A Tissue-Engineered Construct Based on a Decellularized Scaffold and the Islets of Langerhans: A Streptozotocin-Induced Diabetic Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Technology of the DP Scaffold
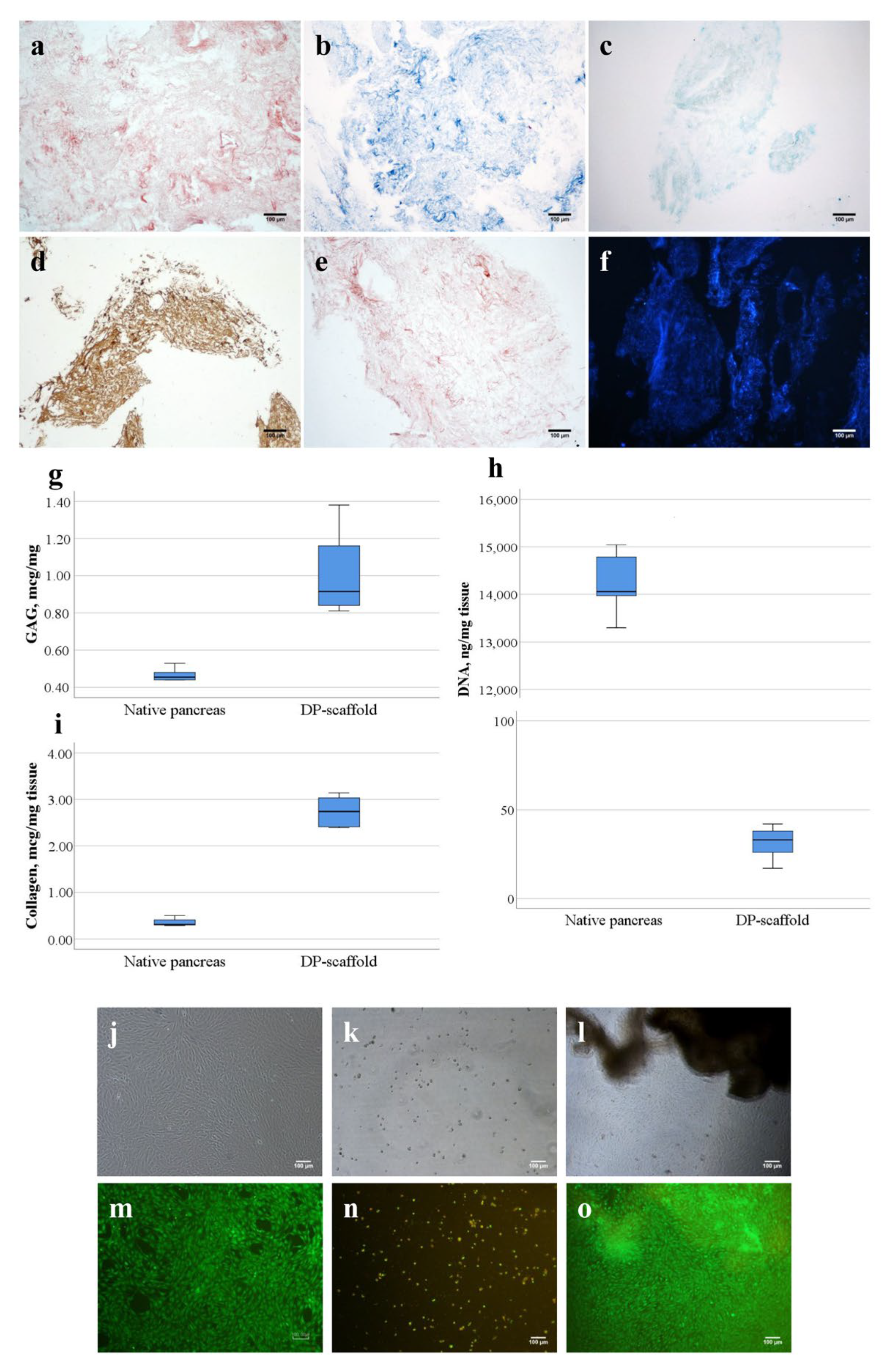
2.2. Biochemical Study of Scaffold from Decellularized Human Pancreas (DP Scaffold)
2.2.1. DNA Concentration Determination
2.2.2. Glycosaminoglycan (GAG) Concentration Determination
2.2.3. Collagen Concentration Determination (Collagen Quantification)
2.2.4. Determination of Cytotoxicity
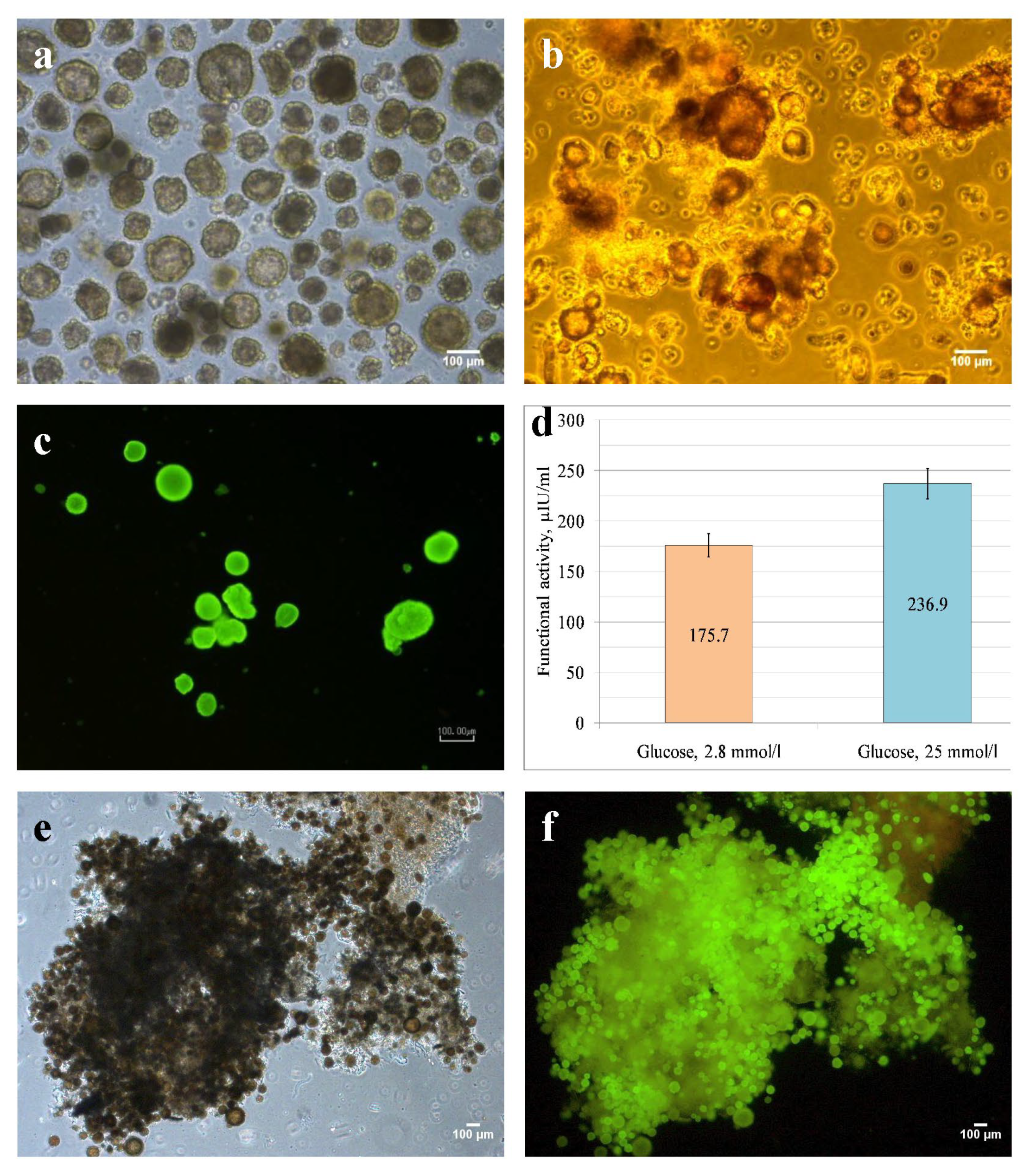
2.3. Rat Islets Isolation, Dithizone Staining
2.4. Cell Viability Assay
2.5. Enzyme Immunoassay (ELISA)
2.6. Rat Model of Type 1 Diabetes Mellitus
2.7. Preparation of Islets and Scaffold for Injection into Rats with Streptozotocin-Induced Diabetic Model
2.8. Intraperitoneal Injection of Islets of Langerhans Alone and Seeded on DP Scaffold into Rats with Streptozotocin-Induced T1DM
- The control group (animals without treatment)—5 rats;
- Experimental group 1 (an intraperitoneal injection of 2000 allogeneic islets of Langerhans alone)—5 rats;
- Experimental group 2 (an intraperitoneal injection of 2000 allogeneic islets of Langerhans seeded on a DP scaffold)—5 rats.
