Mechanisms of Cbl-Mediated Ubiquitination of Proteins in T and Natural Killer Cells and Effects on Immune Cell Functions
Abstract
:1. Introduction
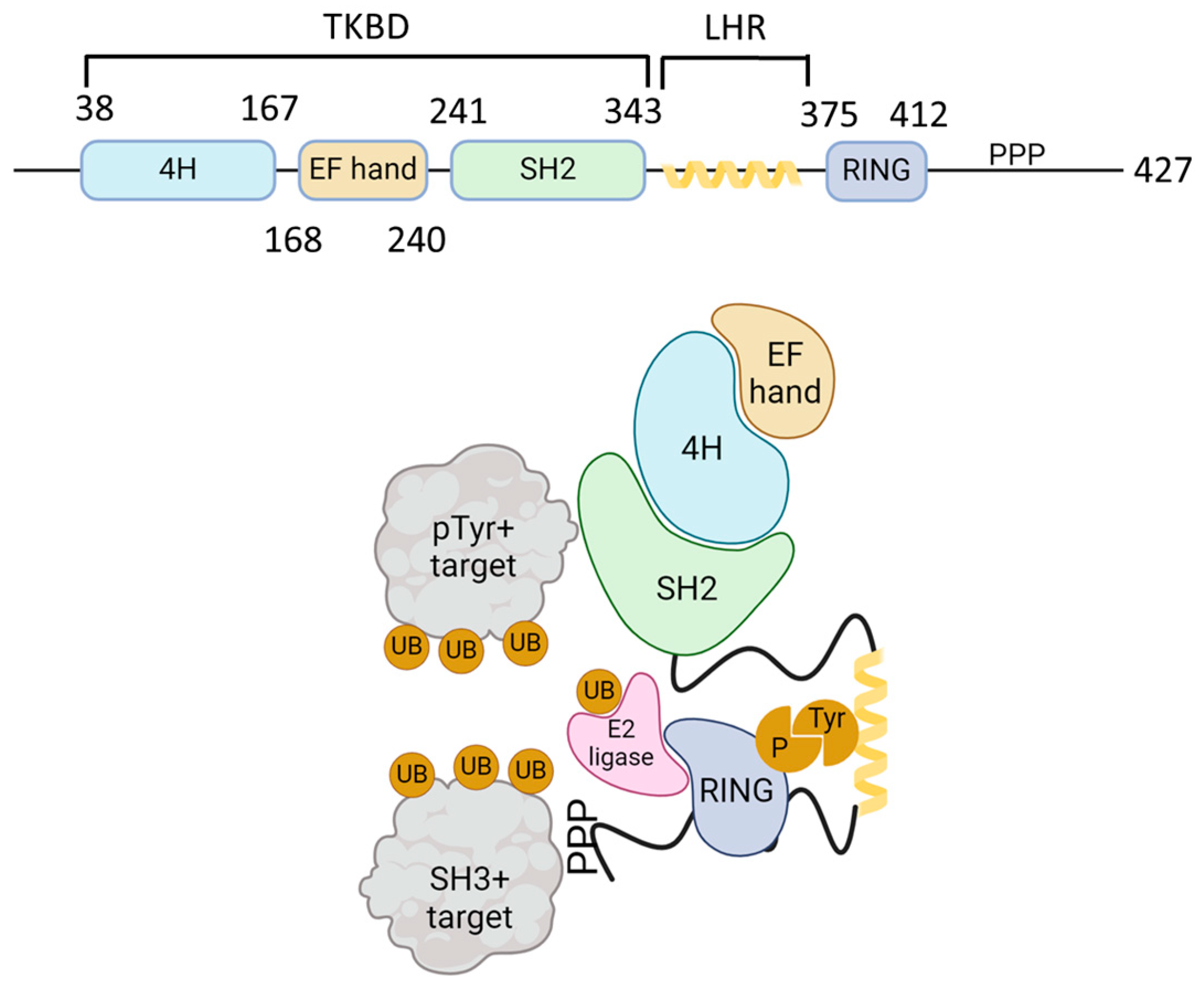
2. Cbl, an E3 Ubiquitin Ligase
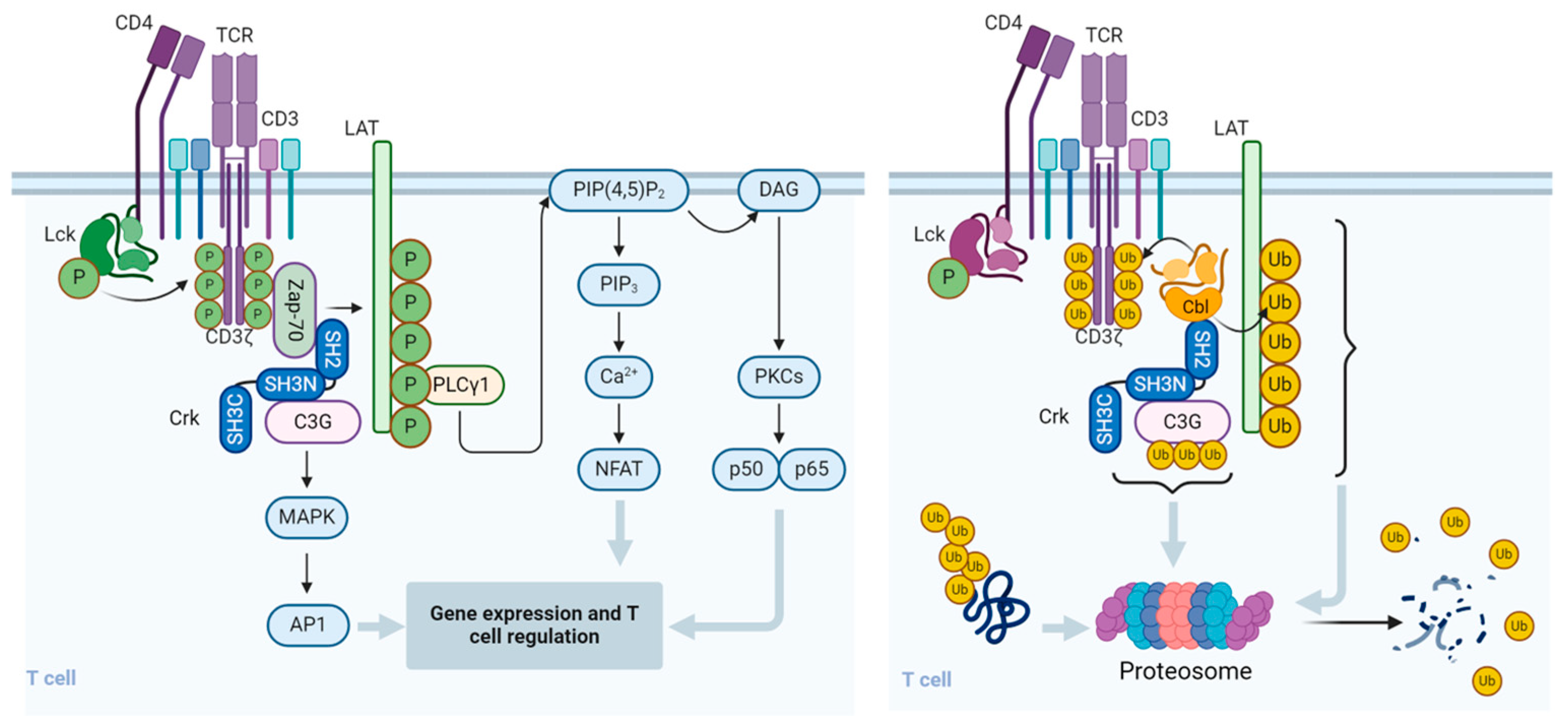
3. Effect of Cbl on T Cells
4. Cbl and Chimeric Antigen Receptor (CAR) T Cells
5. Involvement of Cbl in the Regulation of T Cell Activation
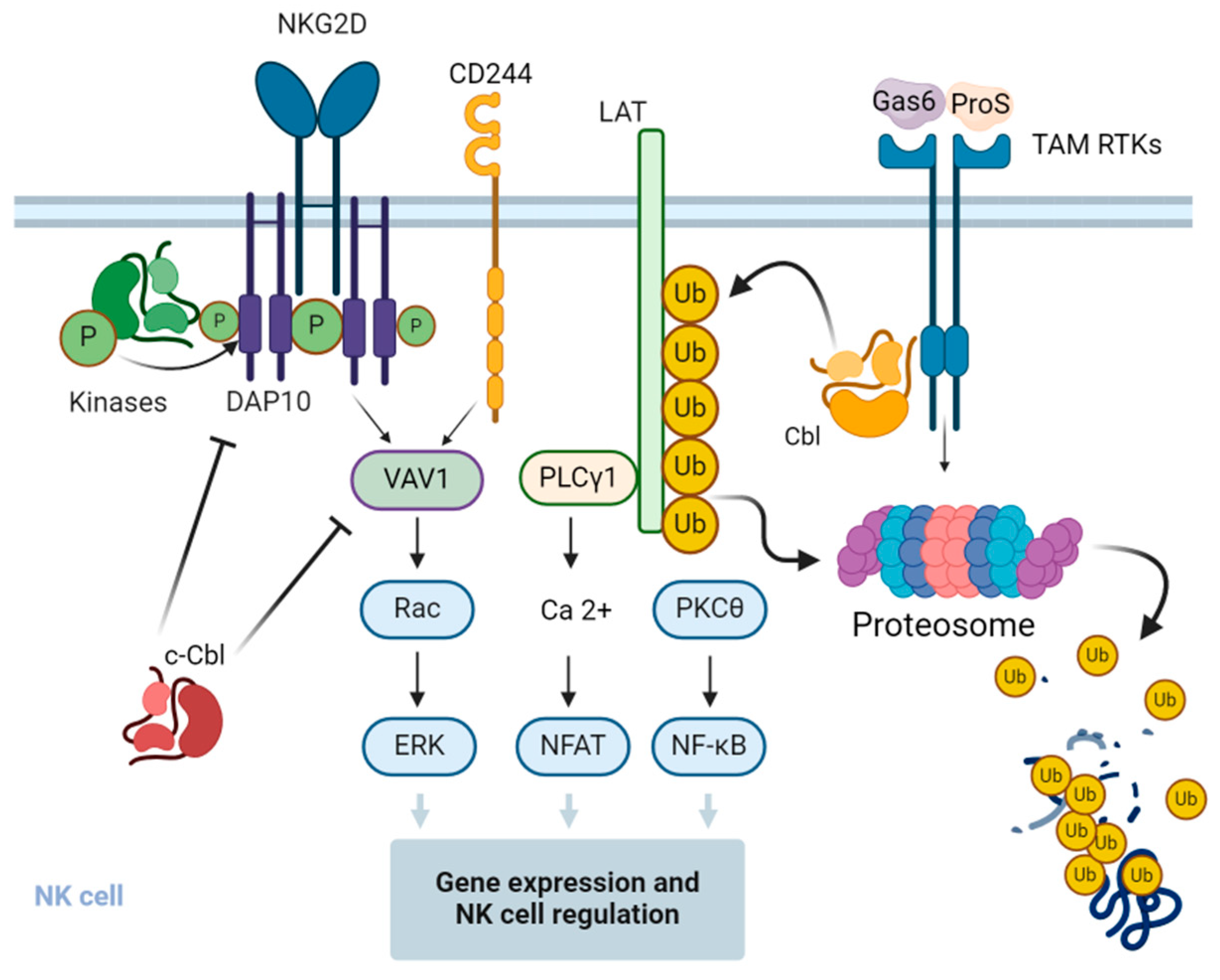
6. Effect of Cbl on Natural Killer (NK) Cells
7. Cbl and NK Cell Exhaustion
8. Cbl Inhibitors and Applications in Immunotherapy
9. Recently Discovered Mechanism of Cbl Regulation in Activated Immune Cells
10. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| APC | antigen-presenting cells |
| C3G | Crk SH3 domain-binding guanine nucleotide-releasing factor |
| CAR | chimeric antigen receptor |
| CARD | caspase recruitment domain |
| CARMA1 | CARD-MAGUK protein 1 |
| Cbl | Casitas B-lineage lymphoma |
| cNK | conventional NK cells |
| CRK | CT-10 regulator of kinases |
| DC | dendritic cell |
| DUB | deubiquitinase |
| GAS6 | growth arrest-specific gene-6 |
| IL | interleukin |
| ILC | innate lymphoid cells |
| IFN-γ | interferon gamma |
| LAT | linker of activation of T cells |
| Lck | cell-specific protein-tyrosine kinase |
| LCMV | lymphocytic choriomeningitis virus |
| LZ | leucine zipper |
| MAGUK | membrane-associated guanylate kinase |
| NK | natural killer |
| PI3K | phosphoinositide-3 kinase |
| PKCθ | protein kinase C theta |
| PLCγ1 | phospholipase C gamma 1 |
| PR | proline rich |
| RING | really interesting group finger domain |
| SLE | systemic lupus erythematosus |
| SLP76 | SH2-domain-containing leukocyte protein of 76 kDa |
| TAM | Tyro3, Axl, and Mer (receptor kinases) |
| TCR | T cell antigen receptor |
| Tfh | follicular helper T cell |
| TGF-β | transforming growth factor beta |
| TILs | tumor-infiltrating lymphocytes |
| TKB | tyrosine kinase binding |
| TNFα | tumor necrosis factor alpha |
| trNK | tissue-resident NK cells |
| UB | ubiquitin |
| ZAP70 | zeta chain-associated protein 70 |
References
- Kuwabara, T.; Matsui, Y.; Ishikawa, F.; Kondo, M. Regulation of T-Cell Signaling by Post-Translational Modifications in Autoimmune Disease. Int. J. Mol. Sci. 2018, 19, 819. [Google Scholar] [CrossRef]
- Raposo, B.; Merky, P.; Lundqvist, C.; Yamada, H.; Urbonaviciute, V.; Niaudet, C.; Viljanen, J.; Kihlberg, J.; Kyewski, B.; Ekwall, O.; et al. T cells specific for post-translational modifications escape intrathymic tolerance induction. Nat. Commun. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 2008, 8, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chai, Q.Y.; Liu, C.H. The ubiquitin system: A critical regulator of innate immunity and pathogen–host interactions. Cell. Mol. Immunol. 2016, 13, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Malynn, B.A.; Ma, A. Ubiquitin Makes Its Mark on Immune Regulation. Immunity 2010, 33, 843–852. [Google Scholar] [CrossRef]
- Hu, H.; Sun, S.-C. Ubiquitin signaling in immune responses. Cell Res. 2016, 26, 457–483. [Google Scholar] [CrossRef]
- Pickart, C.M. Mechanisms Underlying Ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef]
- Haglund, K.; Dikic, I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J. Cell Sci. 2012, 125, 265–275. [Google Scholar] [CrossRef]
