Investigation of Cell Mechanics and Migration on DDR2-Expressing Neuroblastoma Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Lentivirus Preparation and Infection
2.3. Western Blot
2.4. Lentivector Cell Maintenance
2.5. Collagen-Coated Glass Slides
2.6. Cell Spreading Area Measurements
2.7. Kymograph
2.8. Atomic Force Microscopy
2.9. Polyacrylamide Substrate Preparation
2.10. Cell Traction Force Measurements
2.11. 2D Cell Migration Assay
2.12. Statistics
3. Results
3.1. shRNA-Mediated Knockdown of DDR2
3.2. Dependence of Human Neuroblastoma Phenotype on Collagen I and DDR2
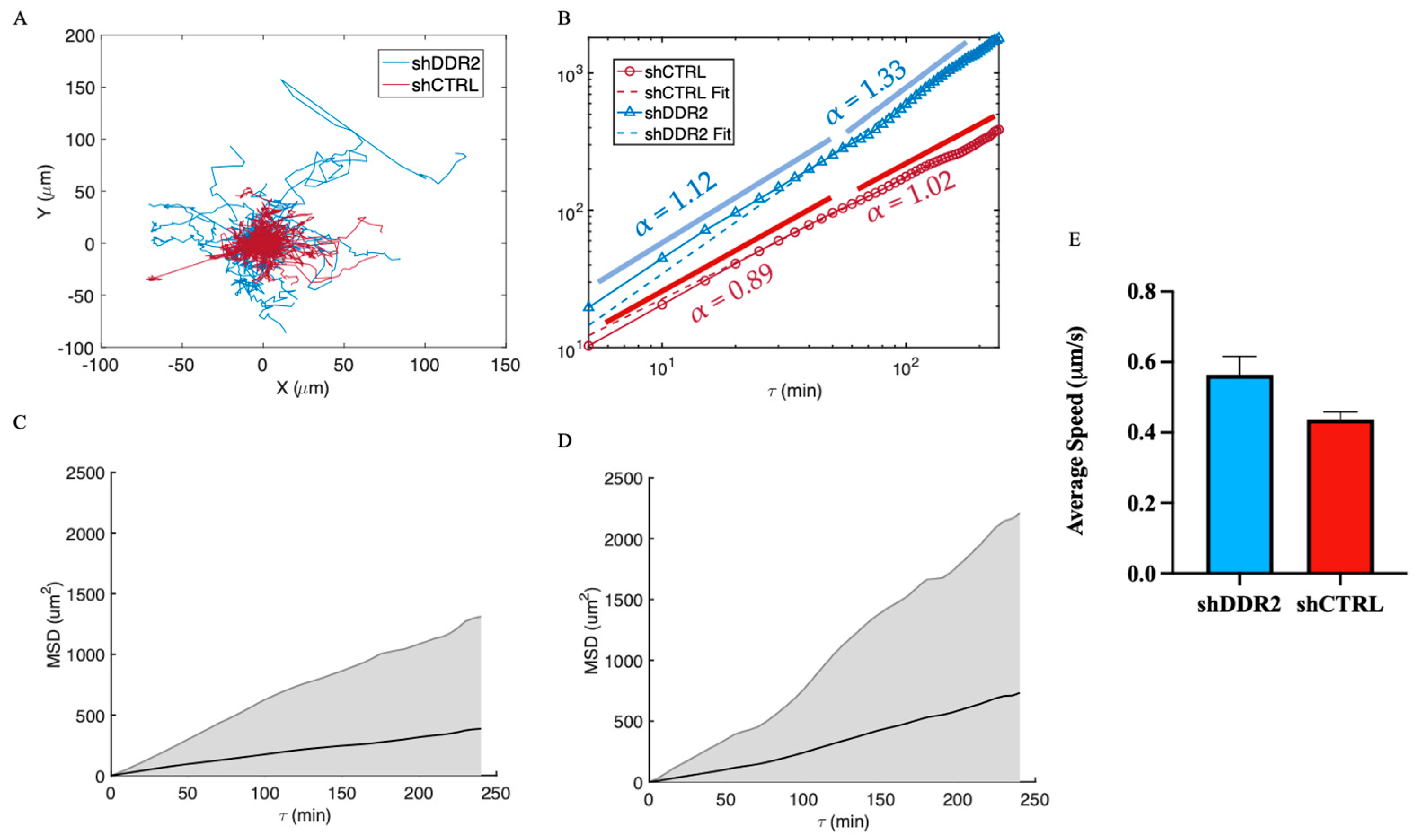
3.3. DDR2 Silencing Altered Cell Migration on 2D Collagen Substrates
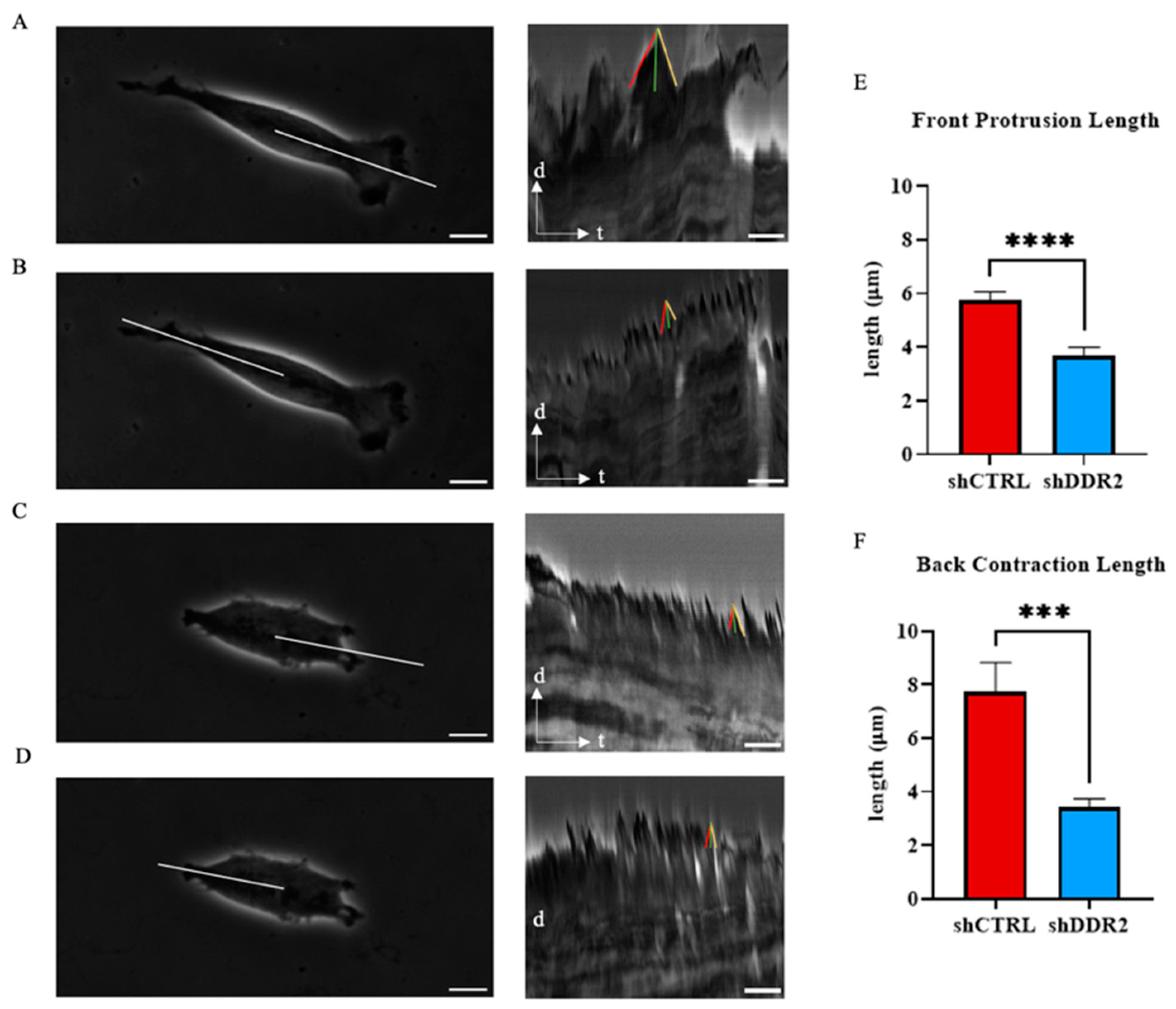
3.4. DDR2 Affects Cellular Protrusion Dynamics
3.5. Regulation of Cell Stiffness with DDR2 Collagen Activation
3.6. Effect of DDR2 Knockdown on Cell Traction Force
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 contributors; et al. International incidence of childhood cancer, 2001–10: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Childhood Cancer Collaborators. The global burden of childhood and adolescent cancer in 2017: An analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019, 20, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. Embo Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Horwacik, I. The Extracellular Matrix and Neuroblastoma Cell Communication—A Complex Interplay and Its Therapeutic Implications. Cells 2022, 11, 3172. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef]
- Li, C.; Qiu, S.; Liu, X.; Guo, F.; Zhai, J.; Li, Z.; Deng, L.; Ge, L.; Qian, H.; Yang, L.; et al. Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling. Signal Transduct. Target. Ther. 2023, 8, 247. [Google Scholar] [CrossRef]
- Blissett, A.R.; Garbellini, D.; Calomeni, E.P.; Mihai, C.; Elton, T.S.; Agarwal, G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. J. Mol. Biol. 2008, 385, 902–911. [Google Scholar] [CrossRef]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Smith, A.W.; Jones, B. Discoidin domain receptors: Micro insights into macro assemblies. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 118496. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. J. Biol. Chem. 2003, 278, 16761–16769. [Google Scholar]