2.9. Histological Staining
2.10. Statistical Analysis
3. Results
3.1. DP-Scaffold Characterization
3.2. Rat Islets Isolated, Characterizied, and Seeded on DP Scaffold
3.3. Streptozotocin-Induced Diabetic Rat Model
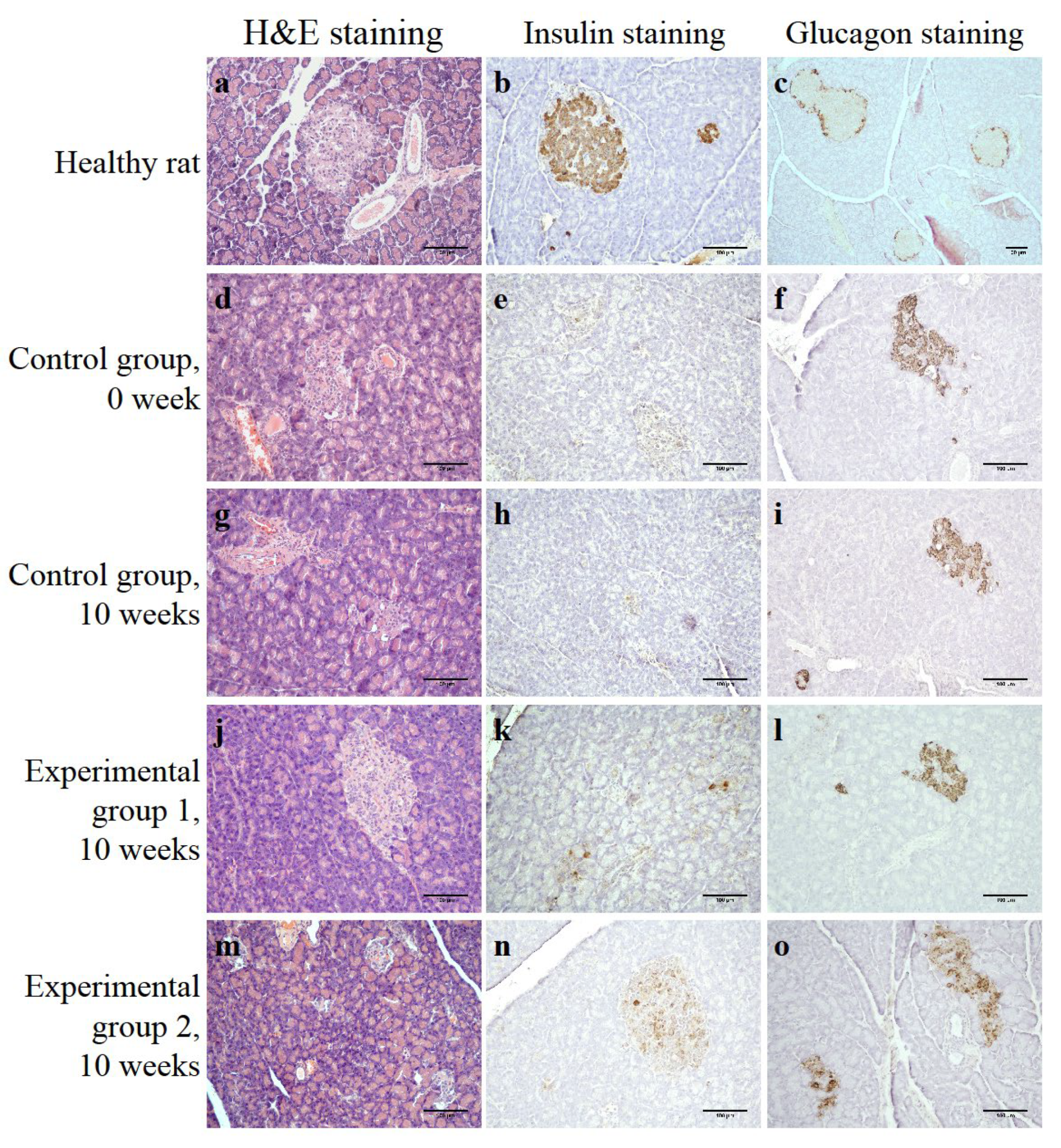
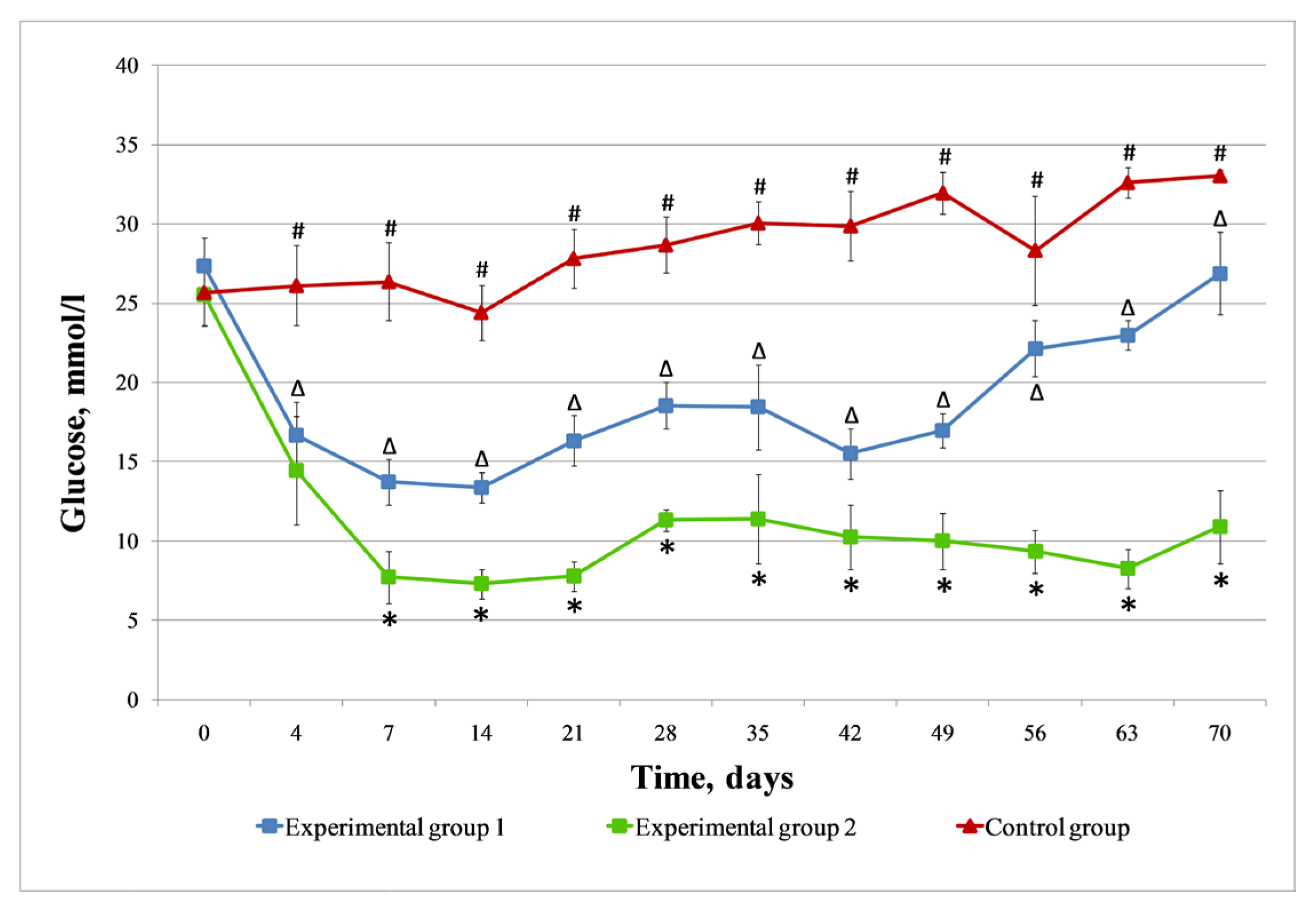
3.4. Injection Islets and Islets Seeded on DP Scaffold in STZ-Induced Diabetic Rats
3.4.1. Control Group
3.4.2. Experimental Group 1 (Injection of Islets of Langerhans)
3.4.3. Experimental Group 2 (Injection of Islets of Langerhans Seeded on DP Scaffold)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, P.; Shapiro, A.M.; Ricordi, C.; Secchi, A. The clinical impact of islet transplantation. Am. J. Transplant. 2008, 8, 1990–1997. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Barton, F.B.; Rickels, M.R.; Alejandro, R.; Hering, B.J.; Wease, S.; Naziruddin, B.; Oberholzer, J.; Odorico, J.S.; Garfinkel, M.R.; Levy, M.; et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012, 35, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Pokrywczynska, A.M.; Ricordi, C. Clinical Pancreatic Islet Transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef]
- Cayabyab, F.; Nih, L.R.; Yoshihara, E. Advances in Pancreatic Islet Transplantation Sites for the Treatment of Diabetes. Front. Endocrinol. 2021, 12, 732431. [Google Scholar] [CrossRef]
- Eguchi, N.; Damyar, K.; Alexander, M.; Dafoe, D.; Lakey, J.R.T.; Ichii, H. Anti-Oxidative Therapy in Islet Cell Transplantation. Antioxidants 2022, 11, 1038. [Google Scholar] [CrossRef]
- Korsgren, O.; Nilsson, B.; Berne, C.; Felldin, M.; Foss, A.; Kallen, R.; Lundgren, T.; Salmela, K.; Tibell, A.; Tufveson, G. Current status of clinical islet transplantation. Transplantation 2005, 79, 1289–1293. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Gallant, H.L.; Hao, E.G.; Lakey, J.R.; McCready, T.; Rajotte, R.V.; Yatscoff, R.W.; Kneteman, N.M. The portal immunosuppressive storm: Relevance to islet transplantation? Ther. Drug Monit. 2005, 27, 35–37. [Google Scholar] [CrossRef]
