Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity
Abstract
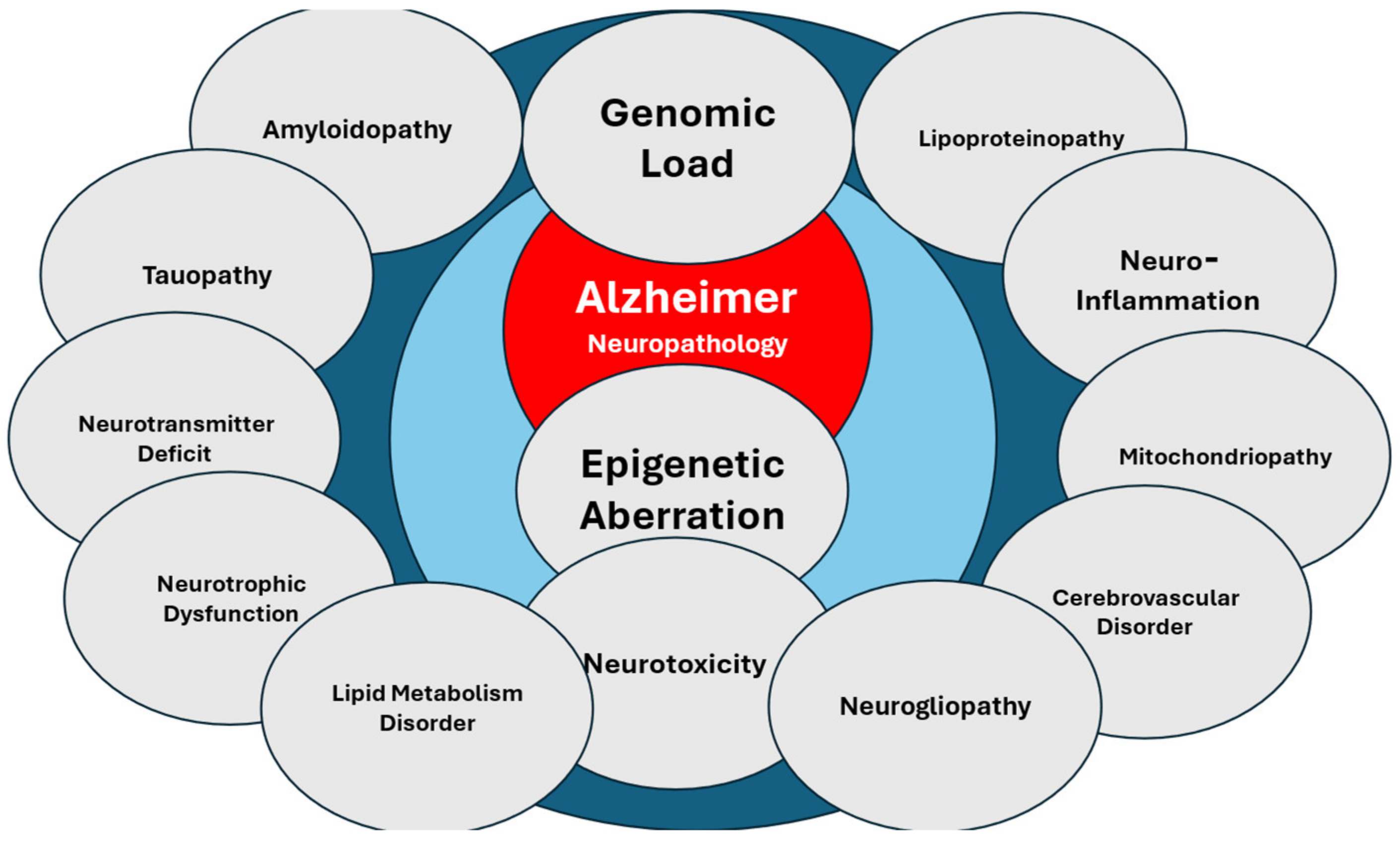
:1. Introduction
2. Drug Development
3. Multi-Target Drugs
3.1. Tacrine Derivatives
3.1.1. Tacrine-Based Cyclopentapyranopyridine– and Tetrahydropyranoquinoline–Kojic Acid Derivatives
3.1.2. Tetrahydroaminoacridine (THA)–Ferulic Acid Hybrids
3.1.3. Tacrine–Resveratrol Fused Hybrids
3.1.4. Tacrine–Benzofuran Hybrids
3.1.5. Tacrine–Hydroxyphenylbenzimidazole Hybrids
3.1.6. Tacrine–Deferiprone Hybrids
3.1.7. Multifunctional Tacrine–Donepezil Hybrids
3.1.8. Conjugates of Tacrine with 1,2,4-Thiadiazole Derivatives
3.1.9. Conjugates of Tacrine and Salicylamide: Salicylimine Derivatives
3.1.10. Tacrine Conjugates with 2-Arylhydrazinylidene-1,3-Diketones
3.1.11. Methylene-Linked 1,2,3,4-Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine Hybrids
3.1.12. Quinolinetrione–Tacrine Hybrids
3.1.13. Tacrine–Selegiline Hybrids
3.1.14. Tacrine–Flavone Hybrids
3.1.15. Capsaicin–Tacrine Hybrids
3.2. Donepezil Derivatives
3.2.1. E2020-NOH
3.2.2. 1-Aryldonepezil Analogs
3.2.3. Multifunctional Aromatic Amine Hybrids of Donepezil
3.2.4. Racemic Trans Propargylamino-Donepezil
3.2.5. Cinnamoyl-N-Acylhydrazone-Donepezil Hybrids
3.2.6. Phenothiazine/Donepezil-like Hybrids
3.2.7. N-Benzyl-piperidinyl-aryl-acylhydrazone Derivatives
3.2.8. Donepezil-Arylsulfonamide Hybrids
3.2.9. Vilazodone–Donepezil Chimeras
3.3. Derivatives of Other AChE Inhibitors
3.3.1. Rivastigmine–Hydroxyphenylbenzimidazole Hybrids
3.3.2. Apigenin–Rivastigmine Hybrids
3.3.3. Galantamine–Memantine Hybrids and Galantamine–Curcumin Hybrids
3.3.4. Huperzine A
3.4. Memantine Derivatives
3.4.1. Tacrine–Adamantanes Hybrids
3.4.2. Galantamine–Memantine Conjugates
3.4.3. Aminoadamantane–Carbazole/Tetrahydrocarbazole Hybrids
3.4.4. Memantine–Antioxidant Hybrids
3.4.5. Memantine–Polyamine Conjugates
3.4.6. H2S-Releasing Memantine Prodrug
3.4.7. Dual P2X7-NMDA Receptor Antagonists
3.5. Diverse Chemical Derivatives with MT Effects
3.5.1. Ladostigil
3.5.2. M30
3.5.3. Memoquin
3.5.4. ASS234
3.5.5. RS-0406
3.5.6. Acridine
3.5.7. Indanone Derivatives
3.5.8. Chromone Derivatives
3.5.9. Quinazolines
3.5.10. Benzyl Pyridinium-2,4-dioxochroman Derivatives
3.5.11. 2,2′-Bipyridyl Derivatives
3.5.12. 8-Hydroxyquinoline Derivatives
3.5.13. Hybrid 8-Hydroxy Quinoline–Indole Derivatives
3.5.14. Lithium
3.5.15. Pregnenolone Derivatives
3.5.16. Phosphazine and Phosphazide Derivatives
3.5.17. N-Benzylpyrrolidine Derivatives
3.5.18. Clioquinol-1-benzyl-1,2,3,6-tetrahydropyridine Hybrids
3.5.19. 2-Substituted Benzo[d]oxazol-5-amine Derivatives
3.5.20. MT Thiazolidinediones
3.5.21. 2-Propargylamino-naphthoquinone Derivatives
3.5.22. MT Tryptamine Derivatives
3.5.23. Deoxyvasicinone–Indole
3.5.24. Benzofurans
3.5.25. Ibudilast
3.5.26. N-Cyclohexylimidazo[1,2-a]pyridine Derivatives
3.5.27. 20(R)-Panaxadiol Derivatives
3.5.28. Doxycycline
3.5.29. Tryptanthrin Derivatives with Benzenesulfonamide Substituents
3.5.30. Dimethyl Fumarate Plus Tranilast-Modified Dithiocarbate
3.5.31. Polysubstituted Pyrazine Derivatives
3.5.32. Thiosemicarbazones
3.5.33. PhenylSulfonyl–Pyrimidine Carboxylate Derivatives
3.5.34. Protriptyline
3.5.35. Amiridine Hybrids
3.5.36. Phosphodiesterase 2 Inhibitors
3.5.37. 2-Arylbenzofuran Derivatives
3.5.38. Salicyladimine Derivatives
3.5.39. 3-Arylbenzofuranone Derivatives
3.5.40. CNI-1493 and C1213
3.5.41. Fluoren-9-Amines
3.5.42. Flavone–Cyanoacetamide Hybrids
3.5.43. Indazole Ethers
3.5.44. Hyaluronan–Carnosine Conjugates
3.5.45. Melatonin-Derived Benzylpyridinium Bromides
3.5.46. Toluidine Blue O
3.5.47. Pyrimidine/Pyrrolidine–Sertraline Based Hybrids
3.5.48. Quinazolinone Derivatives
3.5.49. Histamine H3 Receptor Ligands
3.5.50. Alkyl-Substituted 4-Methoxy Benzaldehyde Thiosemicarbazones
3.5.51. Biomimetic Dendrimer–Peptide Conjugates
3.5.52. MT Anti-Neuroinflammatory Agents
3.6. Anti-Amyloid Agents
3.7. Potential Anti-Tauopathic Drugs
3.7.1. Modulators of Tau Post-Translational Modifications