- Yeung, D.A.; Shanker, N.; Sohail, A.; Weiss, B.A.; Wang, C.; Wellmerling, J.; Das, S.; Ganju, R.K.; Miller, J.L.; Herr, A.B.; et al. Clustering, Spatial Distribution, and Phosphorylation of Discoidin Domain Receptors 1 and 2 in Response to Soluble Collagen I. J. Mol. Biol. 2019, 431, 368–390. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Chung, K.-H.; Spurlin, T.A.; Haynes, R.J.; Elliott, J.T.; Plant, A.L. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials 2009, 30, 6687–6694. [Google Scholar] [CrossRef]
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021, 599, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 2014, 310, 39–87. [Google Scholar]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin domain receptor tyrosine kinases: New players in cancer progression. Cancer Metastasis Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Lu, Q.; Nada, H.; Woo, J.; Quan, G.; Lee, K. The Journey of DDR1 and DDR2 Kinase Inhibitors as Rising Stars in the Fight Against Cancer. Int. J. Mol. Sci. 2021, 22, 6535. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Hammerman, P.S.; Sos, M.L.; Ramos, A.H.; Xu, C.; Dutt, A.; Zhou, W.; Brace, L.E.; Woods, B.A.; Lin, W.; Zhang, J.; et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011, 1, 78–89. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, W.; Liu, X.; Zhao, H.; Zhang, J.; Zhang, J.; Li, X.; Zhang, Y.; Bu, X.; Shi, M.; et al. Discoidin domain receptor 2 (DDR2) promotes breast cancer cell metastasis and the mechanism implicates epithelial-mesenchymal transition programme under hypoxia. J. Pathol. 2014, 234, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Rozen, E.J.; Shohet, J.M. Systematic review of the receptor tyrosine kinase superfamily in neuroblastoma pathophysiology. Cancer Metastasis Rev. 2021, 41, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Herrera, M.L.; Quezada-Calvillo, R. DDR2 plays a role in fibroblast migration independent of adhesion ligand and collagen activated DDR2 tyrosine kinase. Biochem. Biophys. Res. Commun. 2012, 429, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Lee, Y.M.; Kim, D.K. DDR2 inhibition reduces migration and invasion of murine metastatic melanoma cells by suppressing MMP2/9 expression through ERK/NF-kappaB pathway. Acta Biochim. Biophys. Sin. 2015, 47, 292–298. [Google Scholar] [CrossRef]
- von Mässenhausen, A.; Sanders, C.; Brägelmann, J.; Konantz, M.; Queisser, A.; Vogel, W.; Kristiansen, G.; Duensing, S.; Schröck, A.; Bootz, F.; et al. Targeting DDR2 in head and neck squamous cell carcinoma with dasatinib. Int. J. Cancer 2016, 139, 2359–2369. [Google Scholar] [CrossRef]