- Hicke, L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001, 2, 195–201. [Google Scholar] [CrossRef]
- Tsygankov, A.Y.; Teckchandani, A.M.; Feshchenko, E.A.; Swaminathan, G. Beyond the RING: CBL proteins as multivalent adapters. Oncogene 2001, 20, 6382–6402. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Sawasdikosol, S.; Burakoff, S.J.; Eck, M.J. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 1999, 402, 29–34. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Jang, I.K.; Hodes, R.; Gu, H. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J. Clin. Investig. 2007, 117, 1029–1036. [Google Scholar] [CrossRef]
- Wiedemann, A.; Müller, S.; Favier, B.; Penna, D.; Guiraud, M.; Delmas, C.; Champagne, E.; Valitutti, S. T-cell activation is accompanied by an ubiquitination process occurring at the immunological synapse. Immunol. Lett. 2005, 98, 57–61. [Google Scholar] [CrossRef]
- Garcia, G.G.; Miller, R.A. Single-Cell Analyses Reveal Two Defects in Peptide-Specific Activation of Naive T Cells from Aged Mice. J. Immunol. 2001, 166, 3151–3157. [Google Scholar] [CrossRef]
- Bachmaier, K.; Krawczyk, C.; Kozieradzki, I.; Kong, Y.Y.; Sasaki, T.; Oliveira-Dos-Santos, A.; Mariathasan, S.; Bouchard, D.; Wakeham, A.; Itie, A.; et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 2000, 403, 211–216. [Google Scholar] [CrossRef]
- Dou, H.; Buetow, L.; Hock, A.; Sibbet, G.J.; Vousden, K.H.; Huang, D.T. Structural basis for autoinhibition and phosphorylation-dependent activation of c-Cbl. Nat. Struct. Mol. Biol. 2012, 19, 184–192. [Google Scholar] [CrossRef]
- Hou, D.; Cenciarelli, C.; Jensen, J.P.; Nguygen, H.B.; Weissman, A.M. Activation-dependent ubiquitination of a T cell antigen receptor subunit on multiple intracellular lysines. J. Biol. Chem. 1994, 269, 14244–14247. [Google Scholar] [CrossRef] [PubMed]
- Cenciarelli, C.; Hou, D.; Hsu, K.C.; Rellahan, B.L.; Wiest, D.L.; Smith, H.T.; Fried, V.A.; Weissman, A.M. Activation-Induced Ubiquitination of the T Cell Antigen Receptor. Science 1992, 257, 795–797. [Google Scholar] [CrossRef]
- Andoniou, C.E.; Lill, N.L.; Thien, C.B.; Lupher, M.L.; Ota, S.; Bowtell, D.D.L.; Scaife, R.M.; Langdon, W.Y.; Band, H. The Cbl Proto-Oncogene Product Negatively Regulates the Src-Family Tyrosine Kinase Fyn by Enhancing Its Degradation. Mol. Cell. Biol. 2000, 20, 851–867. [Google Scholar] [CrossRef]
- Rao, N.; Miyake, S.; Reddi, A.L.; Douillard, P.; Ghosh, A.K.; Dodge, I.L.; Zhou, P.; Fernandes, N.D.; Band, H. Negative regulation of Lck by Cbl ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2002, 99, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jeon, M.S.; Liao, L.; Yang, C.; Elly, C.; Yates, J.R.; Liu, Y.-C. K33-Linked Polyubiquitination of T Cell Receptor-ζ Regulates Proteolysis-Independent T Cell Signaling. Immunity 2010, 33, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, N.; Mueller, D.L. Casitas B-Lineage Lymphoma b Inhibits Antigen Recognition and Slows Cell Cycle Progression at Late Times during CD4 + T Cell Clonal Expansion. J. Immunol. 2008, 181, 5331–5339. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.J.; Kole, H.K.; Brown, K.; Naramura, M.; Fukuhara, S.; Hu, R.J.; Jang, I.K.; Gutkind, J.S.; Shevach, E.; Gu, H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature 2000, 403, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Loeser, S.; Loser, K.; Bijker, M.S.; Rangachari, M.; Van Der Burg, S.H.; Wada, T.; Beissert, S.; Melief, C.J.M.; Penninger, J.M. Spontaneous tumor rejection by cbl-b–deficient CD8+ T cells. J. Exp. Med. 2007, 204, 879–891. [Google Scholar] [CrossRef]