3.7.2. Tau Aggregation Inhibitors
3.7.3. Promoters of Tau Degradation
3.7.4. Potential MT Anti-Tau Drugs
3.8. Natural Bioproducts
3.9. Epigenetic Drugs
4. Nosustrophine: A Prototype of Epipleiotropic Agent for AD Prevention and Treatment
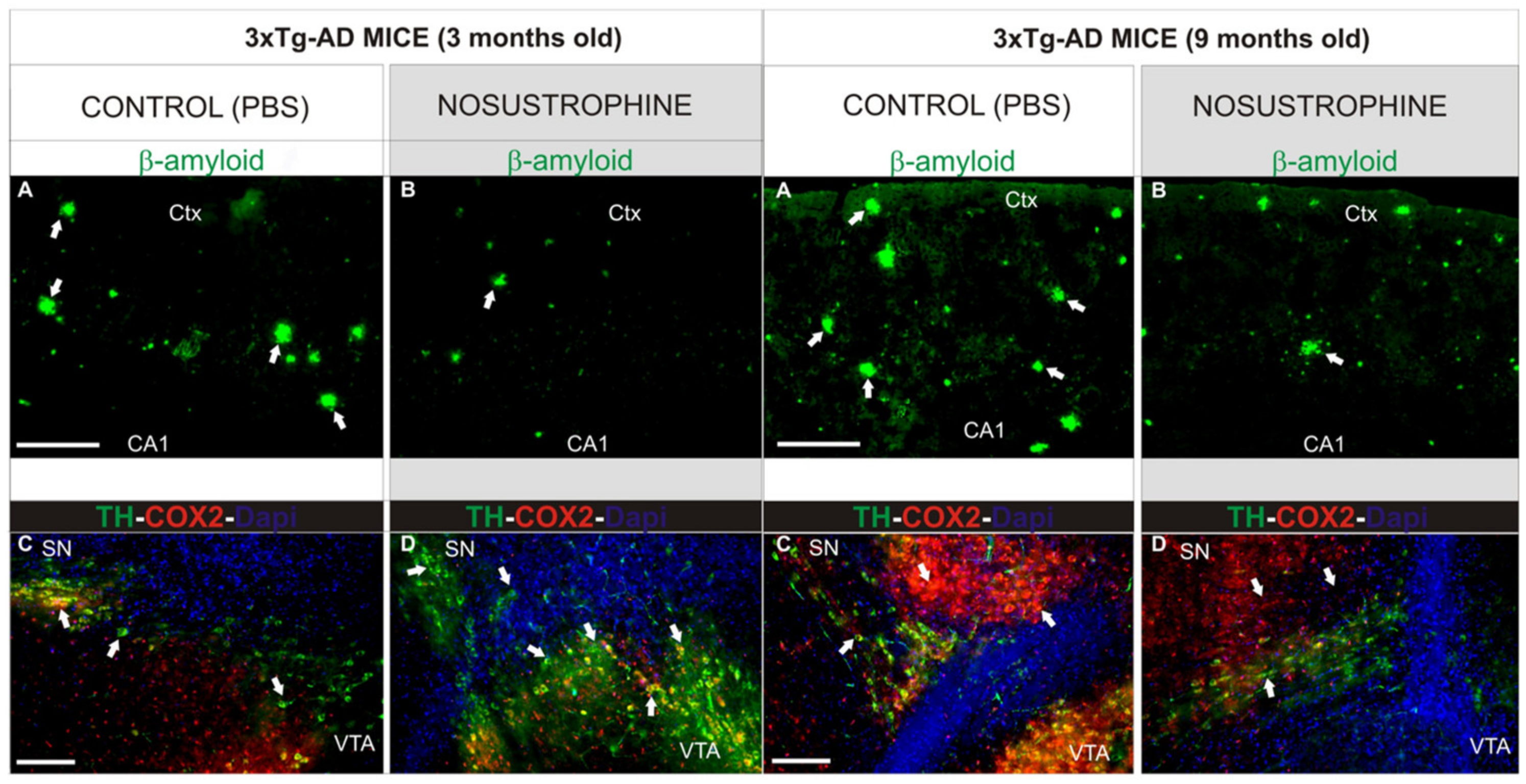
4.1. Neuroprotective, Antioxidant, Anti-Inflammatory, Anti-Amyloid, and Neurotrophic Effects
4.2. Regulation of AD-Related Gene Expression
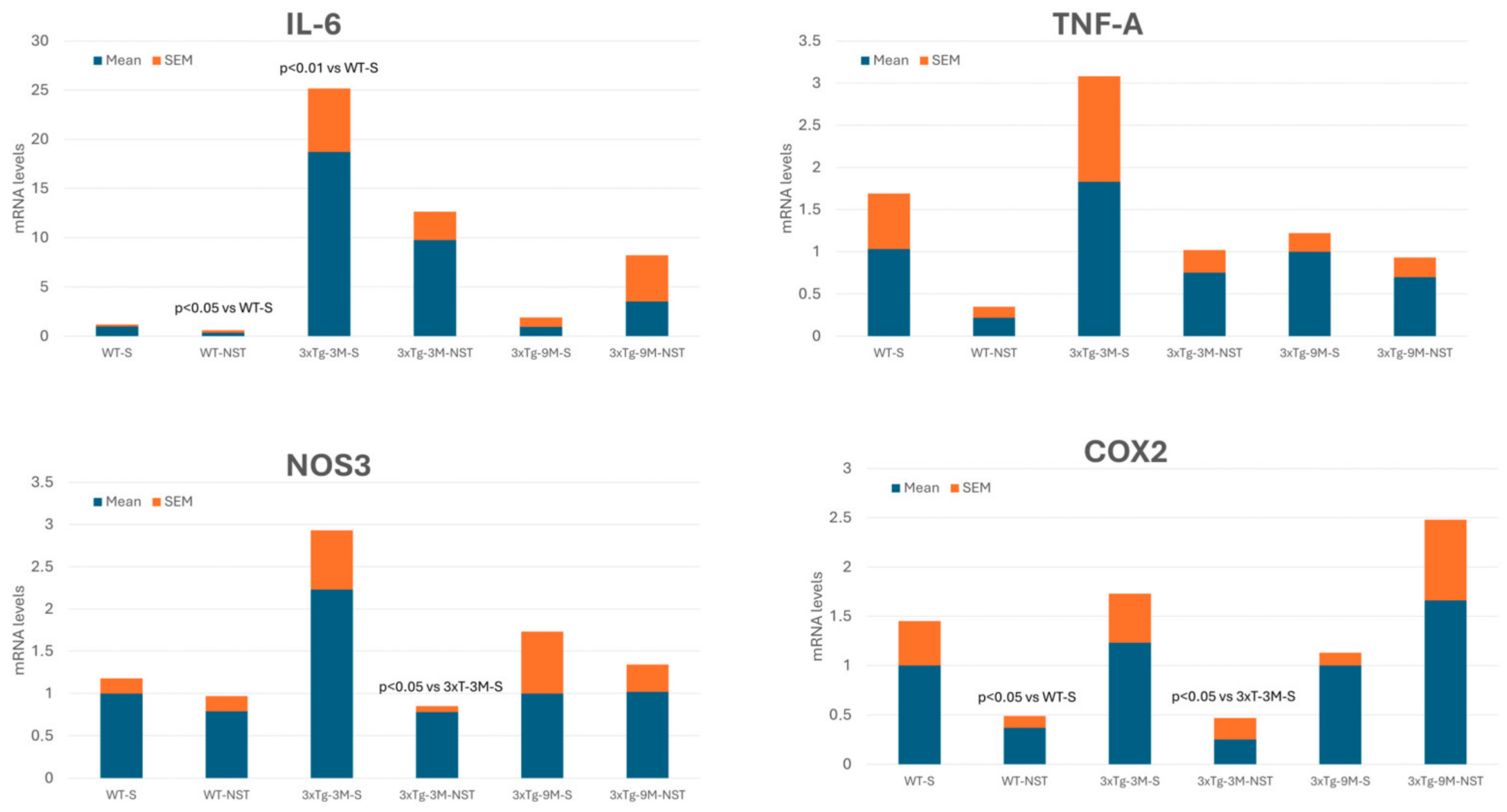
4.3. Regulation of Inflammation-Related Gene Expression
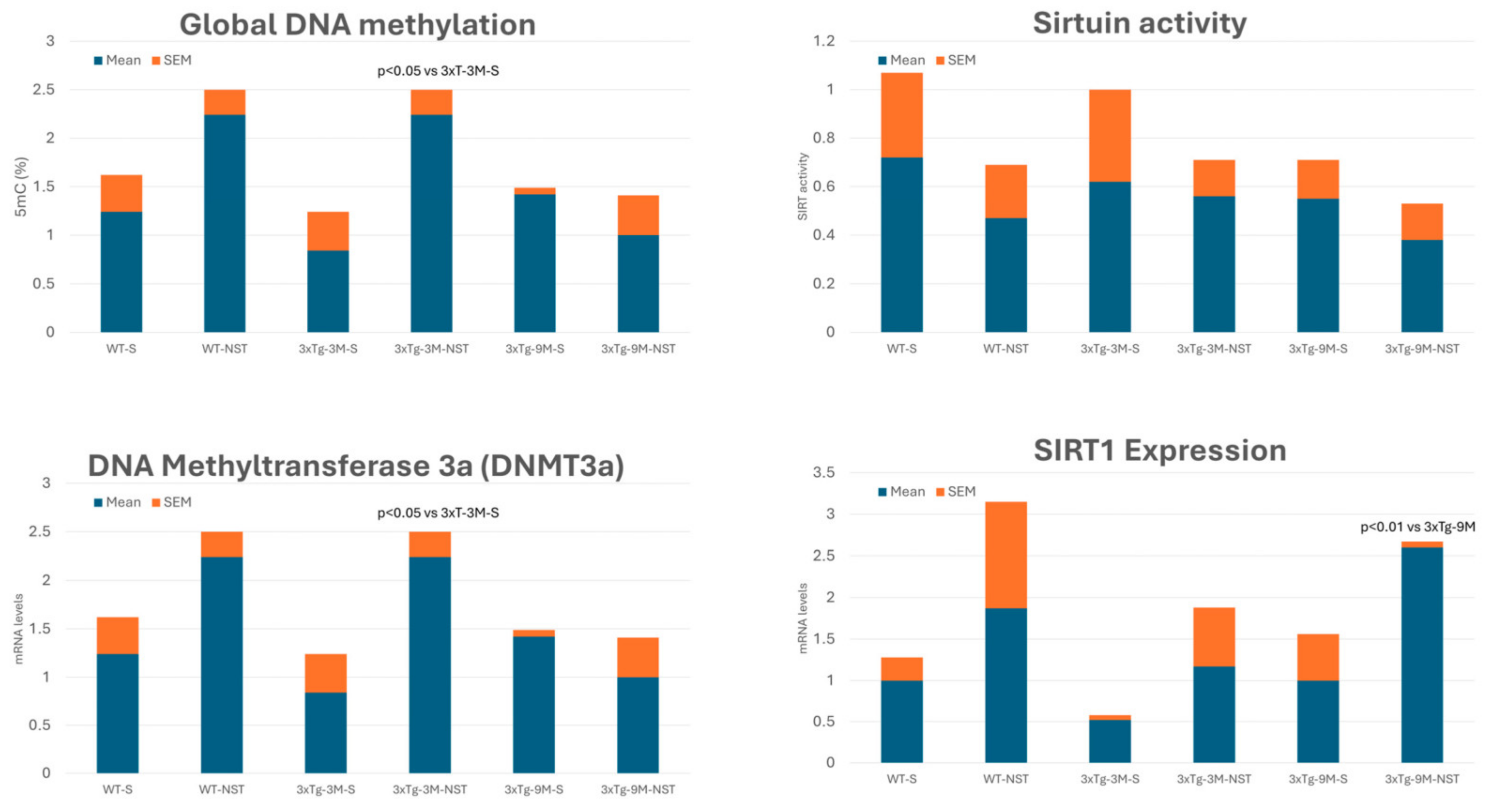
4.4. Epigenetic Effects
4.4.1. DNA Methylation
4.4.2. Modulation of Sirtuin Activity
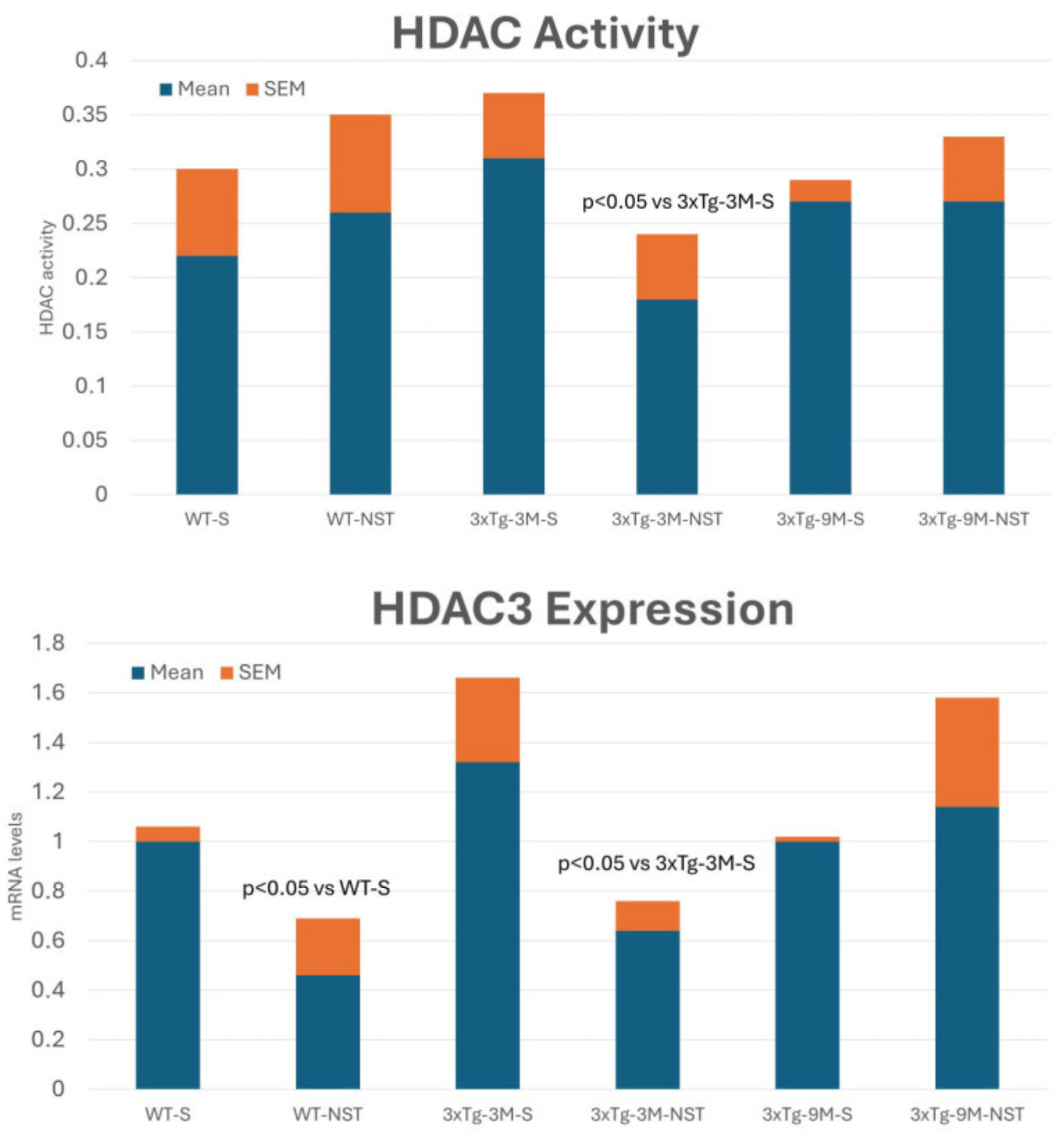
4.4.3. Regulation of Histone Deacetylase (HDAC) Activity
5. Conclusions and Future Trends
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cacabelos, R. What have we learnt from past failures in Alzheimer’s disease drug discovery? Expert. Opin. Drug Discov. 2022, 17, 309–323. [Google Scholar] [PubMed]
- Cacabelos, R. How plausible is an Alzheimer’s disease vaccine? Expert. Opin. Drug Discov. 2020, 15, 1–6. [Google Scholar] [PubMed]
- Cacabelos, R. Pharmacogenetic considerations when prescribing cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert. Opin. Drug Metabol. Toxicol. 2020, 16, 673–701. [Google Scholar]
- Alzheimer’s Association. 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar]
- Wimo, A.; Jönsson, L.; Gustavsson, A.; McDaid, D.; Ersek, K.; Georges, J.; Gulácsi, L.; Karpati, K.; Kenigsberg, P.; Valtonen, H. The economic impact of dementia in Europe in 2008-cost estimates from the Eurocode project. Int. J. Geriatr. Psychiatry 2011, 26, 825–832. [Google Scholar]
- Ministry of Health, Labour and Welfare of Japan. Annual Report on Health and Welfare; Ministry of Health, Labour and Welfare of Japan: Tokyo, Japan, 2018. [Google Scholar]
- Market Research Future. Alzheimer’s Disease Drug Market Research Report—Forecast to 2027; Market Research Future: New York, NY, USA, 2020. [Google Scholar]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar]
- Cacabelos, R.; Naidoo, V.; Martínez-Iglesias, O.; Corzo, L.; Cacabelos, N.; Pego, R.; Carril, J.C. Pharmacogenomics of Alzheimer’s Disease: Novel Strategies for Drug Utilization and Development. Methods Mol. Biol. 2022, 2547, 275–387. [Google Scholar]
- Horgusluoglu, E.; Neff, R.; Song, W.M.; Wang, M.; Wang, Q.; Arnold, M.; Krumsiek, J.; Galindo-Prieto, B.; Ming, C.; Nho, K.; et al. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease. Alzheimers Dement. 2022, 18, 1260–1278. [Google Scholar] [CrossRef]
- Cacabelos, R.; Naidoo, V.; Martínez-Iglesias, O.; Corzo, L.; Cacabelos, N.; Pego, R.; Carril, J.C. Personalized Management and Treatment of Alzheimer’s Disease. Life 2022, 12, 460. [Google Scholar] [CrossRef]
- Cacabelos, R.; Martínez-Iglesias, O.; Cacabelos, N.; Cacabelos, P.; Naidoo, V. Epigenetics and Pharmacoepigenetics of Age-related Neurodegenerative Disorders. In Pharmacoepigenetics; Cacabelos, R., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2024; in press. [Google Scholar]
- Migliore, L.; Coppedè, F. Gene-environment interactions in Alzheimer disease: The emerging role of epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Piehl, N.; Simonton, B.; Parikh, M.; Zhang, Z.; Teregulova, V.; van Olst, L.; Gate, D. Epigenetic dysregulation in Alzheimer’s disease peripheral immunity. Neuron 2024, 112, 1235–1248.e5. [Google Scholar] [CrossRef] [PubMed]
- Takousis, P.; Sadlon, A.; Schulz, J.; Wohlers, I.; Dobricic, V.; Middleton, L.; Lill, C.M.; Perneczky, R.; Bertram, L. Differential expression of microRNAs in Alzheimer’s disease brain, blood, and cerebrospinal fluid. Alzheimers Dement. 2019, 15, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Torrellas, C. Epigenetics of Aging and Alzheimer’s Disease: Implications for Pharmacogenomics and Drug Response. Int. J. Mol. Sci. 2015, 16, 30483–30543. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Xia, Y.; Yassi, N.; Raniga, P.; Bourgeat, P.; Desmond, P.; Doecke, J.; Ames, D.; Laws, S.M.; Fowler, C.; Rainey-Smith, S.R.; et al. Comorbidity of Cerebrovascular and Alzheimer’s Disease in Aging. J. Alzheimers Dis. 2020, 78, 321–334. [Google Scholar] [CrossRef]
