Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness
Abstract
1. Introduction
1.1. Aquaporin Localization and Role in Physiology and Disease
| Aquaporin | Localization | Function | Role in Critical Illness | References |
|---|---|---|---|---|
| AQP0 | Eye lens, skin, male reproductive system | Acts as both a water channel and a structural protein in the lens. Maintains lens transparency and hydration | Impairment may contribute to cataracts and other ocular complications in critically ill patients | [8,48,49] |
| AQP1 | Kidneys, eye, brain, heart, lung, liver, skeletal muscle, blood cells, and various glands | Facilitates water reabsorption, crucial for urine concentration and fluid balance for maintaining plasma volume and cerebrospinal fluid (CSF) production | Altered expression affects fluid retention, edema, and organ function in critical illness | [8] |
| AQP2 | Kidneys (collecting ducts), ear, stomach, intestines, reproductive systems | Regulated by vasopressin; essential in water reabsorption, especially during dehydration | Dysregulation linked to electrolyte imbalances and water retention complications | [8,49,50] |
| AQP3 | Kidney, skin, immune cells, gastrointestinal tract (salivary and pancreatic secretion), lung, spinal cord | Transports water, glycerol, and small solutes, contributing to systemic fluid balance | [8] | |
| AQP4 | Brain, spinal cord, lung, kidney, stomach, skeletal muscle | Regulates osmotic balance in glial cells, critical in brain edema | Key role in cerebral edema management in brain injury and neurological illness | [8] |
| AQP5 | Epithelial tissues (salivary glands, airways), lung, immune cells, pancreas, skin | Facilitates fluid secretion, essential for saliva production and lung function | Altered levels associated with respiratory distress and secretion deficits | [8,51,52] |
| AQP6 | Kidney (intercalated cells), ear, female reproductive system | Transports water and anions, aiding acid-base balance | Potentially impacts acid-base imbalances and renal dysfunction in critical illness | [8,53,54] |
| AQP7 | Adipose tissue, kidney, gastrointestinal tract, heart | Transports glycerol and water, facilitating energy balance by releasing glycerol from adipose tissue for gluconeogenesis | Implicated in metabolic dysregulation and fluid imbalances under critical conditions | [8,55] |
| AQP8 | Liver, pancreas, kidney, gastrointestinal tract | Supports cellular osmoregulation, ammonia detoxification, particularly in the liver, and bile secretion | Dysfunction associated with liver and pancreatic issues in critical illness | [8,56] |
| AQP9 | Liver, immune cells, heart, spinal cord, spleen | Transports water, glycerol, urea; role in metabolic processes | Dysregulation affects immunity, metabolic balance, and organ perfusion | [8,57] |
| AQP10 | Intestine, ear, heart | Facilitates water and solute absorption in intestines | Linked to fluid absorption issues and nutrient transport under stress | [8,57,58] |
| AQP11 | Kidney, heart, gastrointestinal tract, reproductive systems | Associated with renal development and function | Deficiency may contribute to renal and cardiovascular complications | [8,57] |
| AQP12 | Pancreas, female reproductive system | Involved in digestive fluid secretion and pancreatic enzyme secretion regulation | Potential role in pancreatic insufficiency and digestive issues in critical care | [8,59] |
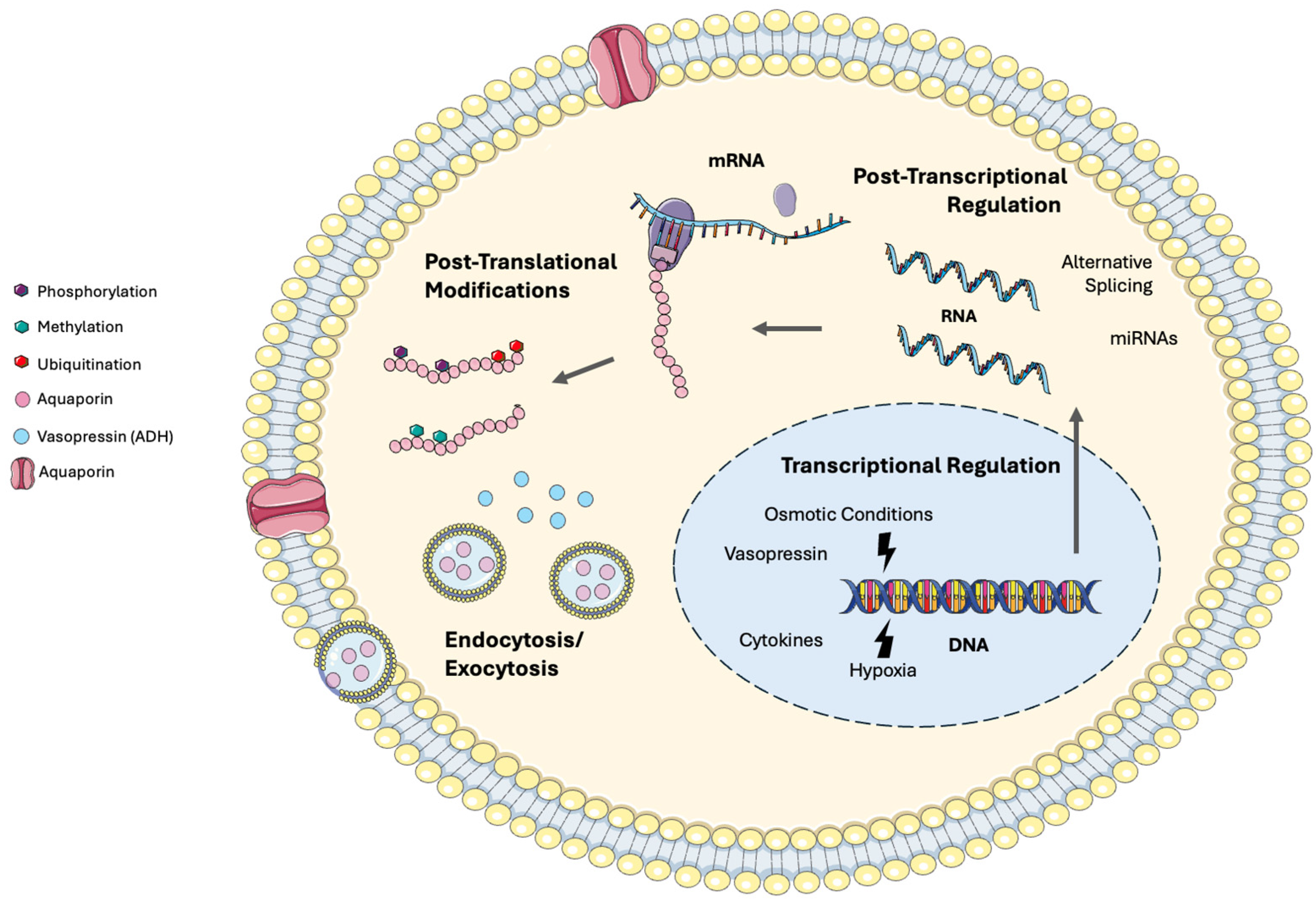
1.2. Aquaporin Structure and Function

1.3. Aquaporin Regulation
2. Aquaporins in Critical Illness
2.1. The Role of Aquaporins in ICU Patients with Sepsis
2.1.1. Sepsis
2.1.2. Aquaporin Expression in Human Immune Cells
2.1.3. Aquaporins and Sepsis
2.1.4. Proposed Mechanisms of Aquaporin Involvement in Sepsis
- (i)
- Increased Vascular Permeability and Edema
- (ii)
- Contribution to Organ Dysfunction
- (iii)
- Inflammatory Modulation
- (iv)
- Barrier Integrity
2.1.5. Aquaporins as Therapeutic Targets in Sepsis
2.1.6. Summary of Aquaporins in Sepsis C
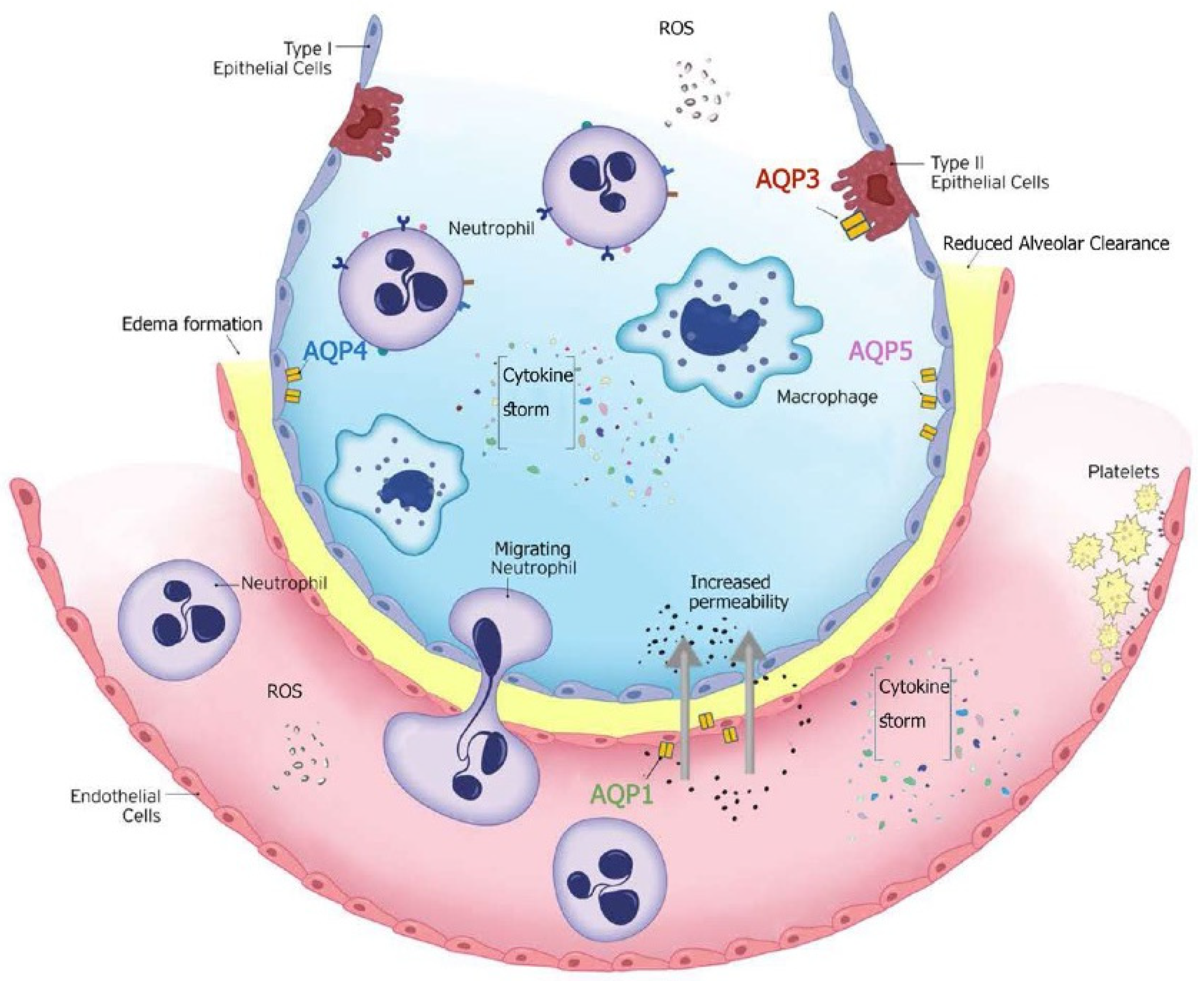
2.2. The Role of Aquaporins in ICU Patients with Clinical Acute Lung Inflammation—Acute Respiratory Distress Syndrome (ARDS)
2.2.1. Clinical Acute Lung Inflammation—Acute Respiratory Distress Syndrome (ARDS)
2.2.2. Overview of Aquaporins in the Lung
2.2.3. Aquaporins and ARDS
2.2.4. Proposed Mechanisms of Aquaporin Involvement in ARDS
- (i)
- Role in Pulmonary Edema Formation
- (ii)
- Role in Pulmonary Edema Clearance
- (iii)
- Role in the Inflammatory Response
- (iv)
- Oxidative Stress and Injury
2.2.5. Aquaporins as Therapeutic Targets in ARDS
2.2.6. Summary of Aquaporins in ARDS
2.3. The Role of Aquaporins in ICU Patients with Acute Kidney Injury
2.3.1. Acute Kidney Injury (AKI)
2.3.2. Localization and Physiology of AQPs in the Kidney
2.3.3. Aquaporins and AKI
2.3.4. Proposed Mechanisms of Aquaporin Involvement in AKI
- (i)
- Water Reabsorption and Urine Concentration
- (ii)
- Inflammation and Oxidative Stress
- (iii)
- Tubular Cell Apoptosis and Necrosis
- (iv)
- Renal Ischemia/Reperfusion-Induced AKI
- (v)
- Edema Formation
2.3.5. Aquaporins as Therapeutic Targets in AKI
2.3.6. Summary of Aquaporins in AKI
2.4. The Role of Aquaporins in ICU Patients with Acute Brain Injury
2.4.1. Acute Brain Injury (ABI)
2.4.2. Localization and Physiology of Aquaporins in the Brain
2.4.3. Aquaporins and ABI
2.4.4. Proposed Mechanisms of Aquaporin Involvement in ABI
- (i)
- Cerebral Edema Formation
- (ii)
- Blood–Brain Barrier Disruption
- (iii)
- Inflammatory Signaling Pathways
- (iv)
- Metabolic and Ionic Homeostasis
- (v)
- Genetic Susceptibility
2.4.5. Aquaporins as Therapeutic Targets in ABI
2.4.6. Summary of Aquaporins in ABI
2.5. The Role of Aquaporins in ICU Patients with Cardiovascular Diseases
Aquaporins and Cardiorenal Interactions
2.6. Molecular Mechanisms of AQP Regulation in Critical Illness
3. Discussion
3.1. Addressing Future Challenges
3.1.1. Available Products Targeting AQPs and Results in Preclinical Studies
3.1.2. Potential Challenges in Targeting AQPs Therapeutically
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Agre, P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. 1993, 265, F463–F476. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Raihan, T.; Ahmed, J.; Hakim, A.; Emon, T.H.; Chowdhury, P.A. Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases. Front. Genet. 2021, 12, 654865. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Tornroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef]
- Verkman, A.S. Physiological importance of aquaporin water channels. Ann. Med. 2002, 34, 192–200. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Mammalian aquaporins: Diverse physiological roles and potential clinical significance. Expert Rev. Mol. Med. 2008, 10, e13. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.M.; Stroka, K.M. The multifaceted role of aquaporins in physiological cell migration. Am. J. Physiol. Cell Physiol. 2023, 325, C208–C223. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Johnston, A.M.; Henning, J. Medical conditions requiring intensive care. J. R. Army Med. Corps 2009, 155, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Joynt, G.M.; Tat, W.W. Ten diseases you need to know if you want to be a critical care specialist in Hong Kong. Intensive Care Med. 2014, 40, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.M.; Verbalis, J.G. Disorders of body water homeostasis in critical illness. Endocrinol. Metab. Clin. N. Am. 2006, 35, 873–894, xi. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.E.; Mehta, R.L. Fluid balance issues in the critically ill patient. Contrib. Nephrol. 2010, 164, 69–78. [Google Scholar] [CrossRef]
- Bollaert, P.E.; Monnier, A.; Schneider, F.; Argaud, L.; Badie, J.; Charpentier, C.; Meziani, F.; Bemer, M.; Quenot, J.P.; Buzzi, M.; et al. Fluid balance control in critically ill patients: Results from POINCARE-2 stepped wedge cluster-randomized trial. Crit. Care 2023, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Robayo-Amortegui, H.; Quintero-Altare, A.; Florez-Navas, C.; Serna-Palacios, I.; Súarez-Saavedra, A.; Buitrago-Bernal, R.; Casallas-Barrera, J.O. Fluid dynamics of life: Exploring the physiology and importance of water in the critical illness. Front. Med. 2024, 11, 1368502. [Google Scholar] [CrossRef] [PubMed]