- Singh, T.P.; Vieyra-Garcia, P.A.; Wagner, K.; Penninger, J.; Wolf, P. Cbl-b deficiency provides protection against UVB-induced skin damage by modulating inflammatory gene signature. Cell Death Dis. 2018, 9, 835. [Google Scholar] [CrossRef]
- Stromnes, I.M.; Blattman, J.N.; Tan, X.; Jeevanjee, S.; Gu, H.; Greenberg, P.D. Abrogating Cbl-b in effector CD8+ T cells improves the efficacy of adoptive therapy of leukemia in mice. J. Clin. Investig. 2010, 120, 3722–3734. [Google Scholar] [CrossRef]
- Paolino, M.; Thien, C.B.F.; Gruber, T.; Hinterleitner, R.; Baier, G.; Langdon, W.Y.; Penninger, J.M. Essential Role of E3 Ubiquitin Ligase Activity in Cbl-b–Regulated T Cell Functions. J. Immunol. 2011, 186, 2138–2147. [Google Scholar] [CrossRef]
- Lutz-Nicoladoni, C.; Wolf, D.; Sopper, S. Modulation of Immune Cell Functions by the E3 Ligase Cbl-b. Front Oncol. 2015, 5, 58. [Google Scholar] [CrossRef]
- Schanz, O.; Cornez, I.; Yajnanarayana, S.P.; David, F.S.; Peer, S.; Gruber, T.; Krawitz, P.; Brossart, P.; Heine, A.; Landsberg, J.; et al. Tumor rejection in Cblb−/− mice depends on IL-9 and Th9 cells. J. Immunother. Cancer 2021, 9, e002889. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, R.; Singh, A.K.; Tsakem, E.L.; Kathania, M.; Riese, M.J.; Theiss, A.L.; Davila, M.L.; Venuprasad, K. Deletion of Cbl-b inhibits CD8+ T-cell exhaustion and promotes CAR T-cell function. J. Immunother. Cancer 2021, 9, e001688. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Wang, Z.E.; Shen, L.; Schroeder, A.; Eckalbar, W.; Weiss, A. Cbl-b deficiency prevents functional but not phenotypic T cell anergy. J. Exp. Med. 2021, 218, e20202477. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.S.; Atfield, A.; Venuprasad, K.; Krawczyk, C.; Sarao, R.; Elly, C.; Yang, C.; Arya, S.; Bachmaier, K.; Su, L.; et al. Essential Role of the E3 Ubiquitin Ligase Cbl-b in T Cell Anergy Induction. Immunity 2004, 21, 167–177. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Callahan, M.K.; Clark, R.B. Resistance to CD4+CD25+ Regulatory T Cells and TGF-β in Cbl-b−/− Mice. J. Immunol. 2004, 173, 1059–1065. [Google Scholar] [CrossRef]
- Fujiwara, M.; Anstadt, E.J.; Clark, R.B. Cbl-b Deficiency Mediates Resistance to Programed Death-Ligand 1/Programed Death-1 Regulation. Front. Immunol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Peer, S.; Baier, G.; Gruber, T. Cblb-deficient T cells are less susceptible to PD-L1-mediated inhibition. Oncotarget 2017, 8, 41841–41853. [Google Scholar] [CrossRef]
- Gronski, M.A.; Boulter, J.M.; Moskophidis, D.; Nguyen, L.T.; Holmberg, K.; Elford, A.R.; Deenick, E.K.; Kim, H.O.; Penninger, J.M.; Odermatt, B.; et al. TCR affinity and negative regulation limit autoimmunity. Nat. Med. 2004, 10, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Choidas, A.; Wallner, S.; Pranjic, B.; Uribesalgo, I.; Loeser, S.; Jamieson, A.M.; Langdon, W.Y.; Ikeda, F.; Fededa, J.P.; et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 2014, 507, 508–512. [Google Scholar] [CrossRef]