- Barcus, C.E.; Hwang, P.Y.; Morikis, V.; Brenot, A.; Pence, P.; Clarke, M.; Longmore, G.D. Tyrosine kinase-independent actions of DDR2 in tumor cells and cancer-associated fibroblasts influence tumor invasion, migration and metastasis. J. Cell Sci. 2021, 134, jcs258431. [Google Scholar] [CrossRef]
- Wang, Y.G.; Xu, L.; Jia, R.R.; Wu, Q.; Wang, T.; Wei, J.; Ma, J.L.; Shi, M.; Li, Z.S. DDR2 Induces Gastric Cancer Cell Activities via Activating mTORC2 Signaling and Is Associated with Clinicopathological Characteristics of Gastric Cancer. Dig. Dis. Sci. 2016, 61, 2272–2283. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar]
- Tsai, M.-C.; Li, W.-M.; Huang, C.-N.; Ke, H.-L.; Li, C.-C.; Yeh, H.-C.; Chan, T.-C.; Liang, P.-I.; Yeh, B.-W.; Wu, W.-J.; et al. DDR2 overexpression in urothelial carcinoma indicates an unfavorable prognosis: A large cohort study. Oncotarget 2016, 7, 78918–78931. [Google Scholar] [CrossRef]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef]
- Zufferey, R.; Nagy, D.; Mandel, R.J.; Naldini, L.; Trono, D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997, 15, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Paganelli, F.; Giuntini, G.; Mattioli, E.; Cappellini, A.; Ramazzotti, G.; Faenza, I.; Maltarello, M.C.; Martelli, A.M.; Scotlandi, K.; et al. Lamin A and Prelamin A Counteract Migration of Osteosarcoma Cells. Cells 2020, 9, 774. [Google Scholar] [CrossRef]
- Thomas, G.; Burnham, N.A.; Camesano, T.A.; Wen, Q. Measuring the mechanical properties of living cells using atomic force microscopy. J. Vis. Exp. 2013, 76, 50497. [Google Scholar]
- Thanh, M.H.; Grella, A.; Kole, D.; Ambady, S.; Wen, Q. Vimentin intermediate filaments modulate cell traction force but not cell sensitivity to substrate stiffness. Cytoskeleton 2021, 78, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Chang, K.-A. Experimental Study of a Horizontal Jet in a Wavy Environment. J. Eng. Mech. 2003, 129, 1149–1155. [Google Scholar] [CrossRef]
- Tarantino, N.; Tinevez, J.-Y.; Crowell, E.F.; Boisson, B.; Henriques, R.; Mhlanga, M.; Agou, F.; Israël, A.; Laplantine, E. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO–IKK supramolecular structures. J. Cell Biol. 2014, 204, 231–245. [Google Scholar] [CrossRef]
- Sasaki, S.; Ueda, M.; Iguchi, T.; Kaneko, M.; Nakayama, H.; Watanabe, T.; Sakamoto, A.; Mimori, K. DDR2 Expression Is Associated with a High Frequency of Peritoneal Dissemination and Poor Prognosis in Colorectal Cancer. Anticancer. Res. 2017, 37, 2587–2591. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Martin, E.E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.-C.; Tanager, K.S.; Yoon, E.; Kidwell, K.M.; et al. Mesenchymal Stem Cell-Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep. 2017, 18, 1215–1228. [Google Scholar] [CrossRef]