- Muresanu, D.F.; Popa-Wagner, A.; Stan, A.; Buga, A.M.; Popescu, B.O. The vascular component of Alzheimer’s disease. Curr. Neurovascular Res. 2014, 11, 168–176. [Google Scholar] [CrossRef]
- Solis, E., Jr.; Hascup, K.N.; Hascup, E.R. Alzheimer’s Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. J. Alzheimers Dis. 2020, 76, 1179–1198. [Google Scholar] [CrossRef]
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; van Veluw, S.J.; et al. The Neurovasculome: Key Roles in Brain Health and Cognitive Impairment: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e251–e271. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Ming, C.; Gao, X.; Yuan, H.; Lin, X.; Mao, X.; Wang, C.; Guo, X.; Du, Y.; et al. P-tau217 correlates with neurodegeneration in Alzheimer’s disease, and targeting p-tau217 with immunotherapy ameliorates murine tauopathy. Neuron 2024, 112, 1676–1693.e12. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Yang, Y.; Murzin, A.G.; Falcon, B.; Kotecha, A.; van Beers, M.; Tarutani, A.; Kametani, F.; Garringer, H.J.; et al. Structure-based classification of tauopathies. Nature 2021, 598, 359–363. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Hong, H.; Hong, L.; Luo, X.; Zeng, Q.; Li, K.; Wang, S.; Jiaerken, Y.; Zhang, R.; Yu, X.; Zhang, Y.; et al. The relationship between amyloid pathology, cerebral small vessel disease, glymphatic dysfunction, and cognition: A study based on Alzheimer’s disease continuum participants. Alzheimers Res. Ther. 2024, 16, 43. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Harris, J.R.; Milton, N.G. Cholesterol in Alzheimer’s disease and other amyloidogenic disorders. Subcell. Biochem. 2010, 51, 47–75. [Google Scholar] [PubMed]
- Vetrivel, K.S.; Thinakaran, G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim. Biophys. Acta 2010, 1801, 860–867. [Google Scholar] [CrossRef]
- Díaz, M.; Fabelo, N.; Ferrer, I.; Marin, R. “Lipid raft aging” in the human frontal cortex during nonpathological aging: Gender influences and potential implications in Alzheimer’s disease. Neurobiol. Aging 2018, 67, 42–52. [Google Scholar] [CrossRef]
- Jiao, F.; Jiang, D.; Li, Y.; Mei, J.; Wang, Q.; Li, X. Amyloidogenesis and Neurotrophic Dysfunction in Alzheimer’s Disease: Do They have a Common Regulating Pathway? Cells 2022, 11, 3201. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Reports 2019, 28, 655–669. [Google Scholar] [CrossRef]
- Greenwald, B.S.; Mohs, R.C.; Davis, K.L. Neurotransmitter deficits in Alzheimer’s disease: Criteria for significance. J. Am. Geriatr. Soc. 1983, 31, 310–316. [Google Scholar] [CrossRef]
- Mann, D.M.; Yates, P.O. Neurotransmitter deficits in Alzheimer’s disease and in other dementing disorders. Hum. Neurobiol. 1986, 5, 147–158. [Google Scholar]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Satpati, A.; Neylan, T.; Grinberg, L.T. Histaminergic neurotransmission in aging and Alzheimer’s disease: A review of therapeutic opportunities and gaps. Alzheimers Dement. 2023, 9, e12379. [Google Scholar] [CrossRef]
- Fernández-Novoa, L.; Cacabelos, R. Histamine function in brain disorders. Behav. Brain Res. 2001, 124, 213–233. [Google Scholar] [CrossRef]
- Cacabelos, R. Have there been improvement in Alzheimer’s disease drug discovery over the past 5 years? Exp. Opin. Drug Discovery 2018, 13, 523–538. [Google Scholar] [CrossRef]
- National Institutes of Health. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC), USA. 2020. Available online: https://report.nih.gov/funding/categorical-spending#/ (accessed on 15 April 2024).
- PhRMA. Biopharmaceutical Research and Development: The Process Behind New Medicines. Washington. 2021. Available online: https://phrma.org/resource-center/Pages/2020-Annual-Research-Overview (accessed on 15 April 2024).
