol. Intensive Ther. 2022, 54, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Qian, Z.; Zhang, H.; Wang, M.; Yu, Y.; Ye, C.; Hu, W.; Gong, S. Diaphragmatic ultrasonography-based rapid shallow breathing index for predicting weaning outcome during a pressure support ventilation spontaneous breathing trial. BMC Pulm. Med. 2022, 22, 337. [Google Scholar] [CrossRef] [PubMed]
- Bissett, B.M.; Wang, J.; Neeman, T.; Leditschke, I.A.; Boots, R.; Paratz, J. Which ICU patients benefit most from inspiratory muscle training? Retrospective analysis of a randomized trial. Physiother. Theory Pract. 2020, 36, 1316–1321. [Google Scholar] [CrossRef]
- Bissett, B.; Gosselink, R.; Van Haren, F.M.P. Respiratory Muscle Rehabilitation in Patients with Prolonged Mechanical Ventilation: A Targeted Approach. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Hoffman, M.; Van Hollebeke, M.; Clerckx, B.; Muller, J.; Louvaris, Z.; Gosselink, R.; Hermans, G.; Langer, D. Can inspiratory muscle training improve weaning outcomes in difficult to wean patients? A protocol for a randomised controlled trial (IMweanT study). BMJ Open 2018, 8, e021091. [Google Scholar] [CrossRef]
| Scheme | Type of Study | Objective | n Total | Sex (%M) | Type of Sports | Age * (n) | Main Findings |
|---|---|---|---|---|---|---|---|
| Brown et al., 2013 (UK) [28] | Control-Case | To investigate the functional changes in the ventilatory system of elite powerlifters | 20 | 50 | Powerlifting | Powerlifting (10): 28 ± 11.3/untrained (10): 25 ± 4.1 | Maximal pulmonary pressures and diaphragm thickness were greater in powerlifters compared to controls. |
| West et al., 2013 (ENG) [27] | Randomized controlled trial | To determinate if inspiratory muscle training (IMT) improves respiratory structure and function and peak exercise responses in highly trained athletes with cervical spinal cord injury (SCI). | 10 | 90 | Wheelchair rugby | Placebo group (5): 27.9 ± 2.8/IMT group (5): 30.5 ± 2.2 | IMT resulted in significant diaphragmatic hypertrophy and increased inspiratory muscle strength in highly trained athletes with cervical SCI. |
| Farias et al., 2023 (BR) [23] | Cross-sectional | Assess the association between diaphragm thickness and the physical performance of athletes and the effects of COVID-19 infection on these parameters. | 63 | 81 | Soccer referee, karate, cycling, soccer, athletics, swimming, running, triathlon, martial arts, and judo | Males (51): 23.44 ± 9.65; Females (12): 16.67 ± 5.03 | No significant association between diagram thickness and maximum oxygen consumption. |
| Ichiba et al., 2020 (JP) [24] | Cross-sectional | Determine the relationship between pulmonary function, pitching distance, and psychologicalcompetitive ability of Japanese boccia players. | 13 | 77 | Boccia | 32.9 ± 12.0 | Significant correlations between diaphragm excursion, weight, and pitching distance. |
| Erail et al., 2022 (TR) [26] | Case–control | Examine the correlation between the aerobic and anaerobic performance of diaphragm thickness in athletes | 40 | 100 | Soccer, basketball, volleyball players, individual athletes, sprinter, wrestling, and judo | TA (15): 21.80 ± 2.40 IA (15): 18.93 ± 2.31 CON (10): 23.60 ± 2.91 | Greater diaphragmatic thickness in athletes correlates with anaerobic activities and thinner diaphragm with higher VO2Max levels. |