- Sartori, C.; Matthay, M.A.; Scherrer, U. Transepithelial sodium and water transport in the lung. Major player and novel therapeutic target in pulmonary edema. Adv. Exp. Med. Biol. 2001, 502, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Matthay, M.A. Bench-to-bedside review: The role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit. Care 2004, 8, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Robriquet, L.; Fang, X. Alveolar epithelium: Role in lung fluid balance and acute lung injury. Proc. Am. Thorac. Soc. 2005, 2, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, E.; Rahimi, S.; Nikmanzar, S.; Nazemi, S.; Naderi Taheri, M.; Alibolandi, Z.; Aschner, M.; Mirzaei, H.; Tamtaji, O.R. Aquaporin 4 in Traumatic Brain Injury: From Molecular Pathways to Therapeutic Target. Neurochem. Res. 2022, 47, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Das, M. Deciphering the role of aquaporins in metabolic diseases: A mini review. Am. J. Med. Sci. 2022, 364, 148–162. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, C.; Piao, Z.; Yuan, H.; Jiang, H.; Wei, H.; Zhou, Y.; Nan, G.; Ji, X. Cerebral edema after ischemic stroke: Pathophysiology and underlying mechanisms. Front. Neurosci. 2022, 16, 988283. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019, 20, 366. [Google Scholar] [CrossRef]
- Kuebler, W.M. Acute Respiratory Distress Syndrome: Biomarkers, Mechanisms, and Water Channels. Anesthesiology 2019, 130, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, K.; Tomita, H.; Hara, A. Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines 2023, 11, 2731. [Google Scholar] [CrossRef] [PubMed]
- Rahmel, T.; Rump, K.; Peters, J.; Adamzik, M. Aquaporin 5 -1364A/C Promoter Polymorphism Is Associated with Pulmonary Inflammation and Survival in Acute Respiratory Distress Syndrome. Anesthesiology 2019, 130, 404–413. [Google Scholar] [CrossRef]
- Szczygielski, J.; Kopańska, M.; Wysocka, A.; Oertel, J. Cerebral Microcirculation, Perivascular Unit, and Glymphatic System: Role of Aquaporin-4 as the Gatekeeper for Water Homeostasis. Front. Neurol. 2021, 12, 767470. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Manitsopoulos, N.; Kardara, M.; Maniatis, N.A.; Orfanos, S.E.; Kotanidou, A. Differential Expression of Aquaporins in Experimental Models of Acute Lung Injury. In Vivo 2017, 31, 885–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Z.; Zhan, J.; Cai, Q.; Xu, F.; Chai, R.; Lam, K.; Luan, Z.; Zhou, G.; Tsang, S.; Kipp, M.; et al. The Water Transport System in Astrocytes-Aquaporins. Cells 2022, 11, 2564. [Google Scholar] [CrossRef] [PubMed]
- Czyżewski, W.; Litak, J.; Sobstyl, J.; Mandat, T.; Torres, K.; Staśkiewicz, G. Aquaporins: Gatekeepers of Fluid Dynamics in Traumatic Brain Injury. Int. J. Mol. Sci. 2024, 25, 6553. [Google Scholar] [CrossRef] [PubMed]
- Messerer, D.A.C.; Schmidt, H.; Frick, M.; Huber-Lang, M. Ion and Water Transport in Neutrophil Granulocytes and Its Impairment during Sepsis. Int. J. Mol. Sci. 2021, 22, 1699. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Garra, S.; Calamita, G.; Soveral, G. The Multifaceted Role of Aquaporin-9 in Health and Its Potential as a Clinical Biomarker. Biomolecules 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Lotsios, N.S.; Keskinidou, C.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E.; Vassiliou, A.G. Aquaporin Expression and Regulation in Clinical and Experimental Sepsis. Int. J. Mol. Sci. 2023, 25, 487. [Google Scholar] [CrossRef]
- Kannan, A.; Panneerselvam, A.; Mariajoseph-Antony, L.F.; Loganathan, C.; Prahalathan, C. Role of Aquaporins in Spermatogenesis and Testicular Steroidogenesis. J. Membr. Biol. 2020, 253, 109–114. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Mentino, D.; De Marco, A.; Del Vecchio, C.; Garra, S.; Cazzato, G.; Foti, C.; Crovella, S.; Calamita, G. Aquaporins Are One of the Critical Factors in the Disruption of the Skin Barrier in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 4020. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am. J. Med. 2006, 119, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W. The role of the kidney in protecting the brain against cerebral edema and neuronal cell swelling. J. Pediatr. 2008, 152, 4–6. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Cruz-Rangel, S. Brain volume regulation: Osmolytes and aquaporin perspectives. Neuroscience 2010, 168, 871–884. [Google Scholar] [CrossRef]
- Nesic, O.; Guest, J.D.; Zivadinovic, D.; Narayana, P.A.; Herrera, J.J.; Grill, R.J.; Mokkapati, V.U.; Gelman, B.B.; Lee, J. Aquaporins in spinal cord injury: The janus face of aquaporin 4. Neuroscience 2010, 168, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz, A.C.; Soyer, B.; Payen, D. Water, water, everywhere: Sodium and water balance and the injured brain. Curr. Opin. Anaesthesiol. 2011, 24, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G. Acquired forms of central diabetes insipidus: Mechanisms of disease. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101449. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.L.; Lin, F.X.; Liu, N.; Chen, R.C. The role of aquaporin 4 (AQP4) in spinal cord injury. Biomed. Pharmacother. 2022, 145, 112384. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Jabeen, S.; Balmus, I.M.; Ciobica, A.; Burlui, V.; Romila, L.; Iordache, A. Exploring the Potential of Exosomal Biomarkers in Mild Traumatic Brain Injury and Post-Concussion Syndrome: A Systematic Review. J. Pers. Med. 2023, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.A.; Jonak, C.R.; Binder, D.K. The Role of Aquaporins in Spinal Cord Injury. Cells 2023, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, L.; Montana, A.; Frisoni, P.; D’Errico, S.; Neri, M. Application of Aquaporins as Markers in Forensic Pathology: A Systematic Review of the Literature. Int. J. Mol. Sci. 2024, 25, 2664. [Google Scholar] [CrossRef]
- Gonen, T.; Sliz, P.; Kistler, J.; Cheng, Y.; Walz, T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 2004, 429, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Yang, Y.; Liu, H.X.; Xiao, S.F.; Qiu, W.X.; Wang, J.X.; Zhao, C.C.; Gui, Y.H.; Liu, G.Z.; Peng, B.; et al. Inner Ear Arginine Vasopressin-Vasopressin Receptor 2-Aquaporin 2 Signaling Pathway Is Involved in the Induction of Motion Sickness. J. Pharmacol. Exp. Ther. 2020, 373, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Cao, R.; Zhang, X.Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Ren. Physiol. 2020, 318, F193–F203. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, H.; Yang, Z.; Du, T.; Zheng, Y.; Lu, Z.; Zhang, K.; Wei, D. AQP5 Is a Novel Prognostic Biomarker in Pancreatic Adenocarcinoma. Front. Oncol. 2022, 12, 890193. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, D.; Takeda, T.; Kakigi, A.; Okada, T.; Nishioka, R.; Kitano, H. Expression and immunolocalization of aquaporin-6 (Aqp6) in the rat inner ear. Acta Oto-Laryngol. 2008, 128, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Im, J.W.; Lee, C.Y.; Kim, D.H.; Bae, H.R. Differential Expressions of Aquaporin Subtypes in Female Reproductive Tract of Mice. Dev. Reprod. 2020, 24, 177–185. [Google Scholar] [CrossRef]
- Liu, J.; Xia, Z.; Peng, S.; Xia, J.; Xu, R.; Wang, X.; Li, F.; Zhu, W. The Important Role of Aquaglyceroporin 7 in Health and Disease. Biomolecules 2024, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Frøkiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Lodder, E.M.; Wilders, R. Aquaporin Channels in the Heart-Physiology and Pathophysiology. Int. J. Mol. Sci. 2019, 20, 2039. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, S.S.; Kim, Y.I.; Kim, S.H.; Yeo, S.G. A Review: Expression of Aquaporins in Otitis Media. Int. J. Mol. Sci. 2017, 18, 2164. [Google Scholar] [CrossRef]
- Ferré-Dolcet, L.; Rivera Del Alamo, M.M. Importance of Water Transport in Mammalian Female Reproductive Tract. Vet. Sci. 2023, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, N.; Siligan, C.; Akimov, S.A.; Pohl, P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015, 1, e1400083. [Google Scholar] [CrossRef]
- Heymann, J.B.; Agre, P.; Engel, A. Progress on the structure and function of aquaporin 1. J. Struct. Biol. 1998, 121, 191–206. [Google Scholar] [CrossRef]
- Strand, L.; Moe, S.E.; Solbu, T.T.; Vaadal, M.; Holen, T. Roles of aquaporin-4 isoforms and amino acids in square array assembly. Biochemistry 2009, 48, 5785–5793. [Google Scholar] [CrossRef]