- Tu, M.M.; Lee, F.Y.F.; Jones, R.T.; Kimball, A.K.; Saravia, E.; Graziano, R.F.; Coleman, B.; Menard, K.; Yan, J.; Michaud, E.; et al. Targeting DDR2 enhances tumor response to anti–PD-1 immunotherapy. Sci. Adv. 2019, 5, eaav2437. [Google Scholar] [CrossRef]
- Kurashige, J.; Hasegawa, T.; Niida, A.; Sugimachi, K.; Deng, N.; Mima, K.; Uchi, R.; Sawada, G.; Takahashi, Y.; Eguchi, H. Integrated Molecular Profiling of Human Gastric Cancer Identifies DDR2 as a Potential Regulator of Peritoneal Dissemination. Sci. Rep. 2016, 6, 22371. [Google Scholar] [CrossRef] [PubMed]
- Terai, H.; Tan, L.; Beauchamp, E.M.; Hatcher, J.M.; Liu, Q.; Meyerson, M.; Gray, N.S.; Hammerman, P.S. Characterization of DDR2 Inhibitors for the Treatment of DDR2 Mutated Nonsmall Cell Lung Cancer. ACS Chem. Biol. 2015, 10, 2687–2696. [Google Scholar] [CrossRef]
- Beauchamp, E.M.; Woods, B.A.; Dulak, A.M.; Tan, L.; Xu, C.; Gray, N.S.; Bass, A.J.; Wong, K.K.; Meyerson, M.; Hammerman, P.S. Acquired resistance to dasatinib in lung cancer cell lines conferred by DDR2 gatekeeper mutation and NF1 loss. Mol. Cancer Ther. 2014, 13, 475–482. [Google Scholar] [CrossRef]
- Yu, H.; Lim, K.P.; Xiong, S.; Tan, L.P.; Shim, W. Functional Morphometric Analysis in Cellular Behaviors: Shape and Size Matter. Adv. Heal. Mater. 2013, 2, 1188–1197. [Google Scholar] [CrossRef]
- Rozen, E.J.; Frantz, W.; Wigglesworth, K.; Vessella, T.; Zhou, H.S.; Shohet, J.M. Blockade of Discoidin Domain Receptor Signaling with Sitravatinib Reveals DDR2 as a Mediator of Neuroblastoma Pathogenesis and Metastasis. Mol. Cancer Ther. 2024, 23, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Berz, D.; Gadgeel, S.M.; Iams, W.T.; Bruno, D.S.; Blakely, C.M.; Spira, A.I.; Patel, M.R.; Waterhouse, D.M.; Richards, D.A.; et al. MRTX-500 Phase 2 Trial: Sitravatinib with Nivolumab in Patients with Nonsquamous NSCLC Progressing on or after Checkpoint Inhibitor Therapy or Chemotherapy. J. Thorac. Oncol. 2023, 18, 907–921. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Prudhomme, S.; Zaman, M.H. Modeling of adhesion, protrusion, and contraction coordination for cell migration simulations. J. Math. Biol. 2014, 68, 267–302. [Google Scholar] [CrossRef] [PubMed]