| Palac et al., 2023 (POL) [25] | Cross-sectional | Determine the relationship between ultrasound imaging of respiratory muscles during tidal breathing and running tests | 22 | 100 | Football players | 17.1 ± 0.29 | Diaphragmatic excursion is positively correlated with 5 m and 10 m speed tests, respectively, in adolescent football players. |
| Scheme | Measure | Subgroups | Variable | Results | p Value | Correlation |
|---|---|---|---|---|---|---|
| Brown et al., 2013 [28] | Respiratory muscle function | Powerlifter | Diaphragm thickness in expiration (mm) | 3.10 ± 0.99 | <0.01 | NR |
| Untrained | 2.06 ± 0.33 | |||||
| Powerlifter | Maximal static inspiratory pressure (cmH2O) | −156.8 ± 24.9 | <0.05 | r = 0.518; p = 0.019 a | ||
| Untrained | −127.7 ± 35.5 | |||||
| Powerlifter | Maximal static expiratory pressure (cmH2O) | 199.9 ± 66.4 | 0.07 | r = 0.671; p = 0.001 a | ||
| Untrained | 153.4 ± 41.6 | |||||
| Sports Performance | Powerlifter | Muscle strength test (kg) | 735 ± 211.3 | <0.05 | r = 0.825; p = 0.03 a | |
| Untrained | no measured | |||||
| West et al., 2013 [27] | Respiratory muscle function | Placebo | Diaphragm thickness (mm) | Pre: 3.42 ± 0.21/Post: 3.32 ± 0.20 | 0.001 | NR |
| IMT | Pre: 3.13 ± 0.17/Post: 3.79 ± 0.06 | |||||
| Placebo | Maximum static inspiratory pressure (cmH2O) | Pre: −122 ± 12/Post: −116 ± 15 | 0.017 | r = 0.25, p = 0.67 a | ||
| IMT | Pre: −121 ± 17/Post: −135 ± 15 | |||||
| Placebo | Maximum static expiratory pressure (cmH2O) | Pre: 72 ± 16/Post: 71 ± 15 | <0.01 | NR | ||
| IMT | Pre: 78 ± 19/Post: 94 ± 19 | |||||
| Sports Performance | Placebo | Work rate (W) | Pre: 53.3 ± 13.7/Post: 54.3 ± 15.3 | 0.034 | NR | |
| IMT | Pre: 54.9 ± 5.9/Post: 63.1 ± 6.1 | |||||
| Placebo | Oxygen uptake-VO2 (L/min) | Pre: 1.11 ± 0.24/Post: 1.08 ± 0.28 | 0.077 | NR | ||
| IMT | Pre: 1.08 ± 0.17/Post: 1.27 ± 0.12 | |||||
| Farias et al., 2023 [23] | Respiratory muscle function | All participants | Diaphragm thickness (%) | Females: 61.00 ± 0.2 | 0.22 | NR |
| Males: 55.00 ± 0.25 | ||||||
| Sports performance | Maximal oxygen uptake-VO2 Max (mL/kg/min) | Females: 39.34 ± 1.74 | r = 0.30; p = 0.22 | |||
| Males: 41.25 ± 6.84 | ||||||
| Ichiba et al., 2020 [24] | Respiratory muscle function | All participants | Diaphragm excursion (mm) | Resting inspiration: 1.76 ± 0.45 | NR | r = 0.598, p = 0.05 b |
| Resting expiration: 1.42 ± 0.47 | NR | r = 0.620, p = 0.05 b | ||||
| Maximal inspiration: 3.9 ± 1.36 | NR | r = 0.589, p = 0.05 b | ||||
| Maximal expiration: 1.05 ± 0.31 | NR | NR | ||||
| Maximum static inspiratory pressure (cm/H2O) | −53.1 ± 25.4 | NR | NR | |||
| Maximum static expiratory pressure (cm/H2O) | 50.7 ± 25.1 | NR | ||||
| Sport performance | Pitching distance (mts) | 18.09 ± 7.86 | NR | |||
| Erail et al., 2022 [26] | Respiratory muscle function | IA | Maximum static inspiratory pressure (cmH2O) | −129.80 ± 28.93 | 0.010 * | r = −0.010 d; r = 0.283 c; p > 0.05 |
| TA | −110.67 ± 24.99 | NR | r = −0.122 d; r = −0.295 c; p > 0.05 | |||