- Bennet, W.; Sundberg, B.; Groth, C.G.; Brendel, M.D.; Brandhorst, D.; Brandhorst, H.; Bretzel, R.G.; Elgue, G.; Larsson, R.; Nilsson, B.; et al. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes 1999, 48, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Joisher, H.; Ganguly, A. Polymeric scaffolds for pancreatic tissue engineering: A review. Rev. Diabet. Stud. 2018, 14, 334–353. [Google Scholar] [CrossRef] [PubMed]
- Riopel, M.; Wang, K. Collagen matrix support of pancreatic islet survival and function. Front. Biosci. 2014, 19, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Lemos, N.E.; de Almeida Brondani, L.; Dieter, C.; Rheinheimer, J.; Bouças, A.P.; Bauermann Leitão, C.; Crispim, D.; Bauer, A.C. Use of additives, scaffolds and extracellular matrix components for improvement of human pancreatic islet outcomes in vitro: A systematic review. Islets 2017, 9, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, M.; Katari, R.; Patel, T.; Peloso, A.; Mugweru, J.; Owusu, K.; Orlando, G. Extracellular matrix scaffold technology for bioartificial pancreas engineering: State of the art and future challenges. J. Diab. Sci. Technol. 2014, 8, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gazia, C.; Gaffley, M.; Asthana, A. Scaffolds for pancreatic tissue engineering. In Handbook of Tissue Engineering Scaffolds; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; Volume 2, pp. 765–786. [Google Scholar] [CrossRef]
- Fisher, S.A.; Tam, R.Y.; Shoichet, M.S. Tissue mimetics: Engineered hydrogel matrices provide biomimetic environments for cell growth. Tissue Eng. Part A 2014, 20, 895–898. [Google Scholar] [CrossRef]