- Hinterleitner, R.; Gruber, T.; Pfeifhofer-Obermair, C.; Lutz-Nicoladoni, C.; Tzankov, A.; Schuster, M.; Penninger, J.M.; Loibner, H.; Lametschwandtner, G.; Wolf, D.; et al. Adoptive Transfer of siRNA Cblb-Silenced CD8+ T Lymphocytes Augments Tumor Vaccine Efficacy in a B16 Melanoma Model. PLoS ONE 2012, 7, e44295. [Google Scholar] [CrossRef]
- Thell, K.; Urban, M.; Harrauer, J.; Haslinger, I.; Kuttke, M.; Brunner, J.S.; Vogel, A.; Schabbauer, G.; Penninger, J.; Gaweco, A. Master checkpoint Cbl-b inhibition: Anti-tumour efficacy in a murine colorectal cancer model following siRNA-based cell therapy. Ann. Oncol. 2019, 30, v503–v504. [Google Scholar] [CrossRef]
- Triozzi, P.; Kooshki, M.; Alistar, A.; Bitting, R.; Neal, A.; Lametschwandtner, G.; Loibner, H. Phase I clinical trial of adoptive cellular immunotherapy with APN401 in patients with solid tumors. J. Immunother. Cancer 2015, 3 (Suppl. S2), P175. [Google Scholar] [CrossRef]
- Qiao, G.; Ying, H.; Zhao, Y.; Liang, Y.; Guo, H.; Shen, H.; Li, Z.; Solway, J.; Tao, E.; Chiang, Y.J.; et al. E3 Ubiquitin Ligase Cbl-b Suppresses Proallergic T Cell Development and Allergic Airway Inflammation. Cell Rep. 2014, 6, 709–723. [Google Scholar] [CrossRef]
- Qiao, G.; Zhao, Y.; Li, Z.; Tang, P.Q.; Langdon, W.Y.; Yang, T.; Zhang, J. T Cell Activation Threshold Regulated by E3 Ubiquitin Ligase Cbl-b Determines Fate of Inducible Regulatory T Cells. J. Immunol. 2013, 191, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chung, D.C.; St Paul, M.; Liu, Z.Q.; Garcia-Batres, C.; Elford, A.R.; Tran, C.W.; Chapatte, L.; Ohashi, P.S. Overproduction of IL-2 by Cbl-b deficient CD4(+) T cells provides resistance against regulatory T cells. OncoImmunology 2020, 9, 1737368. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Huang, M.; Gong, L.; Zhang, X.; Zhong, L.; Calderon, V.; Bian, Z.; He, Y.; Suh, W.-K.; et al. Deficiency of CBL and CBLB ubiquitin ligases leads to hyper T follicular helper cell responses and lupus by reducing BCL6 degradation. Immunity 2024, 57, 1603–1617.e7. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Chen, J.; López-Moyado, I.F.; Seo, H.; Lio, C.W.J.; Hempleman, L.J.; Sekiya, T.; Yoshimura, A.; Scott-Browne, J.P.; Rao, A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019, 567, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Scott-Browne, J.P.; López-Moyado, I.F.; Trifari, S.; Wong, V.; Chavez, L.; Rao, A.; Pereira, R.M. Dynamic Changes in Chromatin Accessibility Occur in CD8 + T Cells Responding to Viral Infection. Immunity 2016, 45, 1327–1340. [Google Scholar] [CrossRef]
- Venuprasad, K. Cbl-b and Itch: Key Regulators of Peripheral T-cell Tolerance. Cancer Res. 2010, 70, 3009–3012. [Google Scholar] [CrossRef]
- Shamim, M.; Nanjappa, S.G.; Singh, A.; Plisch, E.H.; LeBlanc, S.E.; Walent, J.; Svaren, J.; Seroogy, C.; Suresh, M. Cbl-b Regulates Antigen-Induced TCR Down-Regulation and IFN-γ Production by Effector CD8 T Cells without Affecting Functional Avidity. J. Immunol. 2007, 179, 7233–7243. [Google Scholar] [CrossRef]