- Ozu, M.; Galizia, L.; Acuña, C.; Amodeo, G. Aquaporins: More Than Functional Monomers in a Tetrameric Arrangement. Cells 2018, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Walz, T. The structure of aquaporins. Q. Rev. Biophys. 2006, 39, 361–396. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, J.; Carbrey, J.M.; Mukhopadhyay, R.; Agre, P.; Rosen, B.P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 2002, 99, 6053–6058. [Google Scholar] [CrossRef]
- Carbrey, J.M.; Agre, P. Discovery of the aquaporins and development of the field. In Aquaporins, Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 3–28. [Google Scholar] [CrossRef]
- Bienert, G.P.; Bienert, M.D.; Jahn, T.P.; Boutry, M.; Chaumont, F. Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 2011, 66, 306–317. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhattacharjee, H.; Rosen, B.P. Aquaglyceroporins: Generalized metalloid channels. Biochim. Biophys. Acta 2014, 1840, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Perez Di Giorgio, J.; Soto, G.; Alleva, K.; Jozefkowicz, C.; Amodeo, G.; Muschietti, J.P.; Ayub, N.D. Prediction of aquaporin function by integrating evolutionary and functional analyses. J. Membr. Biol. 2014, 247, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ishikawa, T.; Michiue, T.; Zhu, B.L.; Guan, D.W.; Maeda, H. Molecular pathology of brain matrix metalloproteases, claudin5, and aquaporins in forensic autopsy cases with special regard to methamphetamine intoxication. Int. J. Leg. Med. 2014, 128, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Chauvigné, F.; Stavang, J.A.; Belles, X.; Cerdà, J. Insect glycerol transporters evolved by functional co-option and gene replacement. Nat. Commun. 2015, 6, 7814. [Google Scholar] [CrossRef]
- Garneau, A.P.; Carpentier, G.A.; Marcoux, A.A.; Frenette-Cotton, R.; Simard, C.F.; Rémus-Borel, W.; Caron, L.; Jacob-Wagner, M.; Noël, M.; Powell, J.J.; et al. Aquaporins Mediate Silicon Transport in Humans. PLoS ONE 2015, 10, e0136149. [Google Scholar] [CrossRef]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca(2+) and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Jin, L.; Zhang, L.; Cui, X.; Zhang, Z.; Lu, Y.; Yu, L.; Ma, T.; Zhang, H. Aquaporin-8 transports hydrogen peroxide to regulate granulosa cell autophagy. Front. Cell Dev. Biol. 2022, 10, 897666. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Cerdà, J. Evolution and functional diversity of aquaporins. Biol. Bull. 2015, 229, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Litman, T.; Søgaard, R.; Zeuthen, T. Ammonia and urea permeability of mammalian aquaporins. In Aquaporins, Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 327–358. [Google Scholar] [CrossRef]
- Bhattacharjee, H.; Carbrey, J.; Rosen, B.P.; Mukhopadhyay, R. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem. Biophys. Res. Commun. 2004, 322, 836–841. [Google Scholar] [CrossRef]
- Rosen, B.P.; Tamás, M.J. Arsenic transport in prokaryotes and eukaryotic microbes. Adv. Exp. Med. Biol. 2010, 679, 47–55. [Google Scholar] [CrossRef]
- Herrera, M.; Hong, N.J.; Garvin, J.L. Aquaporin-1 transports NO across cell membranes. Hypertension 2006, 48, 157–164. [Google Scholar] [CrossRef]
- Zwiazek, J.J.; Xu, H.; Tan, X.; Navarro-Ródenas, A.; Morte, A. Significance of oxygen transport through aquaporins. Sci. Rep. 2017, 7, 40411. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef] [PubMed]
- Marples, D.; Knepper, M.A.; Christensen, E.I.; Nielsen, S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am. J. Physiol. 1995, 269, C655–C664. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Chou, C.L.; Marples, D.; Christensen, E.I.; Kishore, B.K.; Knepper, M.A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. USA 1995, 92, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef]
- da Silva, I.V.; Cardoso, C.; Martínez-Banaclocha, H.; Casini, A.; Pelegrín, P.; Soveral, G. Aquaporin-3 is involved in NLRP3-inflammasome activation contributing to the setting of inflammatory response. Cell. Mol. Life Sci. 2021, 78, 3073–3085. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Mlinarić, M.; Lourenço, A.R.; Pérez-Garcia, O.; Čipak Gašparović, A.; Soveral, G. Peroxiporins and Oxidative Stress: Promising Targets to Tackle Inflammation and Cancer. Int. J. Mol. Sci. 2024, 25, 8381. [Google Scholar] [CrossRef] [PubMed]
- Schuoler, C.; Haider, T.J.; Leuenberger, C.; Vogel, J.; Ostergaard, L.; Kwapiszewska, G.; Kohler, M.; Gassmann, M.; Huber, L.C.; Brock, M. Aquaporin 1 controls the functional phenotype of pulmonary smooth muscle cells in hypoxia-induced pulmonary hypertension. Basic Res. Cardiol. 2017, 112, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, M.; Jiang, H.; Wang, Q.; He, S.; Chen, J.; Wang, C. Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci. 2018, 193, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Lomora, M.; Kym, U.; Palivan, C.; Holland-Cunz, S.G.; Gros, S.J. AQP1 Is Up-Regulated by Hypoxia and Leads to Increased Cell Water Permeability, Motility, and Migration in Neuroblastoma. Front. Cell Dev. Biol. 2021, 9, 605272. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; da Silva, I.V.; Rodrigues, C.M.P.; Castro, R.E.; Soveral, G. The Emerging Role of microRNAs in Aquaporin Regulation. Front. Chem. 2018, 6, 238. [Google Scholar] [CrossRef]
- Fushimi, K.; Sasaki, S.; Marumo, F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 1997, 272, 14800–14804. [Google Scholar] [CrossRef] [PubMed]
- Leitch, V.; Agre, P.; King, L.S. Altered ubiquitination and stability of aquaporin-1 in hypertonic stress. Proc. Natl. Acad. Sci. USA 2001, 98, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.; van Raak, M.; Breton, S.; Pastor-Soler, N.; Bouley, R.; van der Sluijs, P.; Brown, D.; Deen, P.M. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J. Biol. Chem. 2003, 278, 1101–1107. [Google Scholar] [CrossRef]
- Kamsteeg, E.J.; Hendriks, G.; Boone, M.; Konings, I.B.; Oorschot, V.; van der Sluijs, P.; Klumperman, J.; Deen, P.M. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. USA 2006, 103, 18344–18349. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, J.D.; Nielsen, J.; Yu, M.J.; Pisitkun, T.; Schleicher, S.M.; Nielsen, S.; Knepper, M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am. J. Physiol. Ren. Physiol. 2007, 292, F691–F700. [Google Scholar] [CrossRef]
- Lu, H.J.; Matsuzaki, T.; Bouley, R.; Hasler, U.; Qin, Q.H.; Brown, D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2008, 295, F290–F294. [Google Scholar] [CrossRef]
- Tamma, G.; Robben, J.H.; Trimpert, C.; Boone, M.; Deen, P.M. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am. J. Physiol. Cell Physiol. 2011, 300, C636–C646. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Unterberg, M.; Dahlke, A.; Nowak, H.; Koos, B.; Bergmann, L.; Siffert, W.; Schafer, S.T.; Peters, J.; Adamzik, M.; et al. DNA methylation of a NF-kappaB binding site in the aquaporin 5 promoter impacts on mortality in sepsis. Sci. Rep. 2019, 9, 18511. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Miranda, C.A.; Knepper, M.A. Vasopressin and the regulation of aquaporin-2. Clin. Exp. Nephrol. 2013, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Unger, L.; Abir-Awan, M.; Saadallah, A.; Halsey, A.; Balklava, Z.; Conner, M.; Tornroth-Horsefield, S.; Greenhill, S.D.; Conner, A.; et al. Molecular mechanisms governing aquaporin relocalisation. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183853. [Google Scholar] [CrossRef]
- Kreida, S.; Roche, J.V.; Olsson, C.; Linse, S.; Törnroth-Horsefield, S. Protein-protein interactions in AQP regulation—Biophysical characterization of AQP0-CaM and AQP2-LIP5 complex formation. Faraday Discuss. 2018, 209, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Moon, C.; Rousseau, R.; Soria, J.C.; Hoque, M.O.; Lee, J.; Jang, S.J.; Trink, B.; Sidransky, D.; Mao, L. Aquaporin expression in human lymphocytes and dendritic cells. Am. J. Hematol. 2004, 75, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Maniatis, N.A.; Orfanos, S.E.; Mastora, Z.; Jahaj, E.; Paparountas, T.; Armaganidis, A.; Roussos, C.; Aidinis, V.; Kotanidou, A. Induced expression and functional effects of aquaporin-1 in human leukocytes in sepsis. Crit. Care 2013, 17, R199. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Soveral, G. Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development. Int. J. Mol. Sci. 2021, 22, 1845. [Google Scholar] [CrossRef] [PubMed]
- de Baey, A.; Lanzavecchia, A. The role of aquaporins in dendritic cell macropinocytosis. J. Exp. Med. 2000, 191, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef]