- Van Bokhoven, P.; de Wilde, A.; Vermunt, L.; Leferink, P.S.; Heetveld, S.; Cummings, J.; Scheltens, P.; Vijverberg, E.G.B. The Alzheimer’s disease drug development landscape. Alzheimers Res. Ther. 2021, 13, 186. [Google Scholar] [CrossRef]
- Pirolla, N.F.F.; Batista, V.S.; Dias Viegas, F.P.; Gontijo, V.S.; McCarthy, C.R.; Viegas, C.; Nascimento-Júnior, N.M. Alzheimer’s Disease: Related Targets, Synthesis of Available Drugs, Bioactive Compounds Under Development and Promising Results Obtained from Multi-target Approaches. Curr. Drug Targets 2021, 22, 505–538. [Google Scholar] [CrossRef]
- Turgutalp, B.; Kizil, C. Multi-target drugs for Alzheimer’s disease. Trends Pharmacol. Sci. 2024, 45, 628–638. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, K. Protein-protein interaction network analysis for the identification of novel multi-target inhibitors and target miRNAs against Alzheimer’s disease. Adv. Protein Chem. Struct. Biol. 2024, 139, 405–467. [Google Scholar]
- Liu, K.; Lin, H.H.; Pi, R.; Mak, S.; Han, Y.; Hu, Y. Research and development of anti-Alzheimer’s disease drugs: An update from the perspective of technology flows. Expert. Opin. Ther. Pat. 2018, 28, 341–350. [Google Scholar] [CrossRef]
- Fang, J.; Wang, L.; Wu, T.; Yang, C.; Gao, L.; Cai, H.; Liu, J.; Fang, S.; Chen, Y.; Tan, W.; et al. Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J. Ethnopharmacol. 2017, 196, 281–292. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Dandapat, J.; Dash, U.C.; Kankar, S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018, 215, 42–73. [Google Scholar] [CrossRef]
- Liu, M.; Li, T.; Liang, H.; Zhong, P. Herbal medicines in Alzheimer’s disease and the involvement of gut microbiota. Front. Pharmacol. 2024, 15, 1416502. [Google Scholar] [CrossRef]
- Alarcón-Espósito, J.; Mallea, M.; Rodríguez-Lavado, J. From Hybrids to New Scaffolds: The Latest Medicinal Chemistry Goals in Multi-target Directed Ligands for Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 832–867. [Google Scholar] [CrossRef]
- Fronza, M.G.; Alves, D.; Praticò, D.; Savegnago, L. The neurobiology and therapeutic potential of multi-targeting β-secretase, glycogen synthase kinase 3β and acetylcholinesterase in Alzheimer’s disease. Ageing Res. Rev. 2023, 90, 102033. [Google Scholar] [CrossRef]
- Tian, S.; Huang, Z.; Meng, Q.; Liu, Z. Multi-Target Drug Design of Anti-Alzheimer’s Disease based on Tacrine. Mini Rev. Med. Chem. 2021, 21, 2039–2064. [Google Scholar] [CrossRef]
- Wu, W.Y.; Dai, Y.C.; Li, N.G.; Dong, Z.; Gu, T.; Shi, Z.; Xue, X.; Tang, Y.; Duan, J. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2017, 32, 572–587. [Google Scholar] [CrossRef]
- Yamali, C.; Donmez, S. Recent Developments in Tacrine-based Hybrids as a Therapeutic Option for Alzheimer’s Disease. Mini Rev. Med. Chem. 2023, 23, 869–880. [Google Scholar] [CrossRef]
- Bubley, A.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A.; Krasnovskaya, O. Tacrine-Based Hybrids: Past, Present, and Future. Int. J. Mol. Sci. 2023, 24, 1717. [Google Scholar] [CrossRef]
- Babaee, S.; Chehardoli, G.; Akbarzadeh, T.; Zolfigol, M.A.; Mahdavi, M.; Rastegari, A.; Homayouni Moghadam, F.; Najafi, Z. Design, Synthesis, and Molecular Docking of Some Novel Tacrine Based Cyclopentapyranopyridine- and Tetrahydropyranoquinoline-Kojic Acid Derivatives as Anti-Acetylcholinesterase Agents. Chem. Biodivers. 2021, 18, e2000924. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, H.; Chen, Y.; Lin, H.; Li, Q.; Mo, J.; Bian, Y.; Pei, Y.; Sun, H. Synthesis, pharmacology and molecular docking on multifunctional tacrine-ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018, 33, 496–506. [Google Scholar] [CrossRef]
- Jeřábek, J.; Uliassi, E.; Guidotti, L.; Korábečný, J.; Soukup, O.; Sepsova, V.; Hrabinova, M.; Kuča, K.; Bartolini, M.; Peña-Altamira, L.E.L. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 250–262. [Google Scholar] [CrossRef]
- Fancellu, G.; Chand, K.; Tomás, D.; Orlandini, E.; Piemontese, L.; Silva, D.F.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel tacrine-benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2020, 35, 211–226. [Google Scholar] [CrossRef]
- Hiremathad, A.; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel Tacrine-Hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 148, 255–267. [Google Scholar] [CrossRef]
- Chand, K.; Candeias, E.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Tacrine-deferiprone hybrids as multi-target-directed metal chelators against Alzheimer’s disease: A two-in-one drug. Metallomics 2018, 10, 1460–1475. [Google Scholar] [CrossRef]
- Bayraktar, G.; Bartolini, M.; Bolognesi, M.L.; Erdoğan, M.A.; Armağan, G.; Bayır, E.; Şendemir, A.; Bagetta, D.; Alcaro, S.; Alptüzün, V. Novel multifunctional tacrine-donepezil hybrids against Alzheimer’s disease: Design synthesis and bioactivity studies. Arch. Pharm. 2024, 357, e2300575. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; Rudakova, E.V.; Stupina, T.S.; Terentiev, A.A.; Serkov, I.V.; Proshin, A.N.; Radchenko, E.V.; et al. Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: Synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorg. Chem. 2020, 96, 103563. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Grishchenko, M.V.; Lushchekina, S.V.; Astakhova, T.Y.; Serebryakova, O.G.; Timokhina, E.N.; Zhilina, E.F.; et al. Conjugates of Tacrine and Salicylic Acid Derivatives as New Promising Multitarget Agents for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 2285. [Google Scholar] [CrossRef]
- Elkina, N.A.; Grishchenko, M.V.; Shchegolkov, E.V.; Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Radchenko, E.V.; et al. New Multifunctional Agents for Potential Alzheimer’s Disease Treatment Based on Tacrine Conjugates with 2-Arylhydrazinylidene-1,3-Diketones. Biomolecules 2022, 12, 1551. [Google Scholar] [CrossRef]
- Di Pietro, O.; Pérez-Areales, F.J.; Juárez-Jiménez, J.; Espargaró, A.; Clos, M.V.; Pérez, B.; Lavilla, R.; Sabaté, R.; Luque, F.J.; Muñoz-Torrero, D. Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targeting β-amyloid, tau, and cholinesterase pathologies. Eur. J. Med. Chem. 2014, 84, 107–117. [Google Scholar] [CrossRef]
- Uliassi, E.; Bergamini, C.; Rizzardi, N.; Naldi, M.; Cores, Á.; Bartolini, M.; Carlos Menéndez, J.; Bolognesi, M.L. Quinolinetrione-tacrine hybrids as multi-target-directed ligands against Alzheimer’s disease. Bioorg Med. Chem. 2023, 91, 117419. [Google Scholar] [CrossRef]
- Huang, S.T.; Luo, J.C.; Zhong, G.H.; Teng, L.P.; Yang, C.Y.; Tang, C.L.; Jing, L.; Zhou, Z.B.; Liu, J.; Jiang, N. In vitro and in vivo Biological Evaluation of Newly Tacrine-Selegiline Hybrids as Multi-Target Inhibitors of Cholinesterases and Monoamine Oxidases for Alzheimer’s Disease. Drug Des. Devel Ther. 2024, 18, 133–159. [Google Scholar] [CrossRef]
- Remya, R.S.; Ramalakshmi, N.; Muralidharan, P.; Nalini, C.N. Design, Synthesis, and Pharmacological Evaluation of Novel Tacrine Derivatives as Multi-target ANTI-Alzheimer’s Agents in Rat Models. Cent. Nerv. Syst. Agents Med. Chem. 2023, 23, 175–193. [Google Scholar]
- Long, J.; Qin, F.; Luo, J.; Zhong, G.; Huang, S.; Jing, L.; Yi, T.; Liu, J.; Jiang, N. Design, synthesis, and biological evaluation of novel capsaicin-tacrine hybrids as multi-target agents for the treatment of Alzheimer’s disease. Bioorg Chem. 2024, 143, 107026. [Google Scholar] [CrossRef]
- Kareem, R.T.; Abedinifar, F.; Mahmood, E.A.; Ebadi, A.G.; Rajabi, F.; Vessally, E. The recent development of donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s agents: Highlights from 2010 to 2020. RSC Adv. 2021, 11, 30781–30797. [Google Scholar] [CrossRef] [PubMed]
- Kihara, T.; Shimohama, S.; Sawada, H. New aspects of neurotransmitters in Alzheimer’s disease: Acetylcholine and glutamate. J. Alzheimers Dis. 2004, 6, 245–258. [Google Scholar]
- Luo, J.; Xu, J.J.; Ren, H.J.; Xu, J.B.; Gao, F.; Fang, D.M.; Wan, L.X. Design, synthesis and biological evaluation of 1-aryldonepezil analogues as anti-Alzheimer’s disease agents. Future Med. Chem. 2024, 16, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Wang, F.Q.; Xie, J.; Chen, L.; Zhou, X.L. Design, Synthesis, and Biological Activity of Donepezil: Aromatic Amine Hybrids as Anti-Alzheimerss Drugs. ACS Omega 2023, 8, 21802–21812. [Google Scholar] [CrossRef]
- Guieu, B.; Lecoutey, C.; Legay, R.; Davis, A.; Sopkova de Oliveira Santos, J.; Altomare, C.D.; Catto, M.; Rochais, C.; Dallemagne, P. First Synthesis of Racemic Trans Propargylamino-Donepezil, a Pleiotrope Agent Able to Both Inhibit AChE and MAO-B, with Potential Interest against Alzheimer’s Disease. Molecules 2020, 26, 80. [Google Scholar] [CrossRef]
- Ortiz, C.J.C.; Damasio, C.M.; Pruccoli, L.; Nadur, N.F.; de Azevedo, L.L.; Guedes, I.A.; Dardenne, L.E.; Kümmerle, A.E.; Tarozzi, A.; Viegas, C., Jr. Cinnamoyl-N-Acylhydrazone-Donepezil Hybrids: Synthesis and Evaluation of Novel Multifunctional Ligands Against Neurodegenerative Diseases. Neurochem. Res. 2020, 45, 3003–3020. [Google Scholar] [CrossRef]
- Carocci, A.; Barbarossa, A.; Leuci, R.; Carrieri, A.; Brunetti, L.; Laghezza, A.; Catto, M.; Limongelli, F.; Chaves, S.; Tortorella, P.; et al. Novel Phenothiazine/Donepezil-like Hybrids Endowed with Antioxidant Activity for a Multi-Target Approach to the Therapy of Alzheimer’s Disease. Antioxidants 2022, 11, 1631. [Google Scholar] [CrossRef]
- Dias Viegas, F.P.; de Freitas Silva, M.; Divino da Rocha, M.; Castelli, M.R.; Riquiel, M.M.; Machado, R.P.; Vaz, S.M.; Simões de Lima, L.M.; Mancini, K.C.; Marques de Oliveira, P.C.; et al. Design, synthesis and pharmacological evaluation of N-benzyl-piperidinyl-aryl-acylhydrazone derivatives as donepezil hybrids: Discovery of novel multi-target anti-alzheimer prototype drug candidates. Eur. J. Med. Chem. 2018, 147, 48–65. [Google Scholar] [CrossRef]