- Janssen, E.; Peters, Z.; Alosaimi, M.F.; Smith, E.; Milin, E.; Stafstrom, K.; Wallace, J.G.; Platt, C.D.; Chou, J.; El Ansari, Y.S.; et al. Immune dysregulation caused by homozygous mutations in CBLB. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.; Yakoub-Agha, I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr. Res. Transl. Med. 2017, 65, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiu, S.; Chen, J.; Jiang, S.; Chen, W.; Jiang, J.; Wang, F.; Si, W.; Shu, Y.; Wei, P.; et al. Chimeric Antigen Receptor Designed to Prevent Ubiquitination and Downregulation Showed Durable Antitumor Efficacy. Immunity 2020, 53, 456–470.e6. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M. Transcription Start Regions in the Human Genome Are Favored Targets for MLV Integration. Science 2003, 300, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.R.W.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 Integration in the Human Genome Favors Active Genes and Local Hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef]
- Shah, N.N.; Qin, H.; Yates, B.; Su, L.; Shalabi, H.; Raffeld, M.; Ahlman, M.A.; Stetler-Stevenson, M.; Yuan, C.; Guo, S.; et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019, 3, 2317–2322. [Google Scholar] [CrossRef]
- Naramura, M.; Jang, I.K.; Kole, H.; Huang, F.; Haines, D.; Gu, H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 2002, 3, 1192–1199. [Google Scholar] [CrossRef]
- Krawczyk, C.; Bachmaier, K.; Sasaki, T.; Jones, R.G.; Snapper, S.B.; Bouchard, D.; Kozieradzki, I.; Ohashi, P.S.; Alt, F.W.; Penninger, J.M. Cbl-b Is a Negative Regulator of Receptor Clustering and Raft Aggregation in T Cells. Immunity 2000, 13, 463–473. [Google Scholar] [CrossRef]
- Alcázar, I.; Cortés, I.; Zaballos, A.; Hernandez, C.; Fruman, D.A.; Barber, D.F.; Carrera, A.C. p85β phosphoinositide 3-kinase regulates CD28 coreceptor function. Blood 2009, 113, 3198–3208. [Google Scholar] [CrossRef]
- Fang, D.; Liu, Y.-C. Proteolysis-independent regulation of PI3K by Cbl-b–mediated ubiquitination in T cells. Nat. Immunol. 2001, 2, 870–875. [Google Scholar] [CrossRef]
- Li, D.; Gál, I.; Vermes, C.; Alegre, M.-L.; Chong, A.S.F.; Chen, L.; Shao, Q.; Adarichev, V.; Xu, X.; Koreny, T.; et al. Cutting Edge: Cbl-b: One of the Key Molecules Tuning CD28- and CTLA-4-Mediated T Cell Costimulation. J. Immunol. 2004, 173, 7135–7139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bárdos, T.; Li, D.; Gál, I.; Vermes, C.; Xu, J.; Mikecz, K.; Finnegan, A.; Lipkowitz, S.; Glant, T.T. Cutting Edge: Regulation of T Cell Activation Threshold by CD28 Costimulation Through Targeting Cbl-b for Ubiquitination. J. Immunol. 2002, 169, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Qiao, G.; Tang, J.; Tang, R.; Guo, H.; Warwar, S.; Langdon, W.Y.; Tao, L.; Zhang, J. Protein Tyrosine Phosphatase SHP-1 Modulates T Cell Responses by Controlling Cbl-b Degradation. J. Immunol. 2015, 195, 4218–4227. [Google Scholar] [CrossRef]
- Gruber, T.; Hermann-Kleiter, N.; Hinterleitner, R.; Fresser, F.; Schneider, R.; Gastl, G.; Penninger, J.M.; Baier, G. PKC-θ Modulates the Strength of T Cell Responses by Targeting Cbl-b for Ubiquitination and Degradation. Sci. Signal 2009, 2, ra30. [Google Scholar] [CrossRef]
- Nath, P.R.; Anto, N.P.; Braiman, A.; Isakov, N. Termination of TCR-mediated activation signals is regulated by CrkII-dependent Cbl-mediated ubiquitination and degradation of C3G. Immunobiology 2023, 228, 152342. [Google Scholar] [CrossRef]