- Grady, M.E.; Composto, R.J.; Eckmann, D.M. Cell elasticity with altered cytoskeletal architectures across multiple cell types. J. Mech. Behav. Biomed. Mater. 2016, 61, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.D.; Grashoff, C.; Schwartz, M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011, 475, 316–323. [Google Scholar] [CrossRef]
- Schmidt, S.; Friedl, P. Interstitial cell migration: Integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2009, 339, 83–92. [Google Scholar] [CrossRef]
- Kraning-Rush, C.M.; Califano, J.P.; Reinhart-King, C.A. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef] [PubMed]
- Fruleux, A.; Hawkins, R.J. Physical role for the nucleus in cell migration. J. Phys. Condens. Matter 2016, 28, 363002. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep. Prog. Phys. 2019, 82, 064602. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Sun, X.; Gupta, K.; Wu, B.; Zhang, D.; Yuan, B.; Zhang, X.; Chiang, H.-C.; Zhang, C.; Curiel, T.J.; Bendeck, M.P.; et al. Tumor-extrinsic discoidin domain receptor 1 promotes mammary tumor growth by regulating adipose stromal interleukin 6 production in mice. J. Biol. Chem. 2018, 293, 2841–2849. [Google Scholar] [CrossRef]
- Hur, H.; Ham, I.-H.; Lee, D.; Jin, H.; Aguilera, K.Y.; Oh, H.J.; Han, S.-U.; Kwon, J.E.; Kim, Y.-B.; Ding, K.; et al. Discoidin domain receptor 1 activity drives an aggressive phenotype in gastric carcinoma. BMC Cancer 2017, 17, 87. [Google Scholar] [CrossRef]
- Zhang, K.; Corsa, C.A.; Ponik, S.M.; Prior, J.L.; Piwnica-Worms, D.; Eliceiri, K.W.; Keely, P.J.; Longmore, G.D. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013, 15, 677–687. [Google Scholar] [CrossRef]
- Grither, W.R.; Divine, L.M.; Meller, E.H.; Wilke, D.J.; Desai, R.A.; Loza, A.J.; Zhao, P.; Lohrey, A.; Longmore, G.D.; Fuh, K.C. TWIST1 induces expression of discoidin domain receptor 2 to promote ovarian cancer metastasis. Oncogene 2018, 37, 1714–1729. [Google Scholar] [CrossRef]
- Gao, H.; Chakraborty, G.; Zhang, Z.; Akalay, I.; Gadiya, M.; Gao, Y.; Sinha, S.; Hu, J.; Jiang, C.; Akram, M.; et al. Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell 2016, 166, 47–62. [Google Scholar] [CrossRef]
- Grither, W.R.; Longmore, G.D. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc. Natl. Acad. Sci. USA 2018, 115, E7786–E7794. [Google Scholar] [CrossRef]