- Coronel, M.; Stabler, C. Engineering a local microenvironment for pancreatic islet replacement. Curr. Opin. Biotechnol. 2013, 24, 900–908. [Google Scholar] [CrossRef]
- Abualhassan, N.; Sapozhnikov, L.; Pawlick, R.L.; Kahana, M.; Pepper, A.R.; Bruni, A.; Gala-Lopez, B.; Kin, T.; Mitrani, E.; Shapiro, A.M. Lung-derived microscaffolds facilitate diabetes reversal after mouse and human intraperitoneal islet transplantation. PLoS ONE 2016, 11, e0156053. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Tancos, Z.; Feher, L.Z.; Alfoldi, R.; Kobolak, J.; Dinnyes, A.; Puskas, L.G. Real architecture for 3D Tissue (RAFT) culture system improves viability and maintains insulin and glucagon production of mouse pancreatic islet cells. Cytotechnology 2017, 69, 359–369. [Google Scholar] [CrossRef]
- Amer, L.D.; Mahoney, M.J.; Bryant, S.J. Tissue engineering approaches to cell-based type 1 diabetes therapy. Tissue Eng. Part B Rev. 2014, 20, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, G.; Vermette, P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J. Tissue Eng. Regen. Med. 2018, 12, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-specific decellularization methods: Rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Shirakigawa, N.; Ijima, H. Decellularized tissue engineering. In Advances in Biomaterials for Biomedical Applications; Tripathi, A., Melo, J.S., Eds.; Springer: Singapore, 2017; pp. 185–226. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Basok, Y.B.; Grigoriev, A.M.; Nemets, E.A.; Kirillova, A.D.; Kirsanova, L.A.; Lazhko, A.E.; Subbot, A.; Kravchik, M.V.; Khesuani, Y.D.; et al. Decellularization of cartilage microparticles: Effects of temperature, supercritical carbon dioxide and ultrasound on biochemical, mechanical, and biological properties. J. Biomed. Mater. Res. A 2023, 111, 543–555. [Google Scholar] [CrossRef]