- Queda, F.; Calò, S.; Gwizdala, K.; Magalhães, J.D.; Cardoso, S.M.; Chaves, S.; Piemontese, L.; Santos, M.A. Novel Donepezil-Arylsulfonamide Hybrids as Multitarget-Directed Ligands for Potential Treatment of Alzheimer’s Disease. Molecules 2021, 26, 1658. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Huang, Y.; Gong, Q.; Fu, Y.; Xu, Y.; Huang, J.; You, H.; Zhang, D.; Zhang, D.; et al. The novel therapeutic strategy of vilazodone-donepezil chimeras as potent triple-target ligands for the potential treatment of Alzheimer’s disease with comorbid depression. Eur. J. Med. Chem. 2022, 229, 114045. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Brunetti, L.; Piemontese, L.; Pereira-Santos, A.R.; Cardoso, S.M.; Madrid, Y.; Santos, M.A. Novel Rivastigmine Derivatives as Promising Multi-Target Compounds for Potential Treatment of Alzheimer’s Disease. Biomedicines 2022, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Wang, K.; Shi, J.; Cheng, X.; Zhu, G.; Wei, R.; Ma, Q.; Yu, L.; Zhao, Y.; Tan, Z.; et al. Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 111958. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, R.; Zheleva, D.; Valkova, I.; Stavrakov, G.; Philipova, I.; Atanasova, M.; Doytchinova, I. A Novel Galantamine-Curcumin Hybrid as a Potential Multi-Target Agent against Neurodegenerative Disorders. Molecules 2021, 26, 1865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, G. Huperzine A: A promising drug for Alzheimer’s disease. Acta Pharmacol. Sinica 2010, 31, 587–592. [Google Scholar]
- Marotta, G.; Basagni, F.; Rosini, M.; Minarini, A. Memantine Derivatives as Multitarget Agents in Alzheimer’s Disease. Molecules 2020, 25, 4005. [Google Scholar] [CrossRef]
- Valverde, E.; Sureda, F.X.; Vázquez, S. Novel benzopolycyclic amines with NMDA receptor antagonist activity. Bioorg Med. Chem. 2014, 22, 2678–2683. [Google Scholar] [CrossRef]
- Reggiani, A.M.; Simoni, E.; Caporaso, R.; Meunier, J.; Keller, E.; Maurice, T.; Minarini, A.; Rosini, M.; Cavalli, A. In Vivo Characterization of ARN14140, a Memantine/Galantamine-Based Multi-Target Compound for Alzheimer’s Disease. Sci. Rep. 2016, 6, 33172. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Shevtsova, E.F.; Makhaeva, G.F.; Grigoriev, V.V.; Boltneva, N.P.; Kovaleva, N.V.; Lushchekina, S.V.; Shevtsov, P.N.; Neganova, M.E.; Redkozubova, O.M.; et al. Novel conjugates of aminoadamantanes with carbazole derivatives as potential multitarget agents for AD treatment. Sci. Rep. 2017, 7, 45627. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Caporaso, R.; Basagni, F.; Catanzaro, M.; Abu, I.F.; Fagiani, F.; Fusco, F.; Masuzzo, S.; Albani, D.; et al. Merging memantine and ferulic acid to probe connections between NMDA receptors, oxidative stress and amyloid-β peptide in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 111–120. [Google Scholar] [CrossRef]
- Sozio, P.; Cerasa, L.S.; Laserra, S.; Cacciatore, I.; Cornacchia, C.; Di Filippo, E.S.; Fulle, S.; Fontana, A.; Di Crescenzo, A.; Grilli, M.; et al. Memantine-sulfur containing antioxidant conjugates as potential prodrugs to improve the treatment of Alzheimer’s disease. Eur. J. Pharm. Sci. 2013, 49, 187–198. [Google Scholar] [CrossRef]
- Kumamoto, T.; Nakajima, M.; Uga, R.; Ihayazaka, N.; Kashihara, H.; Katakawa, K.; Ishikawa, T.; Saiki, R.; Nishimura, K.; Igarashi, K. Design, synthesis, and evaluation of polyamine-memantine hybrids as NMDA channel blockers. Bioorg Med. Chem. 2018, 26, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.; Daniele, S.; Pietrobono, D.; Citi, V.; Bellusci, L.; Chiellini, G.; Calderone, V.; Martini, C.; Rapposelli, S. Memantine prodrug as a new agent for Alzheimer’s Disease. Sci. Rep. 2019, 9, 4612. [Google Scholar] [CrossRef] [PubMed]
- Karoutzou, O.; Kwak, S.H.; Lee, S.D.; Martínez-Falguera, D.; Sureda, F.X.; Vázquez, S.; Kim, Y.C.; Barniol-Xicota, M. Towards a Novel Class of Multitarget-Directed Ligands: Dual P2X7-NMDA Receptor Antagonists. Molecules 2018, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.; Shoham, S.; Ashani, Y. Ladostigil: A novel neuroprotective, anti-Alzheimer’s drug with cholinesterase and brain-selective monoamine oxidase inhibitory activity. Annals N. Y. Acad. Sci. 2006, 1074, 352–357. [Google Scholar]
- Dubey, S.; Singh, E. Antioxidants: An approach for restricting oxidative stress induced neurodegeneration in Alzheimer’s disease. Inflammopharmacol 2023, 31, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Reichert, F.; Zohar, K.; Lezmi, E.; Eliyahu, T.; Rotshenker, S.; Linial, M.; Weinstock, M. Ladostigil Reduces the Adenoside Triphosphate/Lipopolysaccharide-Induced Secretion of Pro-Inflammatory Cytokines from Microglia and Modulate-Immune Regulators, TNFAIP3, and EGR1. Biomolec 2024, 14, 112. [Google Scholar] [CrossRef]
- Gal, S.; Zheng, H.; Fridkin, M.; Youdim, M.B. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. Neurotherapeut 2005, 2, 83–91. [Google Scholar]
- Youdim, M.B.H. Site-activated multi target iron chelators with acetylcholinesterase (AChE) and monoamine oxidase (MAO) inhibitory activities for Alzheimer’s disease therapy. J. Neural Transm. 2022, 129, 715–721. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Youdim, M.B.H. The Neuroprotective Activities of the Novel Multi-Target Iron-Chelators in Models of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis and Aging. Cells 2023, 12, 763. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Banzi, R.; Bartolini, M.; Cavalli, A.; Tarozzi, A.; Andrisano, V.; Minarini, A.; Rosini, M.; Tumiatti, V.; Bergamini, C.; et al. Novel class of quinone-bearing polyamines as multi-target-directed ligands to combat Alzheimer’s disease. J. Med. Chem. 2007, 50, 4882–4897. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Masand, N.; Gautam, V.; Kaushik, S.; Wu, D. Multi-Target-Directed Ligand Approach in Anti-Alzheimer’s Drug Discovery. In Deciphering Drug Targets for Alzheimer’s Disease; Kumar, D., Patil, V.M., Wu, D., Thorat, N., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Manivasagam, T.; Surya, R.; Jayalakshmi, M.; Justin Thenmozhi, A.; Prema, A.; Qoronfleh, M.W.; Alharbi, H.F.; Rajamani, Y. Introduction to Alzheimer’s Disease. In Nutraceuticals for Alzheimer’s Disease: A Promising Therapeutic Approach. Nutritional Neurosciences; Thenmozhi, A.J., Manivasagam, T., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Marco-Contelles, J.; Unzeta, M.; Bolea, I.; Esteban, G.; Ramsay, R.R.; Romero, A.; Martínez-Murillo, R.; Carreiras, M.C.; Ismaili, L. ASS234, As a New Multi-Target Directed Propargylamine for Alzheimer’s Disease Therapy. Front. Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Piplani, P. Acridine: A Scaffold for the Development of Drugs for Alzheimer’s Disease. Curr. Top. Med. Chem. 2023, 23, 1260–1276. [Google Scholar] [CrossRef]
- Huang, L.; Miao, H.; Sun, Y.; Meng, F.; Li, X. Discovery of indanone derivatives as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 87, 429–439. [Google Scholar] [CrossRef]
- Abdpour, S.; Jalili-Baleh, L.; Nadri, H.; Forootanfar, H.; Bukhari, S.N.A.; Ramazani, A.; Ebrahimi, S.E.S.; Foroumadi, A.; Khoobi, M. Chromone derivatives bearing pyridinium moiety as multi-target-directed ligands against Alzheimer’s disease. Bioorg. Chem. 2021, 110, 104750. [Google Scholar] [CrossRef]
- Haghighijoo, Z.; Zamani, L.; Moosavi, F.; Emami, S. Therapeutic potential of quinazoline derivatives for Alzheimer’s disease: A comprehensive review. Eur. J. Med. Chem. 2022, 227, 113949. [Google Scholar] [CrossRef]
- Mollazadeh, M.; Mohammadi-Khanaposhtani, M.; Zonouzi, A.; Nadri, H.; Najafi, Z.; Larijani, B.; Mahdavi, M. New benzyl pyridinium derivatives bearing 2,4-dioxochroman moiety as potent agents for treatment of Alzheimer’s disease: Design, synthesis, biological evaluation, and docking study. Bioorg Chem. 2019, 87, 506–515. [Google Scholar] [CrossRef]
- Tan, R.X.; Li, W.H.; Pang, J.M.; Zhong, S.M. Design, synthesis, and evaluation of 2,2′-bipyridyl derivatives as bifunctional agents against Alzheimer’s disease. Mol. Divers. 2024, 28, 1225–1238. [Google Scholar] [CrossRef]
- Yang, X.; Cai, P.; Liu, Q.; Wu, J.; Yin, Y.; Wang, X.; Kong, L. Novel 8-hydroxyquinoline derivatives targeting β-amyloid aggregation, metal chelation and oxidative stress against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 3191–3201. [Google Scholar] [CrossRef]
- Bowroju, S.K.; Mainali, N.; Ayyadevara, S.; Penthala, N.R.; Krishnamachari, S.; Kakraba, S.; Shmookler Reis, R.J.; Crooks, P.A. Design and Synthesis of Novel Hybrid 8-Hydroxy Quinoline-Indole Derivatives as Inhibitors of Aβ Self-Aggregation and Metal Chelation-Induced Aβ Aggregation. Molecules 2020, 25, 3610. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M. The Putative Use of Lithium in Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.; Mobeen, B.; Hussain, F.; Sadiq, A.; Rashid, U. Pregnenolone derivatives for the treatment of Alzheimer’s disease: Synthesis, and in vitro inhibition of amyloid β1-42 peptide aggregation, acetylcholinesterase and carbonic anhydrase-II. RSC Adv. 2024, 14, 14742–14757. [Google Scholar] [CrossRef]
- El-Sayed, N.F.; El-Hussieny, M.; Ewies, E.F.; Fouad, M.A.; Boulos, L.S. New phosphazine and phosphazide derivatives as multifunctional ligands targeting acetylcholinesterase and β-Amyloid aggregation for treatment of Alzheimer’s disease. Bioorg. Chem. 2020, 95, 103499. [Google Scholar] [CrossRef]
- Choubey, P.K.; Tripathi, A.; Sharma, P.; Shrivastava, S.K. Design, synthesis, and multitargeted profiling of N-benzylpyrrolidine derivatives for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2020, 28, 115721. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Zhang, P.; Li, X.; Lu, L.; Sun, Y.; Zhang, B.; Allen, S.; White, L.; Phillips, J.; et al. Discovery of novel hybrids containing clioquinol-1-benzyl-1,2,3,6-tetrahydropyridine as multi-target-directed ligands (MTDLs) against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 244, 114841. [Google Scholar] [CrossRef]