- Valencia, K.; Ormazábal, C.; Zandueta, C.; Luis-Ravelo, D.; Antón, I.; Pajares, M.J.; Agorreta, J.; Montuenga, L.M.; Martínez-Canarias, S.; Leitinger, B.; et al. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin. Cancer Res. 2012, 18, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Regad, T. Targeting RTK Signaling Pathways in Cancer. Cancers 2015, 7, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fayçal, C.; Hatat, A.-S.; Gazzeri, S.; Eymin, B. Splice Variants of the RTK Family: Their Role in Tumour Progression and Response to Targeted Therapy. Int. J. Mol. Sci. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Labrador, J.P.; Azcoitia, V.; Tuckermann, J.; Lin, C.; Olaso, E.; Mañes, S.; Brückner, K.; Goergen, J.; Lemke, G.; Yancopoulos, G.; et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. Embo Rep. 2001, 2, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ying, J.; Dai, M.; Peng, J.; Zhang, D. Co-expression of DDR2 and IFITM1 promotes breast cancer cell proliferation, migration and invasion and inhibits apoptosis. J. Cancer Res. Clin. Oncol. 2022, 148, 3385–3398. [Google Scholar] [CrossRef]
- Kawai, I.; Hisaki, T.; Sugiura, K.; Naito, K.; Kano, K. Discoidin domain receptor 2 (DDR2) regulates proliferation of endochondral cells in mice. Biochem. Biophys. Res. Commun. 2012, 427, 611–617. [Google Scholar] [CrossRef]
- Xu, J.; Lu, W.; Zhang, S.; Zhu, C.; Ren, T.; Zhu, T.; Zhao, H.; Liu, Y.; Su, J. Overexpression of DDR2 contributes to cell invasion and migration in head and neck squamous cell carcinoma. Cancer Biol. Ther. 2014, 15, 612–622. [Google Scholar] [CrossRef]
- Olaso, E.; Labrador, J.-P.; Wang, L.; Ikeda, K.; Eng, F.J.; Klein, R.; Lovett, D.H.; Lin, H.C.; Friedman, S.L. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J. Biol. Chem. 2002, 277, 3606–3613. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Jarai, G. Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices. Fibrogenesis Tissue Repair 2012, 5, 3. [Google Scholar] [CrossRef]
- Vargas, P.; Maiuri, P.; Bretou, M.; Sáez, P.J.; Pierobon, P.; Maurin, M.; Chabaud, M.; Lankar, D.; Obino, D.; Terriac, E.; et al. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 2015, 18, 43–53. [Google Scholar] [CrossRef]
- Harris, T.H.; Banigan, E.J.; Christian, D.A.; Konradt, C.; Wojno, E.D.T.; Norose, K.; Wilson, E.H.; John, B.; Weninger, W.; Luster, A.D.; et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 2012, 486, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Huda, S.; Weigelin, B.; Wolf, K.; Tretiakov, K.V.; Polev, K.; Wilk, G.; Iwasa, M.; Emami, F.S.; Narojczyk, J.W.; Banaszak, M.; et al. Lévy-like movement patterns of metastatic cancer cells revealed in microfabricated systems and implicated in vivo. Nat. Commun. 