- Nath, P.R.; Dong, G.; Braiman, A.; Isakov, N. Immunophilins Control T Lymphocyte Adhesion and Migration by Regulating CrkII Binding to C3G. J. Immunol. 2014, 193, 3966–3977. [Google Scholar] [CrossRef]
- Nolz, J.C.; Nacusi, L.P.; Segovis, C.M.; Medeiros, R.B.; Mitchell, J.S.; Shimizu, Y.; Billadeau, D.D. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL–C3G-mediated activation of Rap1. J. Cell Biol. 2008, 182, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Reedquist, K.A.; Fukazawa, T.; Panchamoorthy, G.; Langdon, W.Y.; Shoelson, S.E.; Druker, B.J.; Band, H. Stimulation through the T Cell Receptor Induces Cbl Association with Crk Proteins and the Guanine Nucleotide Exchange Protein C3G. J. Biol. Chem. 1996, 271, 8435–8442. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, Y.; Fang, D.; Huang, J.; Jeon, M.S.; Liu, Y.C. Negative Regulation of T Cell Antigen Receptor-mediated Crk-L-C3G Signaling and Cell Adhesion by Cbl-b. J. Biol. Chem. 2003, 278, 23978–23983. [Google Scholar] [CrossRef]
- Nath, P.R.; Gangaplara, A.; Pal-Nath, D.; Mandal, A.; Maric, D.; Sipes, J.M.; Cam, M.; Shevach, E.M.; Roberts, D.D. CD47 Expression in Natural Killer Cells Regulates Homeostasis and Modulates Immune Response to Lymphocytic Choriomeningitis Virus. Front. Immunol. 2018, 9, 2985. [Google Scholar] [CrossRef]
- Nath, P.R.; Pal-Nath, D.; Mandal, A.; Cam, M.C.; Schwartz, A.L.; Roberts, D.D. Natural Killer Cell Recruitment and Activation Are Regulated by CD47 Expression in the Tumor Microenvironment. Cancer Immunol. Res. 2019, 7, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.R.; Maclean, M.; Nagarajan, V.; Lee, J.W.; Yakin, M.; Kumar, A.; Nadali, H.; Schmidt, B.; Kaya, K.D.; Kodati, S.; et al. Single-cell profiling identifies a CD8bright CD244bright Natural Killer cell subset that reflects disease activity in HLA-A29-positive birdshot chorioretinopathy. Nat. Commun. 2024, 15, 6443. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, L.; Mansour, A.G.; Yu, M.J.; Brooks, N.; Teng, K.-Y.; Li, Z.; Zhang, J.; Barr, T.; Yu, J.; et al. Cbl-b Is Upregulated and Plays a Negative Role in Activated Human NK Cells. J. Immunol. 2021, 206, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mahlakõiv, T.; Ye, Q.; Somanchi, S.; He, S.; Rana, H.; DiFiglia, A.; Gleason, J.; van der Touw, W.; Hariri, R.; et al. CBLB ablation with CRISPR/Cas9 enhances cytotoxicity of human placental stem cell-derived NK cells for cancer immunotherapy. J. Immunother. Cancer 2021, 9, e001975. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Voskinarian-Berse, V.; Law, E.; Reddin, T.; Bhatia, M.; Hariri, A.; Ning, Y.; Dong, D.; Maguire, T.; Yarmush, M.; et al. Characterization and ex vivo Expansion of Human Placenta-Derived Natural Killer Cells for Cancer Immunotherapy. Front. Immunol. 2013, 4, 101. [Google Scholar] [CrossRef]
- Chirino, L.M.; Kumar, S.; Okumura, M.; Sterner, D.E.; Mattern, M.; Butt, T.R.; Kambayashi, T. TAM receptors attenuate murine NK-cell responses via E3 ubiquitin ligase Cbl-b. Eur. J. Immunol. 2020, 50, 48–55. [Google Scholar] [CrossRef]
- Kojo, S.; Elly, C.; Harada, Y.; Langdon, W.Y.; Kronenberg, M.; Liu, Y.-C. Mechanisms of NKT cell anergy induction involve Cbl-b-promoted monoubiquitination of CARMA1. Proc. Natl. Acad. Sci. USA 2009, 106, 17847–17851. [Google Scholar] [CrossRef]