- Gutti, G.; Kakarla, R.; Kumar, D.; Beohar, M.; Ganeshpurkar, A.; Kumar, A.; Krishnamurthy, S.; Singh, S.K. Discovery of novel series of 2-substituted benzo[d]oxazol-5-amine derivatives as multi-target directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 182, 111613. [Google Scholar] [CrossRef]
- Yang, X.Y.; Wang, L.H.; Chen, T.; Hodge, D.R.; Resau, J.H.; DaSilva, L.; Farrar, W.L. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPAR gamma) agonists—PPAR gamma co-association with transcription factor NFAT. J. Biol. Chem. 2020, 275, 4541–4544. [Google Scholar] [CrossRef]
- Yang, J.; Shi, X.; Wang, Y.; Ma, M.; Liu, H.; Wang, J.; Xu, Z. Multi-Target Neuroprotection of Thiazolidinediones on Alzheimer’s Disease via Neuroinflammation and Ferroptosis. J. Alzheimers Dis. 2023, 96, 927–945. [Google Scholar] [CrossRef]
- Kim, J.H.; Lewin, T.M.; Coleman, R.A. Expression and characterization of recombinant rat acyl-CoA synthetases 1, 4, and 5—Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 2001, 276, 24667–24673. [Google Scholar] [CrossRef]
- Mezeiova, E.; Janockova, J.; Andrys, R.; Soukup, O.; Kobrlova, T.; Muckova, L.; Pejchal, J.; Simunkova, M.; Handl, J.; Micankova, P.; et al. 2-Propargylamino-naphthoquinone derivatives as multipotent agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 211, 113112. [Google Scholar] [CrossRef] [PubMed]
- Asghar, S.; Mushtaq, N.; Ahmed, A.; Anwar, L.; Munawar, R.; Akhtar, S. Potential of Tryptamine Derivatives as Multi-Target Directed Ligands for Alzheimer’s Disease: AChE, MAO-B, and COX-2 as Molecular Targets. Molecules 2024, 29, 490. [Google Scholar] [CrossRef]
- Nerella, A.; Jeripothula, M. Design, synthesis and biological evaluation of novel deoxyvasicinone-indole as multi-target agents for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2021, 49, 128212. [Google Scholar] [CrossRef]
- Cabrera-Pardo, J.R.; Fuentealba, J.; Gavilán, J.; Cajas, D.; Becerra, J.; Napiórkowska, M. Exploring the Multi-Target Neuroprotective Chemical Space of Benzofuran Scaffolds: A New Strategy in Drug Development for Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1679. [Google Scholar] [CrossRef]
- Montanari, S.; Mahmoud, A.M.; Pruccoli, L.; Rabbito, A.; Naldi, M.; Petralla, S.; Moraleda, I.; Bartolini, M.; Monti, B.; Iriepa, I.; et al. Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer’s disease treatment. Eur. J. Med. Chem. 2019, 178, 243–258. [Google Scholar] [CrossRef]
- Oliveros, G.; Wallace, C.H.; Chaudry, O.; Liu, Q.; Qiu, Y.; Xie, L.; Rockwell, P.; Figueiredo-Pereira, M.E.; Serrano, P.A. Repurposing ibudilast to mitigate Alzheimer’s disease by targeting inflammation. Brain 2023, 146, 898–911. [Google Scholar] [CrossRef]
- Haghighijoo, Z.; Akrami, S.; Saeedi, M.; Zonouzi, A.; Iraji, A.; Larijani, B.; Fakherzadeh, H.; Sharifi, F.; Arzaghi, S.M.; Mahdavi, M.; et al. N-Cyclohexylimidazo [1,2-a]pyridine derivatives as multi-target-directed ligands for treatment of Alzheimer’s disease. Bioorg. Chem. 2020, 103, 104146. [Google Scholar] [CrossRef]
- Pang, L.; Li, J.; Liu, Z.; Quan, Y.S.; Sui, H.H.; Jia, Y.; Chen, F.; Lee, J.J.; Liu, P.; Quan, Z.S.; et al. In vitro and in vivo biological evaluation of newly synthesized multi-target 20(R)-panaxadiol derivatives for treating Alzheimer’s disease. Eur. J. Med. Chem. 2022, 244, 114825. [Google Scholar] [CrossRef]
- Balducci, C.; Forloni, G. Doxycycline for Alzheimer’s Disease: Fighting β-Amyloid Oligomers and Neuroinflammation. Front. Pharmacol. 2019, 10, 738. [Google Scholar] [CrossRef]
- Wang, G.; Du, J.; Ma, J.; Liu, P.; Xing, S.; Xia, J.; Dong, S.; Li, Z. Discovery of Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1468. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, M.; Liu, P.; Cao, D.; Luo, J.; Wan, Y.; Fang, Y.; Jin, Y.; Xie, S.S.; Liu, J. A multi-target directed ligands strategy for the treatment of Alzheimer’s disease: Dimethyl fumarate plus Tranilast modified Dithiocarbate as AChE inhibitor and Nrf2 activator. Eur. J. Med. Chem. 2022, 242, 114630. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jing, L.; Tian, Y.; Więckowska, A.; Kang, D.; Meng, B.; Panek, D.; Godyń, J.; Góral, I.; Song, Y.; et al. Multifunctional agents against Alzheimer’s disease based on oxidative stress: Polysubstituted pyrazine derivatives synthesized by multicomponent reactions. Bioorg. Med. Chem. 2023, 96, 117535. [Google Scholar] [CrossRef] [PubMed]
- Palanimuthu, D.; Poon, R.; Sahni, S.; Anjum, R.; Hibbs, D.; Lin, H.Y.; Bernhardt, P.V.; Kalinowski, D.S.; Richardson, D.R. A novel class of thiosemicarbazones show multi-functional activity for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Prajapati, S.K.; Majumdar, S.; Raza, M.K.; Gabr, M.T.; Kumar, S.; Pal, K.; Rashid, H.; Kumar, S.; Krishnamurthy, S.; et al. Discovery of new phenyl sulfonyl-pyrimidine carboxylate derivatives as the potential multi-target drugs with effective anti-Alzheimer’s action: Design, synthesis, crystal structure and in-vitro biological evaluation. Eur. J. Med. Chem. 2021, 215, 113224. [Google Scholar] [CrossRef]
- Bansode, S.B.; Jana, A.K.; Batkulwar, K.B.; Warkad, S.D.; Joshi, R.S.; Sengupta, N.; Kulkarni, M.J. Molecular investigations of protriptyline as a multi-target directed ligand in Alzheimer’s disease. PLoS ONE 2014, 9, e105196. [Google Scholar] [CrossRef]
- Mezeiova, E.; Prchal, L.; Hrabinova, M.; Muckova, L.; Pulkrabkova, L.; Soukup, O.; Misiachna, A.; Janousek, J.; Fibigar, J.; Kucera, T.; et al. Morphing cholinesterase inhibitor amiridine into multipotent drugs for the treatment of Alzheimer’s disease. Biomed. Pharmacother. 2024, 173, 116399. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Han, C.; Zhang, C.; Zhou, Q.; Zhang, B.; Le, M.L.; Huang, M.X.; Wu, Y.; Luo, H.B. Discovery of effective phosphodiesterase 2 inhibitors with antioxidant activities for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2021, 41, 128016. [Google Scholar] [CrossRef]
- Yun, Y.; Miao, Y.; Sun, X.; Sun, J.; Wang, X. Synthesis and biological evaluation of 2-arylbenzofuran derivatives as potential anti-Alzheimer’s disease agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 1346–1356. [Google Scholar] [CrossRef]
- Yang, H.L.; Cai, P.; Liu, Q.H.; Yang, X.L.; Fang, S.Q.; Tang, Y.W.; Wang, C.; Wang, X.B.; Kong, L.Y. Design, synthesis, and evaluation of salicyladimine derivatives as multitarget-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 5917–5928. [Google Scholar] [CrossRef]
- Yang, J.; Yun, Y.; Miao, Y.; Sun, J.; Wang, X. Synthesis and biological evaluation of 3-arylbenzofuranone derivatives as potential anti-Alzheimer’s disease agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 805–814. [Google Scholar] [CrossRef]
- Sankowski, R.; Herring, A.; Keyvani, K.; Frenzel, K.; Wu, J.; Röskam, S.; Noelker, C.; Bacher, M.; Al-Abed, Y. The multi-target effects of CNI-1493: Convergence of anti-amylodogenic and anti-inflammatory properties in animal models of Alzheimer’s disease. Mol. Med. 2016, 22, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Konecny, J.; Misiachna, A.; Hrabinova, M.; Pulkrabkova, L.; Benkova, M.; Prchal, L.; Kucera, T.; Kobrlova, T.; Finger, V.; Kolcheva, M.; et al. Pursuing the Complexity of Alzheimer’s Disease: Discovery of Fluoren-9-Amines as Selective Butyrylcholinesterase Inhibitors and N-Methyl-d-Aspartate Receptor Antagonists. Biomolecules 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.J.; Mohan, P.; Yeggoni, D.P.; Babu, Z.R.; Kumar, P.B.; Rao, A.D.; Subramanyam, R.; Damu, A.G. New Flavone-Cyanoacetamide Hybrids with a Combination of Cholinergic, Antioxidant, Modulation of β-Amyloid Aggregation, and Neuroprotection Properties as Innovative Multifunctional Therapeutic Candidates for Alzheimer’s Disease and Unraveling Their Mechanism of Action with Acetylcholinesterase. Mol. Pharm. 2018, 15, 2206–2223. [Google Scholar] [PubMed]
- González-Naranjo, P.; Pérez-Macias, N.; Campillo, N.E.; Pérez, C.; Arán, V.J.; Girón, R.; Sánchez-Robles, E.; Martín, M.I.; Gómez-Cañas, M.; García-Arencibia, M.; et al. Cannabinoid agonists showing BuChE inhibition as potential therapeutic agents for Alzheimer’s disease. Eur. J. Med. Chem. 2014, 73, 56–72. [Google Scholar] [CrossRef]
- Greco, V.; Naletova, I.; Ahmed, I.M.M.; Vaccaro, S.; Messina, L.; La Mendola, D.; Bellia, F.; Sciuto, S.; Satriano, C.; Rizzarelli, E. Hyaluronan-carnosine conjugates inhibit Aβ aggregation and toxicity. Sci. Rep. 2020, 10, 15998. [Google Scholar] [CrossRef]
- Luo, X.T.; Wang, C.M.; Liu, Y.; Huang, Z.G. New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant, and neuroprotective properties as innovative drugs for Alzheimer’s disease. Eur. J. Med. Chem. 2015, 103, 302–311. [Google Scholar] [CrossRef]
- Onder, S.; Biberoglu, K.; Yuksel, M.; Tacal, O. Toluidine blue O attenuates tau phosphorylation in N2a-APPSwe cells. Chem. Biol. Interact. 2022, 366, 110126. [Google Scholar] [CrossRef]
- Javed, M.A.; Jan, M.S.; Shbeer, A.M.; Al-Ghorbani, M.; Rauf, A.; Wilairatana, P.; Mannan, A.; Sadiq, A.; Farooq, U.; Rashid, U. Evaluation of pyrimidine/pyrrolidine-sertraline based hybrids as multitarget anti-Alzheimer agents: In-vitro, in-vivo, and computational studies. Biomed. Pharmacother. 2023, 159, 114239. [Google Scholar] [CrossRef]
- Moftah, H.K.; Mousa, M.H.A.; Elrazaz, E.Z.; Kamel, A.S.; Lasheen, D.S.; Georgey, H.H. Novel quinazolinone Derivatives: Design, synthesis and in vivo evaluation as potential agents targeting Alzheimer disease. Bioorg. Chem. 2024, 143, 107065. [Google Scholar] [CrossRef]
- Łażewska, D.; Bajda, M.; Kaleta, M.; Zaręba, P.; Doroz-Płonka, A.; Siwek, A.; Alachkar, A.; Mogilski, S.; Saad, A.; Kuder, K.; et al. Rational design of new multitarget histamine H3 receptor ligands as potential candidates for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 207, 112743. [Google Scholar] [CrossRef]