2018, 9, 4539. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.V.; Grither, W.R.; Brenot, A.; Hwang, P.Y.; Barcus, C.E.; Ernst, M.; Pence, P.; Walter, C.; Pathak, A.; Longmore, G.D.; et al. DDR2 controls breast tumor stiffness and metastasis by regulating integrin mediated mechanotransduction in CAFs. eLife 2019, 8, 45508. [Google Scholar] [CrossRef]
- Kim, D.H.; Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 2013, 27, 1351–1361. [Google Scholar] [CrossRef]
- Franchi, M.; Piperigkou, Z.; Karamanos, K.-A.; Franchi, L.; Masola, V. Extracellular Matrix-Mediated Breast Cancer Cells Morphological Alterations, Invasiveness, and Microvesicles/Exosomes Release. Cells 2020, 9, 2031. [Google Scholar] [CrossRef]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef]
- Remmerbach, T.W.; Wottawah, F.; Dietrich, J.; Lincoln, B.; Wittekind, C.; Guck, J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009, 69, 1728–1732. [Google Scholar] [CrossRef]
- Lautscham, L.A.; Kämmerer, C.; Lange, J.R.; Kolb, T.; Mark, C.; Schilling, A.; Strissel, P.L.; Strick, R.; Gluth, C.; Rowat, A.C.; et al. Migration in Confined 3D Environments Is Determined by a Combination of Adhesiveness, Nuclear Volume, Contractility, and Cell Stiffness. Biophys. J. 2015, 109, 900–913. [Google Scholar] [CrossRef]
- Rajendran, A.K.; Sankar, D.; Amirthalingam, S.; Kim, H.D.; Rangasamy, J.; Hwang, N.S. Trends in mechanobiology guided tissue engineering and tools to study cell-substrate interactions: A brief review. Biomater. Res. 2023, 27, 55. [Google Scholar] [CrossRef] [PubMed]
- Bornschlögl, T. How filopodia pull: What we know about the mechanics and dynamics of filopodia. Cytoskeleton 2013, 70, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 1953–1960. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vessella, T.; Rozen, E.J.; Shohet, J.; Wen, Q.; Zhou, H.S. Investigation of Cell Mechanics and Migration on DDR2-Expressing Neuroblastoma Cell Line. Life 2024, 14, 1260. https://doi.org/10.3390/life14101260
Vessella T, Rozen EJ, Shohet J, Wen Q, Zhou HS. Investigation of Cell Mechanics and Migration on DDR2-Expressing Neuroblastoma Cell Line. Life. 2024; 14(10):1260. https://doi.org/10.3390/life14101260
Chicago/Turabian StyleVessella, Theadora, Esteban J. Rozen, Jason Shohet, Qi Wen, and Hong Susan Zhou. 2024. "Investigation of Cell Mechanics and Migration on DDR2-Expressing Neuroblastoma Cell Line" Life 14, no. 10: 1260. https://doi.org/10.3390/life14101260