- Rump, K.; Rahmel, T.; Rustige, A.M.; Unterberg, M.; Nowak, H.; Koos, B.; Schenker, P.; Viebahn, R.; Adamzik, M.; Bergmann, L. The Aquaporin 3 Promoter Polymorphism -1431 A/G is Associated with Acute Graft Rejection and Cytomegalovirus Infection in Kidney Recipients Due to Altered Immune Cell Migration. Cells 2020, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Unterberg, M.; Bergmann, L.; Bankfalvi, A.; Menon, A.; Schäfer, S.; Scherag, A.; Bazzi, Z.; Siffert, W.; Peters, J.; et al. AQP5-1364A/C polymorphism and the AQP5 expression influence sepsis survival and immune cell migration: A prospective laboratory and patient study. J. Transl. Med. 2016, 14, 321. [Google Scholar] [CrossRef]
- Talwar, S.; Munson, P.J.; Barb, J.; Fiuza, C.; Cintron, A.P.; Logun, C.; Tropea, M.; Khan, S.; Reda, D.; Shelhamer, J.H.; et al. Gene expression profiles of peripheral blood leukocytes after endotoxin challenge in humans. Physiol. Genom. 2006, 25, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Tanaka, Y.; Marumo, F.; Sasaki, S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem. Biophys. Res. Commun. 1998, 244, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Ogura, H.; Koh, T.; Shimazu, T.; Sugimoto, H. Enhanced expression of aquaporin 9 in activated polymorphonuclear leukocytes in patients with systemic inflammatory response syndrome. Shock 2014, 42, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Karlsson, T.; Vikström, E. Pseudomonas aeruginosa lasI/rhlI quorum sensing genes promote phagocytosis and aquaporin 9 redistribution to the leading and trailing regions in macrophages. Front. Microbiol. 2015, 6, 915. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A. Clinical Assays in Sepsis: Prognosis, Diagnosis, Outcomes, and the Genetic Basis of Sepsis. In Sepsis; Chapter 6; Vijay, K., Ed.; Intech Open: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Loitto, V.M.; Forslund, T.; Sundqvist, T.; Magnusson, K.E.; Gustafsson, M. Neutrophil leukocyte motility requires directed water influx. J. Leukoc. Biol. 2002, 71, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Thon, P.; Rahmel, T.; Ziehe, D.; Palmowski, L.; Marko, B.; Nowak, H.; Wolf, A.; Witowski, A.; Orlowski, J.; Ellger, B.; et al. AQP3 and AQP9-Contrary Players in Sepsis? Int. J. Mol. Sci. 2024, 25, 1209. [Google Scholar] [CrossRef] [PubMed]
- Ziehe, D.; Marko, B.; Thon, P.; Rahmel, T.; Palmowski, L.; Nowak, H.; von Busch, A.; Wolf, A.; Witowski, A.; Vonheder, J.; et al. The Aquaporin 3 Polymorphism (rs17553719) Is Associated with Sepsis Survival and Correlated with IL-33 Secretion. Int. J. Mol. Sci. 2024, 25, 1400. [Google Scholar] [CrossRef] [PubMed]
- Benga, G. The first discovered water channel protein, later called aquaporin 1: Molecular characteristics, functions and medical implications. Mol. Asp. Med. 2012, 33, 518–534. [Google Scholar] [CrossRef]
- Monzani, E.; Bazzotti, R.; Perego, C.; La Porta, C.A. AQP1 is not only a water channel: It contributes to cell migration through Lin7/beta-catenin. PLoS ONE 2009, 4, e6167. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, J.; Wang, Z.; Zong, H.; Zhang, L.; Zhang, R.; Sun, L. LncRNA H19 functions as an Aquaporin 1 competitive endogenous RNA to regulate microRNA-874 expression in LPS sepsis. Biomed. Pharmacother. 2018, 105, 1183–1191. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Zhu, M.; Cheng, H. Effects and early diagnostic value of lncRNA H19 on sepsis-induced acute lung injury. Exp. Ther. Med. 2022, 23, 279. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, H.; Gao, M.; Ma, N.; Sun, R. Long non-coding RNA CASC2 improved acute lung injury by regulating miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell Biosci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, J.; Wei, Q.; Wang, H.; Zhao, C.; Hu, C.; Han, Y.; Hui, Z.; Yang, L.; Dai, Q.; et al. Potential of circulating lncRNA CASC2 as a biomarker in reflecting the inflammatory cytokines, multi-organ dysfunction, disease severity, and mortality in sepsis patients. J. Clin. Lab. Anal. 2022, 36, e24569. [Google Scholar] [CrossRef]
- Adamzik, M.; Frey, U.H.; Bitzer, K.; Jakob, H.; Baba, H.A.; Schmieder, R.E.; Schneider, M.P.; Heusch, G.; Peters, J.; Siffert, W. A novel-1364A/C aquaporin 5 gene promoter polymorphism influences the responses to salt loading of the renin-angiotensin-aldosterone system and of blood pressure in young healthy men. Basic Res. Cardiol. 2008, 103, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Adamzik, M.; Frey, U.H.; Möhlenkamp, S.; Scherag, A.; Waydhas, C.; Marggraf, G.; Dammann, M.; Steinmann, J.; Siffert, W.; Peters, J. Aquaporin 5 Gene Promoter—1364A/C Polymorphism Associated with 30-day Survival in Severe Sepsis. Anesthesiology 2011, 114, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Nomura, J.; Hisatsune, A.; Miyata, T.; Isohama, Y. The role of CpG methylation in cell type-specific expression of the aquaporin-5 gene. Biochem. Biophys. Res. Commun. 2007, 353, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Spellenberg, T.; von Busch, A.; Wolf, A.; Ziehe, D.; Thon, P.; Rahmel, T.; Adamzik, M.; Koos, B.; Unterberg, M. AQP5-1364A/C Polymorphism Affects AQP5 Promoter Methylation. Int. J. Mol. Sci. 2022, 23, 11813. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wu, D.; Shi, S.; Wang, L. miR-34b-5p promotes renal cell inflammation and apoptosis by inhibiting aquaporin-2 in sepsis-induced acute kidney injury. Ren. Fail. 2021, 43, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Role of aquaporin water channels in kidney and lung. Am. J. Med. Sci. 1998, 316, 310–320. [Google Scholar] [CrossRef]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Role of aquaporins in lung liquid physiology. Respir. Physiol. Neurobiol. 2007, 159, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Reggio, E. Aquaporin and vascular diseases. Curr. Neuropharmacol. 2010, 8, 105–111. [Google Scholar] [CrossRef] [PubMed]
- González-Marrero, I.; Hernández-Abad, L.G.; González-Gómez, M.; Soto-Viera, M.; Carmona-Calero, E.M.; Castañeyra-Ruiz, L.; Castañeyra-Perdomo, A. Altered Expression of AQP1 and AQP4 in Brain Barriers and Cerebrospinal Fluid May Affect Cerebral Water Balance during Chronic Hypertension. Int. J. Mol. Sci. 2022, 23, 12277. [Google Scholar] [CrossRef]
- Sisto, M.; Ribatti, D.; Lisi, S. Aquaporin water channels: New perspectives on the potential role in inflammation. Adv. Protein Chem. Struct. Biol. 2019, 116, 311–345. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Frøkiær, J.; Nielsen, S. Regulation of aquaporin-2 in the kidney: A molecular mechanism of body-water homeostasis. Kidney Res. Clin. Pract. 2013, 32, 96–102. [Google Scholar] [CrossRef]
- Grinevich, V.; Knepper, M.A.; Verbalis, J.; Reyes, I.; Aguilera, G. Acute endotoxemia in rats induces down-regulation of V2 vasopressin receptors and aquaporin-2 content in the kidney medulla. Kidney Int. 2004, 65, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Summer, S.N.; Falk, S.; Wang, W.; Ljubanovic, D.; Schrier, R.W. Role of AQP1 in endotoxemia-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 294, F1473–F1480. [Google Scholar] [CrossRef]
- Olesen, E.T.; de Seigneux, S.; Wang, G.; Lutken, S.C.; Frokiaer, J.; Kwon, T.H.; Nielsen, S. Rapid and segmental specific dysregulation of AQP2, S256-pAQP2 and renal sodium transporters in rats with LPS-induced endotoxaemia. Nephrol. Dial. Transplant. 2009, 24, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.E.; Sanches, T.R.; Volpini, R.A.; Shimizu, M.H.; Kuriki, P.S.; Camara, N.O.; Seguro, A.C.; Andrade, L. Effects of continuous erythropoietin receptor activator in sepsis-induced acute kidney injury and multi-organ dysfunction. PLoS ONE 2012, 7, e29893. [Google Scholar] [CrossRef]
- Ozden, E.S.; Asci, H.; Buyukbayram, H.I.; Sevuk, M.A.; Imeci, O.B.; Dogan, H.K.; Ozmen, O. Dexpanthenol protects against lipopolysaccharide-induced acute kidney injury by restoring aquaporin-2 levels via regulation of the silent information regulator 1 signaling pathway. Korean J. Anesthesiol. 2023, 76, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Adamzik, M. Aquaporins in sepsis- an update. Front. Immunol. 2024, 15, 1495206. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.; Kumai, Y.; Perry, S.F. The role of aquaporin and tight junction proteins in the regulation of water movement in larval zebrafish (Danio rerio). PLoS ONE 2013, 8, e70764. [Google Scholar] [CrossRef]