- Mehta, P.; Miszta, P.; Filipek, S. Molecular Modeling of Histamine Receptors-Recent Advances in Drug Discovery. Molecules 2021, 26, 1778. [Google Scholar] [CrossRef] [PubMed]
- Staszewski, M.; Iwan, M.; Werner, T.; Bajda, M.; Godyń, J.; Latacz, G.; Korga-Plewko, A.; Kubik, J.; Szałaj, N.; Stark, H.; et al. Guanidines: Synthesis of Novel Histamine H3R Antagonists with Additional Breast Anticancer Activity and Cholinesterases Inhibitory Effect. Pharmaceuticals 2023, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Godyń, J.; Zaręba, P.; Łażewska, D.; Stary, D.; Reiner-Link, D.; Frank, A.; Latacz, G.; Mogilski, S.; Kaleta, M.; Doroz-Płonka, A.; et al. Cyanobiphenyls: Novel H3 receptor ligands with cholinesterase and MAO B inhibitory activity as multitarget compounds for potential treatment of Alzheimer’s disease. Bioorg. Chem. 2021, 114, 105129. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.; Ugale, V.; Shaukat, J.; Hollmann, M.; Shete, P.; Shravage, B.; Tayade, S.; Kumbhar, A.; Butcher, R.; Jani, V.; et al. Novel alkyl-substituted 4-methoxy benzaldehyde thiosemicarbazones: Multi-target directed ligands for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2023, 957, 176028. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, T.; Chen, Q.; Li, C.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; Chen, H.; Zhou, Z.; et al. Biomimetic Dendrimer-Peptide Conjugates for Early Multi-Target Therapy of Alzheimer’s Disease by Inflammatory Microenvironment Modulation. Adv. Mater. 2021, 33, e2100746. [Google Scholar] [CrossRef]
- Melchiorri, D.; Merlo, S.; Micallef, B.; Borg, J.J.; Dráfi, F. Alzheimer’s disease and neuroinflammation: Will new drugs in clinical trials pave the way to a multi-target therapy? Front. Pharmacol. 2023, 14, 1196413. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Klegeris, A. Novel multi-target directed ligand-based strategies for reducing neuroinflammation in Alzheimer’s disease. Life Sci. 2018, 207, 314–322. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef]
- Mufson, E.J.; Ward, S.; Binder, L. Prefibrillar tau oligomers in mild cognitive impairment and Alzheimer’s disease. Neurodegener. Dis. 2014, 13, 151–153. [Google Scholar] [CrossRef]
- Bierer, L.M.; Hof, P.R.; Purohit, D.P.; Carlin, L.; Schmeidler, J.; Davis, K.L.; Perl, D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995, 52, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kumar, R.; Pavlov, P.F.; Winblad, B. Small molecule therapeutics for tauopathy in Alzheimer’s disease: Walking on the path of most resistance. Eur. J. Med. Chem. 2021, 209, 112915. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Chen, X.; Tracy, T.E.; Li, Y.; Zhou, Y.; Wang, C.; Shirakawa, K.; Minami, S.S.; Defensor, E.; Mok, S.A.; et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015, 21, 1154–1162. [Google Scholar] [CrossRef]
- Liu, J.Y.; Guo, H.Y.; Quan, Z.S.; Shen, Q.K.; Cui, H.; Li, X. Research progress of natural products and their derivatives against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2023, 38, 2171026. [Google Scholar] [CrossRef]
- Thakral, S.; Yadav, A.; Singh, V.; Kumar, M.; Kumar, P.; Narang, R.; Sudhakar, K.; Verma, A.; Khalilullah, H.; Jaremko, M.; et al. Alzheimer’s disease: Molecular aspects and treatment opportunities using herbal drugs. Ageing Res. Rev. 2023, 88, 101960. [Google Scholar] [CrossRef]
- Fernandes, F.; Barroso, M.F.; De Simone, A.; Emriková, E.; Dias-Teixeira, M.; Pereira, J.P.; Chlebek, J.; Fernandes, V.C.; Rodrigues, F.; Andrisano, V.; et al. Multi-target neuroprotective effects of herbal medicines for Alzheimer’s disease. J. Ethnopharmacol. 2022, 290, 115107. [Google Scholar] [CrossRef]
- Singh, S.; Dhanawat, M.; Gupta, S.; Kumar, D.; Kakkar, S.; Nair, A.; Verma, I.; Sharma, P. Naturally Inspired Pyrimidines Analogues for Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 136–151. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, J.G.; Li, H.; Yang, H.M. Pharmacological effects of active components of chinese herbal medicine in the treatment of Alzheimer’s disease: A review. Am. J. Chin. Med. 2016, 44, 1525–1541. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Wang, Z.; Guo, P.; Liu, A.; Du, G. Identification of Multi-Target Anti-AD Chemical Constituents from Traditional Chinese Medicine Formulae by Integrating Virtual Screening and In Vitro Validation. Front. Pharmacol. 2021, 12, 709607. [Google Scholar] [CrossRef]
- Sun, Z.K.; Yang, H.Q.; Chen, S.D. Traditional Chinese medicine: A promising candidate for the treatment of Alzheimer’s disease. Transl. Neurodegener. 2013, 2, 6. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chen, S.B.; Dong, H.G.; Yu, Z.L.; Dong, J.C.; Long, Z.X.; Fong, W.F.; Han, Y.F.; Ko, K.M. New perspectives on chinese herbal medicine (zhong-yao) research and development. Evid. Based Complement. Alternat. Med. 2011, 2011, 403709. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Song, E.J.; Lee, D.; Park, J.; Nam, Y.; Kim, J.I.; Moon, M. Traditional Oriental Medicines and Alzheimer’s Disease. Aging Dis. 2019, 10, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rai, S.N.; Maurya, A.; Mishra, G.; Awasthi, R.; Shakya, A.; Chellappan, D.K.; Dua, K.; Vamanu, E.; Chaudhary, S.K.; et al. Therapeutic potential of phytoconstituents in management of Alzheimer’s disease. Evid. Based Complement. Alternat. Med. 2021, 2021, 5578574. [Google Scholar] [CrossRef]
- Tripathi, P.N.; Lodhi, A.; Rai, S.N.; Nandi, N.K.; Dumoga, S.; Yadav, P.; Tiwari, A.K.; Singh, S.K.; El-Shorgabi, A.N.A.; Chaudhary, S. Review of Pharmacotherapeutic Targets in Alzheimer’s Disease and Its Management Using Traditional Medicinal Plants. Degener. Neurol. Neuromuscul. Dis. 2024, 14, 47–74. [Google Scholar] [CrossRef]
- Sharma, R.; Kuca, K.; Nepovimova, E.; Kabra, A.; Rao, M.M.; Prajapati, P.K. Traditional Ayurvedic and herbal remedies for Alzheimer’s disease: From bench to bedside. Expert. Rev. Neurotherap. 2019, 19, 359–374. [Google Scholar] [CrossRef]
- Lins Alves, L.K.; Cechinel Filho, V.; de Souza, R.L.R.; Furtado-Alle, L. BChE inhibitors from marine organisms—A review. Chem. Biol. Interact. 2022, 367, 110136. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; Pivac, N. Epigenetics of Alzheimer’s Disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef]
- Xiong, X.; James, B.T.; Boix, C.A.; Park, Y.P.; Galani, K.; Victor, M.B.; Sun, N.; Hou, L.; Ho, L.L.; Mantero, J.; et al. Epigenomic dissection of Alzheimer’s disease pinpoints causal variants and reveals epigenome erosion. Cell 2023, 186, 4422–4437. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Q.; Yao, H.; Tan, J.; Liu, Z.; Zhou, Y.; Zou, Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 911635. [Google Scholar] [CrossRef]
- Ren, G.; Song, S.; Zhang, S.X.; Liu, Y.; Lv, Y.; Wang, Y.H.; Zhao, R.; Li, X.Y. Brain region-specific genome-wide deoxyribonucleic acid methylation analysis in patients with Alzheimer’s disease. Front. Mol. Neurosci. 2023, 16, 971565. [Google Scholar] [CrossRef]
- Cacabelos, R.; Torrellas, C. Epigenetic drug discovery for Alzheimer’s disease. Expert. Opin. Drug Discov. 2014, 9, 1059–1086. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Naidoo, V.; Tellado, I.; Cacabelos, N.; Cacabelos, P.; Martínez-Iglesias, O.; Segre, R. Epigenetic Drugs. In Pharmacoepigenetics, 2nd ed.; Cacabelos, R., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2024; in press. [Google Scholar]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Targeting epigenetics: A novel promise for Alzheimer’s disease treatment. Ageing Res. Rev. 2023, 90, 102003. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Carril, J.C.; Cacabelos, N.; Kazantsev, A.G.; Vostrov, A.V.; Corzo, L.; Cacabelos, P.; Goldgaber, D. Sirtuins in Alzheimer’s Disease: SIRT2-Related GenoPhenotypes and Implications for PharmacoEpiGenetics. Int. J. Mol. Sci. 2019, 20, E1249. [Google Scholar] [CrossRef] [PubMed]
- Soltan, O.M.; Abdelrahman, K.S.; Bass, A.K.A.; Takizawa, K.; Narumi, A.; Konno, H. Design of Multi-Target drugs of HDACs and other Anti-Alzheimer related Targets: Current strategies and future prospects in Alzheimer’s diseases therapy. Bioorg. Chem. 2024, 151, 107651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Ye, Q.; Fu, Y.; Li, X.; Yang, K.; Gao, F.; Zhou, A.; Wei, Y.; Tian, S.; et al. Histone deacetylase inhibitors VPA and WT161 ameliorate the pathological features and cognitive impairments of the APP/PS1 Alzheimer’s disease mouse model by regulating the expression of APP secretases. Alzheimers Res. Ther. 2024, 16, 15. [Google Scholar] [CrossRef]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [Google Scholar] [CrossRef]
- Yamashita, Y.; Itoh, Y.; Takada, Y.; Suzuli, T. Epigenetic Inhibitors as Alzheimer’s Disease Therapeutic Agents. Chem. Pharm. Bull. 2024, 72, 630–637. [Google Scholar] [CrossRef]
- Noce, B.; Di Bello, E.; Fioravanti, R.; Mai, A. LSD1 inhibitors for cancer treatment: Focus on multi-target agents and compounds in clinical trials. Front. Pharmacol. 2023, 14, 1120911. [Google Scholar] [CrossRef]
- Yang, S.; Fan, L.; Zhang, R.; Song, C.; Shi, J.; Wang, J.; Zhang, P.; Wang, H.; Zhang, Y. Smilagenin induces expression and epigenetic remodeling of BDNF in alzheimer’s disease. Phytomedicine 2023, 118, 154956. [Google Scholar] [CrossRef]