- Augustin, R.C.; Bao, R.; Luke, J.J. Targeting Cbl-b in cancer immunotherapy. J. Immunother. Cancer 2023, 11, e006007. [Google Scholar] [CrossRef]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell. Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef]
- Da Silva, I.P.; Gallois, A.; Jimenez-Baranda, S.; Khan, S.; Anderson, A.C.; Kuchroo, V.K.; Osman, I.; Bhardwaj, N. Reversal of NK-Cell Exhaustion in Advanced Melanoma by Tim-3 Blockade. Cancer Immunol. Res. 2014, 2, 410–422. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jeon, I.; Kim, B.S.; Park, M.; Bae, E.A.; Song, B.; Koh, C.-H.; Shin, K.-S.; Kim, I.-K.; Choi, K.; et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 2017, 8, 15776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- MacFarlane, A.W.; Jillab, M.; Plimack, E.R.; Hudes, G.R.; Uzzo, R.G.; Litwin, S.; Dulaimi, E.; Al-Saleem, T.; Campbell, K.S. PD-1 Expression on Peripheral Blood Cells Increases with Stage in Renal Cell Carcinoma Patients and Is Rapidly Reduced after Surgical Tumor Resection. Cancer Immunol. Res. 2014, 2, 320–331. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Q.; Huang, M.; Wen, H.; Lin, R.; Zheng, M.; Qu, K.; Li, K.; Wei, H.; Xiao, W.; et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 2019, 70, 168–183. [Google Scholar] [CrossRef]
- Wiesmayr, S.; Webber, S.A.; Macedo, C.; Popescu, I.; Smith, L.; Luce, J.; Metes, D. Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur. J. Immunol. 2012, 42, 541–550. [Google Scholar] [CrossRef]
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinderlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018, 3, e96219. [Google Scholar] [CrossRef]
- Alvarez, M.; Simonetta, F.; Baker, J.; Pierini, A.; Wenokur, A.S.; Morrison, A.R.; Murphy, W.J.; Negrin, R.S. Regulation of murine NK cell exhaustion through the activation of the DNA damage repair pathway. JCI Insight 2019, 4, e127729. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.M.; Li, S.R.; Twelkmeyer, T.; Wang, W.H.; Zhang, S.Y.; Wang, S.-F.; Chen, J.-Z.; Jin, X.; Wu, Y.-Z.; et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 2019, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Wahl, S.M. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J. Exp. Med. 1998, 188, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Langdon, W.Y.; Zhang, J. Regulation of immune responses by E3 ubiquitin ligase Cbl-b. Cell. Immunol. 2019, 340, 103878. [Google Scholar] [CrossRef]
- Dang, C.V.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Hao, Y.; Chen, S.; Pang, B.; Zou, L.; Han, X.; Wang, W.; Liu, L.; Shen, M.; et al. Cbl-b inhibition promotes less differentiated phenotypes of T cells with enhanced cytokine production. Cell. Immunol. 2024, 403–404, 104863. [Google Scholar] [CrossRef]
- Clark, M.A.; Acharya, R.A.; Arico-Muendel, C.C.; Belyanskaya, S.L.; Benjamin, D.R.; Carlson, N.R.; Centrella, P.A.; Chiu, C.H.; Creaser, S.P.; Cuozzo, J.W.; et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 2009, 5, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Rountree, R.; Cohen, F.; Tenn-McClellan, A.; Borodovsky, A.; Gallotta, M.; Stokes, J.; Romo, J.G.; Karim, C.; Hansen, G.M.; Guiducci, C.; et al. Abstract 1595: Small molecule inhibition of the ubiquitin ligase CBL-B results in potent T and NK cell mediated anti-tumor response. Cancer Res. 2021, 81, 1595. [Google Scholar] [CrossRef]