- Tesse, A.; Gena, P.; Rützler, M.; Calamita, G. Ablation of Aquaporin-9 Ameliorates the Systemic Inflammatory Response of LPS-Induced Endotoxic Shock in Mouse. Cells 2021, 10, 435. [Google Scholar] [CrossRef]
- Rump, K.; Koos, B.; Ziehe, D.; Thon, P.; Rahmel, T.; Palmowski, L.; Marko, B.; Wolf, A.; Witowski, A.; Bazzi, Z.; et al. Methazolamide Reduces the AQP5 mRNA Expression and Immune Cell Migration-A New Potential Drug in Sepsis Therapy? Int. J. Mol. Sci. 2024, 25, 610. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Galán Cobo, A.; Echevarría, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting aquaporin function: Potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, X.; Yu, N.; Li, Y.; Kan, J. Long non-coding RNA FGD5-AS1/microRNA-133a-3p upregulates aquaporin 1 to decrease the inflammatory response in LPS-induced sepsis. Mol. Med. Rep. 2021, 24, 784. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yang, G.Y. Aquaporin-4: A Potential Therapeutic Target for Cerebral Edema. Int. J. Mol. Sci. 2016, 17, 1413. [Google Scholar] [CrossRef] [PubMed]
- Abo El Gheit, R.E.; Atef, M.M.; Badawi, G.A.; Elwan, W.M.; Alshenawy, H.A.; Emam, M.N. Role of serine protease inhibitor, ulinastatin, in rat model of hepatic encephalopathy: Aquaporin 4 molecular targeting and therapeutic implication. J. Physiol. Biochem. 2020, 76, 573–586. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Huppert, L.A.; Matthay, M.A.; Ware, L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Kotanidou, A.; Dimopoulou, I.; Orfanos, S.E. Endothelial Damage in Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 8793. [Google Scholar] [CrossRef] [PubMed]
- Keskinidou, C.; Vassiliou, A.G.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Mechanistic Understanding of Lung Inflammation: Recent Advances and Emerging Techniques. J. Inflamm. Res. 2022, 15, 3501–3546. [Google Scholar] [CrossRef]
- Wittekindt, O.H.; Dietl, P. Aquaporins in the lung. Pflug. Arch. 2019, 471, 519–532. [Google Scholar] [CrossRef]
- Matthay, M.A.; Folkesson, H.G.; Clerici, C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002, 82, 569–600. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Fukuda, N.; Song, Y.; Ma, T.; Matthay, M.A.; Verkman, A.S. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Investig. 1999, 103, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fukuda, N.; Song, Y.; Matthay, M.A.; Verkman, A.S. Lung fluid transport in aquaporin-5 knockout mice. J. Clin. Investig. 2000, 105, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fukuda, N.; Bai, C.; Ma, T.; Matthay, M.A.; Verkman, A.S. Role of aquaporins in alveolar fluid clearance in neonatal and adult lung, and in oedema formation following acute lung injury: Studies in transgenic aquaporin null mice. J. Physiol. 2000, 525, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Knock-out models reveal new aquaporin functions. In Aquaporins, Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 359–381. [Google Scholar] [CrossRef]
- Meli, R.; Pirozzi, C.; Pelagalli, A. New Perspectives on the Potential Role of Aquaporins (AQPs) in the Physiology of Inflammation. Front. Physiol. 2018, 9, 101. [Google Scholar] [CrossRef]
- Lai, K.N.; Leung, J.C.; Metz, C.N.; Lai, F.M.; Bucala, R.; Lan, H.Y. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J. Pathol. 2003, 199, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, N.; Bayraktar, M.; Ozturk, A.; Ibrahim, B. Evaluation of the Relationship Between Aquaporin-1, Hepcidin, Zinc, Copper, and İron Levels and Oxidative Stress in the Serum of Critically Ill Patients with COVID-19. Biol. Trace Elem. Res. 2022, 200, 5013–5021. [Google Scholar] [CrossRef]
- Rahmel, T.; Nowak, H.; Rump, K.; Siffert, W.; Peters, J.; Adamzik, M. The aquaporin 5 -1364A/C promoter polymorphism impacts on resolution of acute kidney injury in pneumonia evoked ARDS. PLoS ONE 2018, 13, e0208582. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, J.; Hu, W.; Ye, C.; Ren, P.; Wang, Y.; Li, X. Whole Transcriptomic Analysis of Key Genes and Signaling Pathways in Endogenous ARDS. Dis. Markers 2022, 2022, 1614208. [Google Scholar] [CrossRef]
- Liu, F.; Hu, S.; Zhao, N.; Shao, Q.; Li, Y.; Jiang, R.; Chen, J.; Peng, W.; Qian, K. LncRNA-5657 silencing alleviates sepsis-induced lung injury by suppressing the expression of spinster homology protein 2. Int. Immunopharmacol. 2020, 88, 106875. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.A.; Qiu, X.L.; Wang, X.Z.; Zhao, N.; Qian, K.J. Reducing LncRNA-5657 expression inhibits the brain inflammatory reaction in septic rats. Neural Regen. Res. 2021, 16, 1288–1293. [Google Scholar] [CrossRef]
- Zhu, D.D.; Huang, Y.L.; Guo, S.Y.; Li, N.; Yang, X.W.; Sui, A.R.; Wu, Q.; Zhang, Y.; Kong, Y.; Li, Q.F.; et al. AQP4 Aggravates Cognitive Impairment in Sepsis-Associated Encephalopathy through Inhibiting Na(v) 1.6-Mediated Astrocyte Autophagy. Adv. Sci. 2023, 10, e2205862. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Pei, L.; Bai, T.; Wang, J. Down-regulation of microRNA-126-5p contributes to overexpression of VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol. Lett. 2016, 38, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, Y.; Xu, W. miR-126-5p expression in the plasma of patients with sepsis-induced acute lung injury and its correlation with inflammation and immune function. Clin. Respir. J. 2023, 17, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, D.; Wang, G.; Dong, C.; Wang, X.; Bai, C. Expression of AQP-1, AQP-3, AQP-4 and AQP-5 in pulmonary tissues of mice with endotoxin-induced acute lung injury. Acad. J. Second. Mil. Med. Univ. 2008, 29, 131–135. [Google Scholar] [CrossRef]
- Rump, K.; Brendt, P.; Frey, U.H.; Schäfer, S.T.; Siffert, W.; Peters, J.; Adamzik, M. Aquaporin 1 and 5 expression evoked by the β2 adrenoreceptor agonist terbutaline and lipopolysaccharide in mice and in the human monocytic cell line THP-1 is differentially regulated. Shock 2013, 40, 430–436. [Google Scholar] [CrossRef]
- Hasan, B.; Li, F.-S.; Siyit, A.; Tuyghun, E.; Luo, J.-H.; Upur, H.; Ablimit, A. Expression of aquaporins in the lungs of mice with acute injury caused by LPS treatment. Respir. Physiol. Neurobiol. 2014, 200, 40–45. [Google Scholar] [CrossRef]
- Hong-Min, F.; Chun-Rong, H.; Rui, Z.; Li-Na, S.; Ya-Jun, W.; Li, L. CGRP 8-37 enhances lipopolysaccharide-induced acute lung injury and regulating aquaporin 1 and 5 expressions in rats. J. Physiol. Biochem. 2016, 73, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Liu, L.; Wang, N.; Wang, W.; Jiang, J.; Zhang, J. Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs. J. Surg. Res. 2016, 202, 291–298. [Google Scholar] [CrossRef]
- Li, B.; Liu, C.; Tang, K.; Dong, X.; Xue, L.; Su, G.; Zhang, W.; Jin, Y. Aquaporin-1 attenuates macrophage-mediated inflammatory responses by inhibiting p38 mitogen-activated protein kinase activation in lipopolysaccharide-induced acute kidney injury. Inflamm. Res. 2019, 68, 1035–1047. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Xia, X.; Zhang, Y. Estradiol attenuates LPS-induced acute lung injury via induction of aquaporins AQP1 and AQP5. Eur. J. Inflamm. 2021, 19, 20587392211049197. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhou, X.; Xia, X.; Teng, W.; Sheng, L.; Ding, J. Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats. Open Life Sci. 2023, 18, 20220560. [Google Scholar] [CrossRef] [PubMed]
- Nieman, G.F.; Gatto, L.A.; Andrews, P.; Satalin, J.; Camporota, L.; Daxon, B.; Blair, S.J.; Al-Khalisy, H.; Madden, M.; Kollisch-Singule, M.; et al. Prevention and treatment of acute lung injury with time-controlled adaptive ventilation: Physiologically informed modification of airway pressure release ventilation. Ann. Intensive Care 2020, 10, 3. [Google Scholar] [CrossRef]
- Yamashita, A.; Ito, Y.; Osada, M.; Matsuda, H.; Hosono, K.; Tsujikawa, K.; Okamoto, H.; Amano, H. RAMP1 Signaling Mitigates Acute Lung Injury by Distinctively Regulating Alveolar and Monocyte-Derived Macrophages. Int. J. Mol. Sci. 2024, 25, 10107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Zhang, Y.; Peng, P.; Li, J.; Xin, X. miR-96 and miR-330 overexpressed and targeted AQP5 in lipopolysaccharide-induced rat lung damage of disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 2014, 25, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, L.; Dong, L. Tanshinol upregulates the expression of aquaporin 5 in lung tissue of rats with sepsis. Oncol. Lett. 2018, 16, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Ba, F.; Zhou, X.; Zhang, Y.; Wu, C.; Xu, S.; Wu, L.; Li, J.; Yin, Y.; Gu, X. Lipoxin A4 ameliorates alveolar fluid clearance disturbance in lipopolysaccharide-induced lung injury via aquaporin 5 and MAPK signaling pathway. J. Thorac. Dis. 2019, 11, 3599–3608. [Google Scholar] [CrossRef]