- Mirmalek-Sani, S.H.; Orlando, G.; McQuilling, J.P.; Pareta, R.; Mack, D.L.; Salvatori, M.; Farney, A.C.; Stratta, R.J.; Atala, A.; Opara, E.C.; et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials 2013, 34, 5488–5495. [Google Scholar] [CrossRef]
- Lim, L.Y.; Ding, S.S.L.; Muthukumaran, P.; Teoh, S.H.; Koh, Y.; Teo, A.K.K. Tissue engineering of decellularized pancreas scaffolds for regenerative medicine in diabetes. Acta Biomater. 2023, 157, 49–66. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Ponomareva, A.S.; Baranova, N.V.; Kirsanova, L.A.; Basok, Y.B.; Nemets, E.A.; Kruglov, D.N.; Miloserdov, I.A.; Gautier, S.V. Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes. Int. J. Mol. Sci. 2023, 24, 119. [Google Scholar] [CrossRef]
- Wu, D.; Wan, J.; Huang, Y.; Guo, Y.; Xu, T.; Zhu, M.; Fan, X.; Zhu, S.; Ling, C.; Li, X.; et al. 3D Culture of MIN-6 Cells on Decellularized Pancreatic Scaffold: In Vitro and In Vivo Study. Biomed. Res. Int. 2015, 2015, 432645. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, Y.; Huang, C.; Li, J. Research Progress on Animal Models of Diabetes Mellitus. Austin J. Cardiovasc. Dis. Atheroscler. 2023, 10, 1055. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Baranova, N.V.; Kirsanova, L.A.; Ponomareva, A.S.; Basok, Y.B.; Nemets, E.A.; Gautier, S.V. Comparative Analysis of the Influence of Extracellular Matrix Biomimetics on the Viability and Insulin-Producing Function of Isolated Pancreatic Islets. J. Genet. Eng. Biotechnol. Res. 2021, 3, 17–25. [Google Scholar]
- Karaoz, E.; Genç, Z.S.; Demircan, P.Ç.; Aksoy, A.; Duruksu, G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2010, 1, e36. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-2:2009; Medical Devices. Assessment of the Biological Effect of Medical Devices—Part 2. Requirements for the Treatment of Animals. ISO: Geneva, Switzerland, 2009.