| CON | −94.8 ± 26.36 | NR | r = −0.211 d; r = 0.082 c; p > 0.05 | |||
| IA | Maximum static expiratory pressure (cmH2O) | 168.87 ± 48.73 | 0.001 * | r = 0.156 d; r = 0.064 c; p > 0.05 | ||
| TA | 134.67 ± 26.07 | NR | r = −0.009 d; r = 0.333 c; p > 0.05 | |||
| CON | 102.6 ± 29.31 | NR | r = −0.269 d; r = −0.011 c; p > 0.05 | |||
| IA | Diaphragmatic thickness in inspiration (mm) | 5.67 ± 1.19 | 0.048 | Reported correlations with other parameters with letter c | ||
| TA | 4.87 ± 1.16 | |||||
| CON | 4.56 ± 1.19 | |||||
| IA | Diaphragmatic thickness in expiration (mm) | 1.94 ± 0.43 | 0.001 | Reported correlations with other parameters with letter d | ||
| TA | 1.61 ± 0.21 | |||||
| CON | 1.40 ± 0.25 | |||||
| Sport performance | IA | Average power (W) | 619.99 ± 127.74 | 0.008 * | r = 0.483 c; p < 0.05 | |
| TA | 556.37 ± 96.05 | NR | r = 0.085 d; r = 0.306 c; p > 0.05 | |||
| CON | 469.71 ± 93.94 | NR | r = 0.054 d; r = 0.180 c; p > 0.05 | |||
| IA | Peak power (W) | 878.33 ± 207.59 | 0.008 * | r = 0.495 c; p < 0.05 | ||
| TA | 749.47 ± 137.41 | NR | r = 0.477 c; p < 0.05 | |||
| CON | 649.25 ± 159.08 | NR | r = −0.04; r = −0.049; p > 0.05 | |||
| IA | Maximal oxygen uptake-VO2 Max (mL/kg/min) | 54.39 ± 7.07 | 0.006 * | r = −0.551 c; p < 0.05 | ||
| TA | 57.56 ± 6.93 | 0.001 * | r = 0.169 d; r = -0.230 c; p > 0.05 | |||
| CON | 46.76 ± 3.68 | NR | r = −0.095 d; r = 0.049 c; p > 0.05 | |||
| Palac et al., 2023 [25] | Respiratory muscle function | All participants | Diaphragm excursion (cm) | 4.73 ± 1.45 | NR | r = 0.46; p = 0.04 e |
| Diaphragm thickness end of tidal inspiration (mm) | 2.09 ± 0.85 | NR | Reported correlations with other parameters with letter c | |||
| Diaphragm thickness end of tidal expiration (mm) | 1.71 ± 0.59 | NR | Reported correlations with other parameters with letter d | |||
| Sport performance | Shuttle run test (total number of completed 20 m repetitions) | 127 ± 13.2 | NR | r = 0.01, p = 0.98 c; r = 0.10, p = 0.66 d | ||
| Maximal oxygen uptake-VO2 Max (mL/kg/min) | 56.2 ± 3.54 | NR | r = 0.17, p = 0.45 c; r = 0.29, p = 0.18 d | |||
| Speed test-distance 5 m (s) | 1.03 ± 0.005 | NR | r = −0.07, p = 0.75 c r = −0.27, p = 0,23 d | |||
| Speed test-distance 10 m (s) | 1.87 ± 0.52 | NR | r = −0.06, p = 0.8 c; r = −0.12, p = 0.6 d | |||
| Speed test-distance 30 m (s) | 4.19 ± 0.20 | NR | r = 0,22, p = 0.34 c; r = 0.25, p = 0.25 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payán-Salcedo, H.A.; Arias-Coronel, F.; Estela-Zape, J.L.; Serna-Orozco, M.F. Diaphragmatic Ultrasonography in Sports Performance: A Systematic Review. Life 2024, 14, 1250. https://doi.org/10.3390/life14101250
Payán-Salcedo HA, Arias-Coronel F, Estela-Zape JL, Serna-Orozco MF. Diaphragmatic Ultrasonography in Sports Performance: A Systematic Review. Life. 2024; 14(10):1250. https://doi.org/10.3390/life14101250
Chicago/Turabian StylePayán-Salcedo, Harold Andrés, Florencio Arias-Coronel, Jose Luis Estela-Zape, and Maria Fernanda Serna-Orozco. 2024. "Diaphragmatic Ultrasonography in Sports Performance: A Systematic Review" Life 14, no. 10: 1250. https://doi.org/10.3390/life14101250