- Wang, J.J.; Kong, H.; Xu, J.; Wang, Y.L.; Wang, H.; Xie, W.P. Fasudil alleviates LPS-induced lung injury by restoring aquaporin 5 expression and inhibiting inflammation in lungs. J. Biomed. Res. 2019, 33, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Villandre, J.; White, V.; Lear, T.B.; Chen, Y.; Tuncer, F.; Vaiz, E.; Tuncer, B.; Lockwood, K.; Camarco, D.; Liu, Y.; et al. A Repurposed Drug Screen for Compounds Regulating Aquaporin 5 Stability in Lung Epithelial Cells. Front. Pharmacol. 2022, 13, 828643. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Bhatt, M.; Pannu, N.; Tonelli, M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 2020, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Pickkers, P.; Darmon, M.; Hoste, E.; Joannidis, M.; Legrand, M.; Ostermann, M.; Prowle, J.R.; Schneider, A.; Schetz, M. Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intensive Care Med. 2021, 47, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.L.; Knepper, M.A.; Hoek, A.N.; Brown, D.; Yang, B.; Ma, T.; Verkman, A.S. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J. Clin. Investig. 1999, 103, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, T.; Verkman, A.S. Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3. J. Biol. Chem. 2001, 276, 624–628. [Google Scholar] [CrossRef]
- Verkman, A.S.; Yang, B. Aquaporin gene delivery to kidney. Kidney Int. 2002, 61, S120–S124. [Google Scholar] [CrossRef]
- Ren, H.; Yang, B.; Molina, P.A.; Sands, J.M.; Klein, J.D. NSAIDs Alter Phosphorylated Forms of AQP2 in the Inner Medullary Tip. PLoS ONE 2015, 10, e0141714. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Huang, M.; Su, L.; Xie, D.; Mamuya, F.A.; Ham, O.; Tsuji, K.; Paunescu, T.G.; Yang, B.; Lu, H.A.J. Manganese promotes intracellular accumulation of AQP2 via modulating F-actin polymerization and reduces urinary concentration in mice. Am. J. Physiol. Ren. Physiol. 2018, 314, F306–F316. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yang, B.; Ruiz, J.A.; Efe, O.; Ilori, T.O.; Sands, J.M.; Klein, J.D. Phosphatase inhibition increases AQP2 accumulation in the rat IMCD apical plasma membrane. Am. J. Physiol. Ren. Physiol. 2016, 311, F1189–F1197. [Google Scholar] [CrossRef]
- Kavanagh, C.; Uy, N.S. Nephrogenic Diabetes Insipidus. Pediatr. Clin. N. Am. 2019, 66, 227–234. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 7454. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle 2011, 10, 1548–1556. [Google Scholar] [CrossRef]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress Regulates Aquaporin-8 Permeability to Impact Cell Growth and Survival. Antioxid. Redox Signal. 2016, 24, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Watari, M.; Saito, T.; Morishita, Y.; Ishibashi, K. Enhanced Autophagy in Polycystic Kidneys of AQP11 Null Mice. Int. J. Mol. Sci. 2016, 17, 1993. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Saito, T.; Fukagawa, A.; Higashiyama, M.; Nakamura, T.; Kusaka, I.; Nagasaka, S.; Honda, K.; Saito, T. Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antidiuresis in hyponatremia in elderly subjects. J. Clin. Endocrinol. Metab. 2001, 86, 1665–1671. [Google Scholar] [CrossRef]
- Ivarsen, P.; Frokiaer, J.; Aagaard, N.K.; Hansen, E.F.; Bendtsen, F.; Nielsen, S.; Vilstrup, H. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Gut 2003, 52, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Buemi, M.; D’Anna, R.; Di Pasquale, G.; Floccari, F.; Ruello, A.; Aloisi, C.; Leonardi, I.; Frisina, N.; Corica, F. Urinary excretion of aquaporin-2 water channel during pregnancy. Cell Physiol. Biochem. 2001, 11, 203–208. [Google Scholar] [CrossRef]
- Rossi, L.; Nicoletti, M.C.; Carmosino, M.; Mastrofrancesco, L.; Di Franco, A.; Indrio, F.; Lella, R.; Laviola, L.; Giorgino, F.; Svelto, M.; et al. Urinary Excretion of Kidney Aquaporins as Possible Diagnostic Biomarker of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 4360357. [Google Scholar] [CrossRef] [PubMed]
- Bräsen, J.H.; Mederacke, Y.S.; Schmitz, J.; Diahovets, K.; Khalifa, A.; Hartleben, B.; Person, F.; Wiech, T.; Steenbergen, E.; Großhennig, A.; et al. Cholemic Nephropathy Causes Acute Kidney Injury and Is Accompanied by Loss of Aquaporin 2 in Collecting Ducts. Hepatology 2019, 69, 2107–2119. [Google Scholar] [CrossRef]
- Chan, M.J.; Chen, Y.C.; Fan, P.C.; Lee, C.C.; Kou, G.; Chang, C.H. Predictive Value of Urinary Aquaporin 2 for Acute Kidney Injury in Patients with Acute Decompensated Heart Failure. Biomedicines 2022, 10, 613. [Google Scholar] [CrossRef]
- Suh, S.H.; Lee, K.E.; Kim, I.J.; Kim, O.; Kim, C.S.; Choi, J.S.; Choi, H.I.; Bae, E.H.; Ma, S.K.; Lee, J.U.; et al. Alpha-lipoic acid attenuates lipopolysaccharide-induced kidney injury. Clin. Exp. Nephrol. 2015, 19, 82–91. [Google Scholar] [CrossRef]
- Höcherl, K.; Schmidt, C.; Kurt, B.; Bucher, M. Inhibition of NF-kappaB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am. J. Physiol. Ren. Physiol. 2010, 298, F196–F204. [Google Scholar] [CrossRef]
- Lv, W.; Xue, L.; Liang, L.; Liu, D.; Li, C.; Liao, J.; Jin, Y. Endotoxin induced acute kidney injury modulates expression of AQP1, P53 and P21 in rat kidney, heart, lung and small intestine. PLoS ONE 2023, 18, e0288507. [Google Scholar] [CrossRef] [PubMed]
- Tod, P.; Róka, B.; Kaucsár, T.; Szatmári, K.; Vizovišek, M.; Vidmar, R.; Fonovič, M.; Szénási, G.; Hamar, P. Time-Dependent miRNA Profile during Septic Acute Kidney Injury in Mice. Int. J. Mol. Sci. 2020, 21, 5316. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Tang, K.; Dong, X.; Xue, L.; Su, G.; Jin, Y. Aquaporin 1 alleviates acute kidney injury via PI3K-mediated macrophage M2 polarization. Inflamm. Res. 2020, 69, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liao, J.; Li, C.; Liu, D.; Luo, X.; Diao, R.; Wang, Y.; Jin, Y. Aquaporin 1 is renoprotective in septic acute kidney injury by attenuating inflammation, apoptosis and fibrosis through inhibition of P53 expression. Front. Immunol. 2024, 15, 1443108. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Asvapromtada, S.; Sonoda, H.; Kinouchi, M.; Oshikawa, S.; Takahashi, S.; Hoshino, Y.; Sinlapadeelerdkul, T.; Yokota-Ikeda, N.; Matsuzaki, T.; Ikeda, M. Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am. J. Physiol. Ren. Physiol. 2018, 314, F584–F601. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, W.; Kwon, T.H.; Jonassen, T.; Li, C.; Ring, T.; Froki, A.J.; Nielsen, S. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004, 66, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Vukićević, T.; Schulz, M.; Faust, D.; Klussmann, E. The Trafficking of the Water Channel Aquaporin-2 in Renal Principal Cells-a Potential Target for Pharmacological Intervention in Cardiovascular Diseases. Front. Pharmacol. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Casini, A. Aquaporin modulators: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 49–62. [Google Scholar] [CrossRef]
- Abdeen, A.; Sonoda, H.; El-Shawarby, R.; Takahashi, S.; Ikeda, M. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am. J. Physiol. Ren. Physiol. 2014, 307, F1227–F1237. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhang, X.; Zhou, Q.; Li, J.M.; Berger, S.; Borok, Z.; Zhou, B.; Xiao, Z.; Yin, H.; et al. Aqp5 is a new transcriptional target of Dot1a and a regulator of Aqp2. PLoS ONE 2013, 8, e53342. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, L.; Zhao, B.; Xiao, Z.; Meng, T.; Zhou, Q.; Zhang, W. Urine AQP5 is a potential novel biomarker of diabetic nephropathy. J. Diabetes Complicat. 2016, 30, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, V.; Muehlschlegel, S.; Sonneville, R. Neurological diseases in intensive care. Intensive Care Med. 2023, 49, 987–990. [Google Scholar] [CrossRef]
- Robba, C.; Zanier, E.R.; Lopez Soto, C.; Park, S.; Sonneville, R.; Helbolk, R.; Sarwal, A.; Newcombe, V.F.J.; van der Jagt, M.; Gunst, J.; et al. Mastering the brain in critical conditions: An update. Intensive Care Med. Exp. 2024, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Krishna, S.; Verkman, A.S. Aquaporin water channels and brain edema. Mt. Sinai J. Med. 2002, 69, 242–248. [Google Scholar]