- GOST 33215-2014; Guidelines for the Accommodation and Care of Laboratory Animals. Environment, Housing and Management. MKS 13.020.01. GOSTPEREVOD, Ltd.: London, UK, 2016.
- GOST 33216-2014; Guidelines for the Accommodation and Care of Laboratory Animals. Species-Specific Provisions for Laboratory Rodents and Rabbits. MKS 13.020.01. GOSTPEREVOD, Ltd.: London, UK, 2016.
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- London, N.J.; Swift, S.M.; Clayton, H.A. Isolation, culture and functional evaluation of islets of Langerhans. Diabetes Metab. 1998, 24, 200–207. [Google Scholar] [PubMed]
- Sevastianov, V.I.; Basok, Y.B. Biomimetics of Extracellular Matrices for Cell and Tissue Engineered Medical Products; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2023; p. 339. [Google Scholar]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef]
- Smink, A.M.; de Vos, P. Therapeutic strategies for modulating the extracellular matrix to improve pancreatic islet function and survival after transplantation. Curr. Diab. Rep. 2018, 18, 39. [Google Scholar] [CrossRef]
- Casale, J.; Crane, J.S. Biochemistry, Glycosaminoglycans. In StatPearls; StatPearls Publishing: Treasure Island, USA, 2023. [Google Scholar]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef]
- Xia, C.; Mei, S.; Gu, C.; Zheng, L.; Fang, C.; Shi, Y.; Wu, K.; Lu, T.; Jin, Y.; Lin, X.; et al. Decellularized cartilage as a prospective scaffold for cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 588–595. [Google Scholar] [CrossRef]
- Schneider, C.; Lehmann, J.; van Osch, G.J.; Hildner, F.; Teuschl, A.; Monforte, X.; Miosga, D.; Heimel, P.; Priglinger, E.; Redl, H.; et al. Systematic comparison of protocols for the preparation of human articular cartilage for use as scaffold material in cartilage tissue engineering. Tissue Eng. Part. C Methods 2016, 22, 1095–1107. [Google Scholar] [CrossRef]
- Bautista, C.A.; Park, H.J.; Mazur, C.M.; Aaron, R.K.; Bilgen, B. Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PLoS ONE 2016, 11, e0158976. [Google Scholar] [CrossRef]
- Dubus, M.; Scomazzon, L.; Chevrier, J.; Ledouble, C.; Baldit, A.; Braux, J.; Gindraux, F.; Boulagnon, C.; Audonnet, S.; Colin, M.; et al. Antibacterial and Immunomodulatory Properties of Acellular Wharton’s Jelly Matrix. Biomedicines 2022, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, M.; Evans, T.; Gouon-Evans, V. Orchestrating liver development. Development 2015, 142, 2094–2108. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Ashcroft, F.M. Pancreatic beta-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef] [PubMed]
- Yayla, M.; Binnetoğlu, D. Experimental approaches to diabetes mellitus. Eurasian J. Med. 2022, 54, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Federiuk, I.F.; Casey, H.M.; Quinn, M.J.; Wood, M.D.; Ward, W.K. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: Route of administration, pitfalls, and insulin treatment. Comp. Med. 2004, 54, 252–257. [Google Scholar]