- Shademan, B.; Avci, C.B.; Karamad, V.; Sogutlu, F.; Nourazarian, A. MicroRNAs as a New Target for Alzheimer’s Disease Treatment. Microrna 2023, 12, 3–12. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Nosustrophine: An Epinutraceutical Bioproduct with Effects on DNA Methylation, Histone Acetylation and Sirtuin Expression in Alzheimer’s Disease. Pharmaceutics 2022, 14, 2447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Chan, C.H.; Ma, Q.H.; Xu, X.H.; Xiao, Z.C.; Tan, E.K. The roles of amyloid precursor protein (APP) in neurogenesis, implications to pathogenesis and therapy of Alzheimer disease (AD). Cell Adh. Migr. 2011, 5, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, U.V.; Varma, V.R.; Griswold, M.E.; Blackshear, C.T.; An, Y.; Oommen, A.M.; Varma, S.; Troncoso, J.C.; Pletnikova, O.; O’Brien, R.; et al. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study. PLoS Med. 2020, 17, e1003012. [Google Scholar]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Sadik, G.; Kaji, H.; Takeda, K.; Yamagata, F.; Kameoka, Y.; Hashimoto, K.; Miyanaga, K.; Shinoda, T. In vitro processing of amyloid precursor protein by cathepsin D. Int. J. Biochem. Cell Biol. 1999, 31, 1327–1337. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Helwig, M.; Hoshino, A.; Berridge, C.; Lee, S.N.; Lorenzen, N.; Otzen, D.E.; Eriksen, J.L.; Lindberg, I. The neuroendocrine protein 7B2 suppresses the aggregation of neurodegenerative disease-related proteins. J. Biol. Chem. 2013, 288, 1114–1124. [Google Scholar] [CrossRef]
- Xing, S.; Hu, Y.; Huang, X.; Shen, D.; Chen, C. Nicotinamide phosphoribosyltransferase-related signaling pathway in early Alzheimer’s disease mouse models. Mol. Med. Rep. 2019, 20, 5163–5171. [Google Scholar] [CrossRef]
- De Strooper, B.; Iwatsubo, T.; Wolfe, M.S. Presenilins and γ-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006304. [Google Scholar] [CrossRef]
- Carrera, I.; Lombardi, V.; Naidoo, V.; Martinez-Iglesias, O.; Corzo, L.; Cacabelos, R. Neuronal Protective Effect of Nosustrophine in Cell Culture Models. J. Explor. Res. Pharmacol. 2023, 8, 276–285. [Google Scholar] [CrossRef]
- Carrera, I.; Corzo, L.; Martínez-Iglesias, O.; Naidoo, V.; Cacabelos, R. Neuroprotective Effect of Nosustrophine in a 3xTg Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1306. [Google Scholar] [CrossRef] [PubMed]
- Carrera, I.; Fernández-Novoa, L.; Teijido, O.; Cacabelos, R. Comparative Characterization Profile of Transgenic Mouse Models of Alzheimer ‘s Disease. J. Genom. Med. Pharm. 2017, 2, 331–337. [Google Scholar]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Cacabelos, R. Epigenetic studies in the male APP/BIN1/COPS5 triple-transgenic mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 2446. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and Dopamine Receptors in Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [CrossRef]
- Caligiore, D.; Silvetti, M.; D’Amelio, M.; Puglisi-Allegra, S.; Baldassarre, G. Computational Modeling of Catecholamines Dysfunction in Alzheimer’s Disease at Pre-Plaque Stage. J. Alzheimers Dis. 2020, 77, 275–290. [Google Scholar] [CrossRef]
- Ambrée, O.; Richter, H.; Sachser, N.; Lewejohann, L.; Dere, E.; de Souza Silva, M.A.; Herring, A.; Keyvani, K.; Paulus, W.; Schäbitz, W.R. Levodopa ameliorates learning and memory deficits in a murine model of Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1192–1204. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- El Idrissi, F.; Gressier, B.; Devos, D.; Belarbi, K. A Computational Exploration of the Molecular Network Associated to Neuroinflammation in Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 630003. [Google Scholar] [CrossRef]
- O’Banion, M.K. COX-2 and Alzheimer’s disease: Potential roles in inflammation and neurodegeneration. Expert. Opin. Investig. Drugs 1999, 8, 1521–1536. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Carrera, I.; Carril, J.C.; Fernández-Novoa, L.; Cacabelos, N.; Cacabelos, R. DNA Methylation in Neurodegenerative and Cerebrovascular Disorders. Int. J. Mol. Sci. 2020, 21, 2220. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Naidoo, V.; Cacabelos, N.; Cacabelos, R. Epigenetic Biomarkers as Diagnostic Tools for Neurodegenerative Disorders. Int. J. Mol. Sci. 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.; Donmez, G. The role of sirtuins in Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.K.M.; Wijayasinghe, Y.S. Sirtuins as Potential Therapeutic Targets for Mitigating Neuroinflammation Associated with Alzheimer’s Disease. Front. Cell Neurosci. 2021, 15, 746631. [Google Scholar] [CrossRef]
- Xu, K.; Dai, X.L.; Huang, H.C.; Jiang, Z.F. Targeting HDACs: A promising therapy for Alzheimer’s disease. Oxid. Med. Cell Longev. 2011, 2011, 143269. [Google Scholar] [CrossRef]
- González, J.F.; Alcántara, A.R.; Doadrio, A.L.; Sanchez-Montero, J.M. Developments with multi-target drugs for Alzheimer’s disease: An overview of the current discovery approaches. Expert. Opin. Drug Discov. 2019, 14, 879–891. [Google Scholar] [CrossRef]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; Caraci, F. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: A strategy for individualized therapeutic approaches? J. Neuroinflamm. 2024, 21, 187. [Google Scholar] [CrossRef]
- Sameem, B.; Saeedi, M.; Mahdavi, M.; Shafiee, A. A review on tacrine-based scaffolds as multi-target drugs (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 128, 332–345. [Google Scholar] [CrossRef]
- Martens, Y.A.; Zhao, N.; Liu, C.C.; Kanekiyo, T.; Yang, A.J.; Goate, A.M.; Holtzman, D.M.; Bu, G. ApoE Cascade Hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 2022, 110, 1304–1317. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, C.C.; Qiao, W.; Bu, G. Apolipoprotein, E Receptors, and Modulation of Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 347–357. [Google Scholar] [CrossRef]
- McCorkindale, A.N.; Mundell, H.D.; Guennewig, B.; Suntherland, G.T. Vascular Dysfunction Is Central to Alzheimer’s Disease Pathogenesis in APOE e4 Carriers. Int. J. Mol. Sci. 2022, 23, 7106. [Google Scholar] [CrossRef]
- Cacabelos, R.; Carrera, I.; Martínez-Iglesias, O.; Cacabelos, N.; Naidoo, V. What is the gold standard model for Alzheimer’s disease drug discovery and development? Expert. Opin. Drug Discov. 2021, 16, 1415–1440. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Naidoo, V.; Corzo, L.; Cacabelos, N.; Carril, J.C. Genophenotypic Factors and Pharmacogenomics in Adverse Drug Reactions. Int. J. Mol. Sci. 2021, 22, 13302. [Google Scholar] [CrossRef]
- Argueta, N.; Notari, E.; Szigeti, K. Role of Pharmacogenomics in Individualizing Treatment for Alzheimer’s Disease. CNS Drugs. 2022, 36, 365–376. [Google Scholar] [CrossRef]
- Cacabelos, R.; Carril, J.C.; Corzo, L.; Fernández-Novoa, L.; Pego, R.; Cacabelos, N.; Cacabelos, P.; Alcaraz, M.; Tellado, I.; Naidoo, V. Influence of Pathogenic and Metabolic Genes on the Pharmacogenetics of Mood Disorders in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 366. [Google Scholar] [CrossRef]
- Cacabelos, R.; Carril, J.C.; Corzo, L.; Pego, R.; Cacabelos, N.; Alcaraz, M.; Muñiz, A.; Martínez-Iglesias, O.; Naidoo, V. Pharmacogenetics of anxiety and depression in Alzheimer’s disease. Pharmacogenomics 2023, 24, 27–57. [Google Scholar] [CrossRef]
- Kolli, N.; Lu, M.; Maiti, P.; Rossignol, J.; Dunbar, G.L. Application of the gene editing tool, CRISPR-Cas9, for treating neurodegenerative diseases. Neurochem. Int. 2018, 112, 187–196. [Google Scholar] [CrossRef]
- Pahan, K. A Broad application of CRISPR Cas9 in infectious, inflammatory and neurodegenerative diseases. J. NeuroImmune Pharmacol. 2019, 14, 534–536. [Google Scholar] [CrossRef]
- Fayazi, N.; Sheykhhasan, M.; Asl, S.S.; Najafi, R. Stem Cell-Derived Exosomes: A New Strategy of Neurodegenerative Disease Treatment. Mol. Neurobiol. 2021, 58, 3494–3514. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kesari, K.K.; Rachamalla, M.; Mani, S.; Ashraf, G.M.; Jha, S.K.; Kumar, P.; Ambasta, R.K.; Dureja, H.; Devkota, H.P.; et al. CRISPR/Cas9 gene editing: New hope for Alzheimer’s disease therapeutics. J. Adv. Res. 2022, 40, 207–221. [Google Scholar] [CrossRef]
- Mehta, S.L.; Arruri, V.; Vemuganti, R. Role of transcription factors, noncoding RNAs, epitranscriptomics, and epigenetics in post-ischemic neuroinflammation. J. Neurochem. 2024, 168, 3430–3448. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Ge, A.; Wang, S.; Zeng, J.; Yuan, X.; Mei, Z.; Wang, G.; Ge, J. A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury. Front. Immunol. 2022, 13, 930171. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022, 19, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Lee, S.; Lee, H.J.; Min, J.W.; Iwatsubo, T.; Teunissen, C.E.; Cho, H.J.; Ryu, J.H. Targeting MicroRNA-485-3p Blocks Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2022, 23, 3566. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Macyczko, J.R.; Liu, C.C.; Bu, G. ApoE4 reduction: An emerging and promising therapeutic strategy for Alzheimer’s disease. Neurobiol. Aging. 2022, 115, 20–28. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2024, 30, 304. [Google Scholar] [CrossRef]
- Wang, X.; Xie, J.; Tan, L.; Lu, Y.; Shen, N.; Li, J.; Hu, H.; Li, H.; Li, X.; Cheng, L. N6-methyladenosine-modified circRIMS2 mediates synaptic and memory impairments by activating GluN2B ubiquitination in Alzheimer’s disease. Transl. Neurodegener. 2023, 12, 53. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacabelos, R.; Martínez-Iglesias, O.; Cacabelos, N.; Carrera, I.; Corzo, L.; Naidoo, V. Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity. Life 2024, 14, 1555. https://doi.org/10.3390/life14121555
Cacabelos R, Martínez-Iglesias O, Cacabelos N, Carrera I, Corzo L, Naidoo V. Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity. Life. 2024; 14(12):1555. https://doi.org/10.3390/life14121555
Chicago/Turabian StyleCacabelos, Ramón, Olaia Martínez-Iglesias, Natalia Cacabelos, Iván Carrera, Lola Corzo, and Vinogran Naidoo. 2024. "Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity" Life 14, no. 12: 1555. https://doi.org/10.3390/life14121555
APA StyleCacabelos, R., Martínez-Iglesias, O., Cacabelos, N., Carrera, I., Corzo, L., & Naidoo, V. (2024). Therapeutic Options in Alzheimer’s Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity. Life, 14(12), 1555. https://doi.org/10.3390/life14121555