- Deng, S.; Chen, X.; Lei, Q.; Lu, W. AQP2 Promotes Astrocyte Activation by Modulating the TLR4/NFκB-p65 Pathway Following Intracerebral Hemorrhage. Front. Immunol. 2022, 13, 847360. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007, 22, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Szu, J.I.; Binder, D.K. The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory. Front. Integr. Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Binder, D.K.; Papadopoulos, M.C.; Verkman, A.S. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience 2004, 129, 983–991. [Google Scholar] [CrossRef]
- Yao, X.; Uchida, K.; Papadopoulos, M.C.; Zador, Z.; Manley, G.T.; Verkman, A.S. Mildly Reduced Brain Swelling and Improved Neurological Outcome in Aquaporin-4 Knockout Mice following Controlled Cortical Impact Brain Injury. J. Neurotrauma 2015, 32, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Binder, D.K.; Manley, G.T.; Krishna, S.; Verkman, A.S. Molecular mechanisms of brain tumor edema. Neuroscience 2004, 129, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kurimoto, T.; Miki, A.; Maeda, H.; Kusuhara, S.; Nakamura, M. Aqp9 Gene Deletion Enhances Retinal Ganglion Cell (RGC) Death and Dysfunction Induced by Optic Nerve Crush: Evidence that Aquaporin 9 Acts as an Astrocyte-to-Neuron Lactate Shuttle in Concert with Monocarboxylate Transporters To Support RGC Function and Survival. Mol. Neurobiol. 2020, 57, 4530–4548. [Google Scholar] [CrossRef]
- Dardiotis, E.; Paterakis, K.; Tsivgoulis, G.; Tsintou, M.; Hadjigeorgiou, G.F.; Dardioti, M.; Grigoriadis, S.; Simeonidou, C.; Komnos, A.; Kapsalaki, E.; et al. AQP4 tag single nucleotide polymorphisms in patients with traumatic brain injury. J. Neurotrauma 2014, 31, 1920–1926. [Google Scholar] [CrossRef]
- Nekludov, M.; Bellander, B.M.; Gryth, D.; Wallen, H.; Mobarrez, F. Brain-Derived Microparticles in Patients with Severe Isolated TBI. Brain Inj. 2017, 31, 1856–1862. [Google Scholar] [CrossRef]
- Lo Pizzo, M.; Schiera, G.; Di Liegro, I.; Di Liegro, C.M.; Pál, J.; Czeiter, E.; Sulyok, E.; Dóczi, T. Aquaporin-4 distribution in control and stressed astrocytes in culture and in the cerebrospinal fluid of patients with traumatic brain injuries. Neurol. Sci. 2013, 34, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Q.; Liu, H.; Jiang, L.; Liu, Q.; Lian, W.; Huang, J. Effect of blood pressure on early neurological deterioration of acute ischemic stroke patients with intravenous rt-PA thrombolysis may be mediated through oxidative stress induced blood-brain barrier disruption and AQP4 upregulation. J. Stroke Cerebrovasc. Dis. 2020, 29, 104997. [Google Scholar] [CrossRef]
- Czyżewski, W.; Korulczyk, J.; Szymoniuk, M.; Sakwa, L.; Litak, J.; Ziemianek, D.; Czyżewska, E.; Mazurek, M.; Kowalczyk, M.; Turek, G.; et al. Aquaporin 2 in Cerebral Edema: Potential Prognostic Marker in Craniocerebral Injuries. Int. J. Mol. Sci. 2024, 25, 6617. [Google Scholar] [CrossRef]
- Yang, Z.H.; Yin, X.J.; Fu, G.Y. The correlation between CT findings of diffuse axonal injury and the expression of neuronal aquaporin in patients with craniocerebral injury. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6871–6878. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yao, H.T.; Zhang, W.P.; Zhang, L.; Ding, W.; Zhang, S.H.; Chen, Z.; Wei, E.Q. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J. Zhejiang Univ. Sci. B 2005, 6, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Sevao, M.; Keil, S.A.; Gino, E.; Wang, M.X.; Lee, J.; Haveliwala, M.A.; Klein, E.; Agarwal, S.; Pedersen, T.; et al. Macroscopic changes in aquaporin-4 underlie blast traumatic brain injury-related impairment in glymphatic function. Brain 2024, 147, 2214–2229. [Google Scholar] [CrossRef]
- Suzuki, R.; Okuda, M.; Asai, J.; Nagashima, G.; Itokawa, H.; Matsunaga, A.; Fujimoto, T.; Suzuki, T. Astrocytes co-express aquaporin-1, -4, and vascular endothelial growth factor in brain edema tissue associated with brain contusion. Acta Neurochir. Suppl. 2006, 96, 398–401. [Google Scholar] [CrossRef]
- Aoki, K.; Uchihara, T.; Tsuchiya, K.; Nakamura, A.; Ikeda, K.; Wakayama, Y. Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol. 2003, 106, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Mehta, R.I.; Ivanova, S.; Yu, E.; Gerzanich, V.; Simard, J.M. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol. Commun. 2015, 3, 61. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, R.; Qi, J.; Cong, Y.; Wang, D.; Liu, T.; Gu, Y.; Ban, X.; Huang, Q. The expression and the role of protease nexin-1 on brain edema after intracerebral hemorrhage. J. Neurol. Sci. 2008, 270, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Reeves, C.; Pradim-Jardim, A.; Sisodiya, S.M.; Thom, M.; Liu, J.Y.W. Spatiotemporal dynamics of PDGFRβ expression in pericytes and glial scar formation in penetrating brain injuries in adults. Neuropathol. Appl. Neurobiol. 2019, 45, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.; Tabunoki, H.; Yamamura, T.; Arima, K.; Konno, H. Human astrocytes express aquaporin-1 and aquaporin-4 in vitro and in vivo. Neuropathology 2007, 27, 245–256. [Google Scholar] [CrossRef]
- Roşu, G.C.; Pirici, I.; Istrate-Ofiţeru, A.M.; Iovan, L.; Tudorică, V.; Mogoantă, L.; Gîlceavă, I.C.; Pirici, D. Expression patterns of aquaporins 1 and 4 in stroke. Rom. J. Morphol. Embryol. 2019, 60, 823–830. [Google Scholar] [PubMed]
- Luo, C.; Yao, X.; Li, J.; He, B.; Liu, Q.; Ren, H.; Liang, F.; Li, M.; Lin, H.; Peng, J.; et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis. 2016, 7, e2160. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Brunet, J.F.; Grollimund, L.; Hamou, M.F.; Magistretti, P.J.; Villemure, J.G.; Regli, L. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir. Suppl. 2003, 86, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Jin, F.; Zhu, Y.; Wang, J.; Tang, L.; Wang, Y.; Liebeskind, D.S.; Scalzo, F.; He, Z. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J. Biol. Chem. 2018, 293, 20041–20050. [Google Scholar] [CrossRef]
- Davies, D.C. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002, 200, 639–646. [Google Scholar] [CrossRef]
- Tong, D.M.; Zhou, Y.T.; Wang, G.S.; Chen, X.D.; Yang, T.H. Early prediction and outcome of septic encephalopathy in acute stroke patients with nosocomial coma. J. Clin. Med. Res. 2015, 7, 534–539. [Google Scholar] [CrossRef][Green Version]
- Tauber, S.C.; Eiffert, H.; Brück, W.; Nau, R. Septic encephalopathy and septic encephalitis. Expert Rev. Anti-Infect. Ther. 2017, 15, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.D.; Shields, J.S.; Sukumari-Ramesh, S.; Kimbler, D.E.; Fessler, R.D.; Shakir, B.; Youssef, P.; Yanasak, N.; Vender, J.R.; Dhandapani, K.M. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 2014, 62, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Yan, L.; Wang, C.; Han, A.; Qin, Y.; Cui, L.; Xiang, Q. Associations between Aquaglyceroporin Gene Polymorphisms and Risk of Stroke among Patients with Hypertension. Biomed. Res. Int. 2020, 2020, 9358290. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Krishna, S. Water transport becomes uncoupled from K+ siphoning in brain contusion, bacterial meningitis, and brain tumours: Immunohistochemical case review. J. Clin. Pathol. 2003, 56, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Kleffner, I.; Bungeroth, M.; Schiffbauer, H.; Schäbitz, W.R.; Ringelstein, E.B.; Kuhlenbäumer, G. The role of aquaporin-4 polymorphisms in the development of brain edema after middle cerebral artery occlusion. Stroke 2008, 39, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Bruce, S.; Duren, A.; Piazza, M.; Monahan, A.; Christophe, B.; Zoller, S.; LoPresti, M.; Connolly, E.S. Aquaporin-4 gene variant independently associated with oedema after intracerebral haemorrhage. Neurol. Res. 2015, 37, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Siokas, V.; Marogianni, C.; Aloizou, A.M.; Sokratous, M.; Paterakis, K.; Dardioti, M.; Grigoriadis, S.; Brotis, A.; Kapsalaki, E.; et al. AQP4 tag SNPs in patients with intracerebral hemorrhage in Greek and Polish population. Neurosci. Lett. 2019, 696, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Farr, G.W.; Hall, C.H.; Farr, S.M.; Wade, R.; Detzel, J.M.; Adams, A.G.; Buch, J.M.; Beahm, D.L.; Flask, C.A.; Xu, K.; et al. Functionalized Phenylbenzamides Inhibit Aquaporin-4 Reducing Cerebral Edema and Improving Outcome in Two Models of CNS Injury. Neuroscience 2019, 404, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, H.; Fan, L.; Yan, W.; Yan, Y.F. Treatment Effects of Acetazolamide on Ischemic Stroke: A Meta-Analysis and Systematic Review. World Neurosurg. 2024, 185, e750–e757. [Google Scholar] [CrossRef]