- Zhang, Y.; Zhang, J.; Hong, M.; Huang, J.; Wang, R.; Tan, B.; Huang, P.; Cao, H. Study on the Optimization and Stability of Single-dose Streptozotocin-induced Diabetic Modelling in Rats. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Fajarwati, I.; Solihin, D.D.; Wresdiyati, T.; Batubara, I. Self-recovery in diabetic Sprague Dawley rats induced by intraperitoneal alloxan and streptozotocin. Heliyon 2023, 9, e15533. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Zhang, W.; Qiang, M.; Luo, Y. A bilaminated decellularized scaffold for islet transplantation: Structure, properties and functions in diabetic mice. Biomaterials 2017, 138, 80–90. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Kong, H.; He, Q.; Sun, H.; Bhugul, P.A.; Zhang, Q.; Chen, B.; Zhou, M. The rat pancreatic body tail as a source of a novel extracellular matrix scaffold for endocrine pancreas bioengineering. J. Biol. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Uday Chandrika, K.; Tripathi, R.; Kameshwari, Y.; Rangaraj, N.; Mahesh Kumar, J.; Singh, S. Refunctionalization of Decellularized Organ Scaffold of Pancreas by Recellularization: Whole Organ Regeneration into Functional Pancreas. Tissue Eng. Regen. Med. 2021, 18, 99–112. [Google Scholar] [CrossRef]
- Citro, A.; Moser, P.T.; Dugnani, E.; Rajab, T.K.; Ren, X.; Evangelista-Leite, D.; Charest, J.M.; Peloso, A.; Podesser, B.K.; Manenti, F.; et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials 2019, 199, 40–51. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastianov, V.I.; Ponomareva, A.S.; Baranova, N.V.; Belova, A.D.; Kirsanova, L.A.; Nikolskaya, A.O.; Kuznetsova, E.G.; Chuykova, E.O.; Skaletskiy, N.N.; Skaletskaya, G.N.; et al. A Tissue-Engineered Construct Based on a Decellularized Scaffold and the Islets of Langerhans: A Streptozotocin-Induced Diabetic Model. Life 2024, 14, 1505. https://doi.org/10.3390/life14111505
Sevastianov VI, Ponomareva AS, Baranova NV, Belova AD, Kirsanova LA, Nikolskaya AO, Kuznetsova EG, Chuykova EO, Skaletskiy NN, Skaletskaya GN, et al. A Tissue-Engineered Construct Based on a Decellularized Scaffold and the Islets of Langerhans: A Streptozotocin-Induced Diabetic Model. Life. 2024; 14(11):1505. https://doi.org/10.3390/life14111505
Chicago/Turabian StyleSevastianov, Victor I., Anna S. Ponomareva, Natalia V. Baranova, Aleksandra D. Belova, Lyudmila A. Kirsanova, Alla O. Nikolskaya, Eugenia G. Kuznetsova, Elizaveta O. Chuykova, Nikolay N. Skaletskiy, Galina N. Skaletskaya, and et al. 2024. "A Tissue-Engineered Construct Based on a Decellularized Scaffold and the Islets of Langerhans: A Streptozotocin-Induced Diabetic Model" Life 14, no. 11: 1505. https://doi.org/10.3390/life14111505
APA StyleSevastianov, V. I., Ponomareva, A. S., Baranova, N. V., Belova, A. D., Kirsanova, L. A., Nikolskaya, A. O., Kuznetsova, E. G., Chuykova, E. O., Skaletskiy, N. N., Skaletskaya, G. N., Nemets, E. A., Basok, Y. B., & Gautier, S. V. (2024). A Tissue-Engineered Construct Based on a Decellularized Scaffold and the Islets of Langerhans: A Streptozotocin-Induced Diabetic Model. Life, 14(11), 1505. https://doi.org/10.3390/life14111505