- Sheik Amamuddy, O.; Veldman, W.; Manyumwa, C.; Khairallah, A.; Agajanian, S.; Oluyemi, O.; Verkhivker, G.M.; Tastan Bishop, Ö. Integrated Computational Approaches and Tools forAllosteric Drug Discovery. Int. J. Mol. Sci. 2020, 21, 847. [Google Scholar] [CrossRef]
- Kuai, J.; Bi, Y.; Qi, Y.; Conrady, D.; Govindaraj, R.; Hone, G.; Denny, R.A.; Carson, K.; Harriman, G.; Wang, F. 864 Identification of a novel allosteric oral Cbl-b inhibitor that augmented T cell response and enhanced NK cell killing in vitro and in vivo. J. Immunother. Cancer 2021, 9 (Suppl. S2), A905. [Google Scholar]
- Loibner, H.; Lametschwandtner, G.; Westritschnig, K.; Mutschlechner, O.; Dohnal, A.; Salzberg, M.O.; Triozzi, P.L. Adoptive cellular immunotherapy with APN401, autologous cbl-b silenced peripheral blood mononuclear cells: Data from a phase I study in patients with solid tumors. J. Clin. Oncol. 2018, 36, 3055. [Google Scholar] [CrossRef]
- Wang, H.Y.; Altman, Y.; Fang, D.; Elly, C.; Dai, Y.; Shao, Y.; Liu, Y.-C. Cbl Promotes Ubiquitination of the T Cell Receptor ζ through an Adaptor Function of Zap-70. J. Biol. Chem. 2001, 276, 26004–26011. [Google Scholar] [CrossRef]
- Lupher, M.L.; Reedquist, K.A.; Miyake, S.; Langdon, W.Y.; Band, H. A Novel Phosphotyrosine-binding Domain in the N-terminal Transforming Region of Cbl Interacts Directly and Selectively with ZAP-70 in T Cells. J. Biol. Chem. 1996, 271, 24063–24068. [Google Scholar] [CrossRef] [PubMed]
- Thien, C.B.F.; Langdon, W.Y. c-Cbl and Cbl-b ubiquitin ligases: Substrate diversity and the negative regulation of signalling responses. Biochem. J. 2005, 391, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Gu, H. Cbl and Cbl-b in T-cell regulation. Trends Immunol. 2002, 23, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C. Ubiquitin Ligases and the Immune Response. Annu. Rev. Immunol. 2004, 22, 81–127. [Google Scholar] [CrossRef]
- Smit, L.; Van Der Horst, G.; Borst, J. Sos, Vav, and C3G Participate in B Cell Receptor-induced Signaling Pathways and Differentially Associate with Shc-Grb2, Crk, and Crk-L Adaptors. J. Biol. Chem. 1996, 271, 8564–8569. [Google Scholar] [CrossRef]
- Uemura, N.; Griffin, J.D. The Adapter Protein Crkl Links Cbl to C3G after Integrin Ligation and Enhances Cell Migration. J. Biol. Chem. 1999, 274, 37525–37532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, P.R.; Isakov, N. Mechanisms of Cbl-Mediated Ubiquitination of Proteins in T and Natural Killer Cells and Effects on Immune Cell Functions. Life 2024, 14, 1592. https://doi.org/10.3390/life14121592
Nath PR, Isakov N. Mechanisms of Cbl-Mediated Ubiquitination of Proteins in T and Natural Killer Cells and Effects on Immune Cell Functions. Life. 2024; 14(12):1592. https://doi.org/10.3390/life14121592
Chicago/Turabian StyleNath, Pulak Ranjan, and Noah Isakov. 2024. "Mechanisms of Cbl-Mediated Ubiquitination of Proteins in T and Natural Killer Cells and Effects on Immune Cell Functions" Life 14, no. 12: 1592. https://doi.org/10.3390/life14121592
APA StyleNath, P. R., & Isakov, N. (2024). Mechanisms of Cbl-Mediated Ubiquitination of Proteins in T and Natural Killer Cells and Effects on Immune Cell Functions. Life, 14(12), 1592. https://doi.org/10.3390/life14121592