- Zheng, L.; Cheng, W.; Wang, X.; Yang, Z.; Zhou, X.; Pan, C. Overexpression of MicroRNA-145 Ameliorates Astrocyte Injury by Targeting Aquaporin 4 in Cerebral Ischemic Stroke. Biomed. Res. Int. 2017, 2017, 9530951. [Google Scholar] [CrossRef]
- Kuwahara-Otani, S.; Maeda, S.; Tanaka, K.; Hayakawa, T.; Seki, M. Systemic administration of lipopolysaccharide increases the expression of aquaporin-4 in the rat anterior pituitary gland. J. Vet. Med. Sci. 2013, 75, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Masterman, E.; Ahmed, Z. Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Cells 2021, 10, 2682. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Meng, Y.; Lv, X.; Guo, L.; Wang, X.; Su, Z.; Li, L.; Li, N.; Zhao, S.; Zhao, L.; et al. Dexamethasone attenuates LPS-induced changes in expression of urea transporter and aquaporin proteins, ameliorating brain endotoxemia in mice. Int. J. Clin. Exp. Pathol. 2014, 7, 8443–8452. [Google Scholar] [PubMed]
- Mogoanta, L.; Ciurea, M.; Pirici, I.; Margaritescu, C.; Simionescu, C.; Ion, D.A.; Pirici, D. Different dynamics of aquaporin 4 and glutamate transporter-1 distribution in the perineuronal and perivascular compartments during ischemic stroke. Brain Pathol. 2014, 24, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, L.; Simats, A.; Penalba, A.; Garcia-Tornel, A.; Rovira, A.; Mancha, F.; Bustamante, A.; Montaner, J. Circulating Aquaporin-4 as A biomarker of early neurological improvement in stroke patients: A pilot study. Neurosci. Lett. 2020, 714, 134580. [Google Scholar] [CrossRef]
- Sfera, A.; Price, A.I.; Gradini, R.; Cummings, M.; Osorio, C. Proteomic and epigenomic markers of sepsis-induced delirium (SID). Front. Mol. Biosci. 2015, 2, 59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Shangzu, Z.; Dingxiong, X.; ChengJun, M.; Yan, C.; Yangyang, L.; Zhiwei, L.; Ting, Z.; Zhiming, M.; Yiming, Z.; Liying, Z.; et al. Aquaporins: Important players in the cardiovascular pathophysiology. Pharmacol. Res. 2022, 183, 106363. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Jiang, J.; Geng, Y.J. Attenuated expression of gelsolin in association with induction of aquaporin-1 and nitric oxide synthase in dysfunctional hearts of aging mice exposed to endotoxin. Int. J. Immunopathol. Pharmacol. 2012, 25, 911–922. [Google Scholar] [CrossRef]
- Mohammad, S.; O’Riordan, C.E.; Verra, C.; Aimaretti, E.; Alves, G.F.; Dreisch, K.; Evenas, J.; Gena, P.; Tesse, A.; Rutzler, M.; et al. RG100204, A Novel Aquaporin-9 Inhibitor, Reduces Septic Cardiomyopathy and Multiple Organ Failure in Murine Sepsis. Front. Immunol. 2022, 13, 900906. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Uaratanawong, R.; Choudhary, V.; Hardin, M.; Zhang, C.; Melnyk, S.; Chen, X.; Bollag, W.B. Advanced Glycation End Products and Activation of Toll-like Receptor-2 and -4 Induced Changes in Aquaporin-3 Expression in Mouse Keratinocytes. Int. J. Mol. Sci. 2023, 24, 1376. [Google Scholar] [CrossRef]
- Keskinidou, C.; Lotsios, N.S.; Vassiliou, A.G.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. The Interplay between Aquaporin-1 and the Hypoxia-Inducible Factor 1alpha in a Lipopolysaccharide-Induced Lung Injury Model in Human Pulmonary Microvascular Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 10588. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Q.; Pei, Y.; Gong, M.; Cui, X.; Pan, J.; Zhang, Y.; Liu, Y.; Liu, Y.; Yuan, X.; et al. Aqp-1 Gene Knockout Attenuates Hypoxic Pulmonary Hypertension of Mice. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 48–62. [Google Scholar] [CrossRef]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin Membrane Channels in Oxidative Stress, Cell Signaling, and Aging: Recent Advances and Research Trends. Oxidative Med. Cell. Longev. 2018, 2018, 1501847. [Google Scholar] [CrossRef]
- D’Agostino, C.; Parisis, D.; Chivasso, C.; Hajiabbas, M.; Soyfoo, M.S.; Delporte, C. Aquaporin-5 Dynamic Regulation. Int. J. Mol. Sci. 2023, 24, 1889. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Glogauer, M.; Ellen, R.P.; Loitto, V.M.; Magnusson, K.E.; Magalhães, M.A. Aquaporin 9 phosphorylation mediates membrane localization and neutrophil polarization. J. Leukoc. Biol. 2011, 90, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Morrell, E.D.; Kellum, J.A.; Hallows, K.R.; Pastor-Soler, N.M. Epithelial transport during septic acute kidney injury. Nephrol. Dial. Transplant. 2014, 29, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E.; Golldack, A.; Rothert, M.; von Bülow, J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol. Ther. 2015, 155, 22–35. [Google Scholar] [CrossRef]
- Jelen, S.; Wacker, S.; Aponte-Santamaría, C.; Skott, M.; Rojek, A.; Johanson, U.; Kjellbom, P.; Nielsen, S.; de Groot, B.L.; Rützler, M. Aquaporin-9 protein is the primary route of hepatocyte glycerol uptake for glycerol gluconeogenesis in mice. J. Biol. Chem. 2011, 286, 44319–44325. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Biol. Chem. 2019, 294, 7377–7387. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Zhang, H.; Saadoun, S.; Phuan, P.W.; Lam, C.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012, 71, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Tanaka, M.; Verkman, A.S.; Yasui, M. Inhibition of aquaporin-3 in macrophages by a monoclonal antibody as potential therapy for liver injury. Nat. Commun. 2020, 11, 5666. [Google Scholar] [CrossRef]
- Duan, T.; Tradtrantip, L.; Phuan, P.W.; Bennett, J.L.; Verkman, A.S. Affinity-matured ’aquaporumab’ anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders. Neuropharmacology 2020, 162, 107827. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Asavapanumas, N.; Verkman, A.S. Emerging therapeutic targets for neuromyelitis optica spectrum disorder. Expert Opin. Ther. Targets 2020, 24, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yasui, M.; Hara-Chikuma, M. Aquaporin 3 inhibition suppresses the mitochondrial respiration rate and viability of multiple myeloma cells. Biochem. Biophys. Res. Commun. 2023, 676, 158–164. [Google Scholar] [CrossRef]
- Charlestin, V.; Tan, E.; Arias-Matus, C.E.; Wu, J.; Miranda-Vergara, M.C.; Lee, M.; Wang, M.; Nannapaneni, D.T.; Tennakoon, P.; Blagg, B.S.J.; et al. Evaluation of the Mammalian Aquaporin Inhibitors Auphen and Z433927330 in Treating Breast Cancer. Cancers 2024, 16, 2714. [Google Scholar] [CrossRef]



| AQP | Disease/ Condition | Study Objective | Findings | References |
|---|---|---|---|---|
| AQP1 | Sepsis | To study the involvement of AQP1 in immune response regulation in critically ill patients during infection acquired in the ICU. AQP1 mRNA expression was measured in leukocytes of 16 critically ill patients who develeoped sepsis and septic shock vs 13 non sepsis critically ill patients | Leukocyte AQP1 mRNA expression was induced at the onset of sepsis (median 1.71-fold increase from baseline, p = 0.012) and was further increased upon septic shock (median 3.00-fold increase, p = 0.023 from sepsis | [107] |
| Sepsis | To investigate the potential mechanism of AQP1, miRNA-874, and lncRNA H19 in sepsis and the anti-inflammatory responses related to sepsis myocardial dysfunction. AQP1 mRNA expression was measured in venous blood samples of 69 sepsis patients vs 57 healthy controls | H19 and AQP1 decreased and accompanied with elevated miR-874 expression in the sepsis samples. There was a negative relationship between expression of H19 and miR-874, and a positive correlation between H19 and AQP1 expression | [125] | |
| ARDS | To measure macrophage MIF and AQP1 expression levels in post-mortem lung tissues samples from 15 non-smoking ARDS patients vs postmortem lung tissues from 15 age- and sex-comparable non-smoking patients who had died of non-pulmonary diseases in the ICU | AQP1 was found constitutively expressed in the alveolar endothelium, while its expression was enhanced in alveolar capillary endothelium in lung tissues from ARDS patients | [166] | |
| ARDS | To identify genes and/or cellular pathways involved in the pathogenesis of ICU-acquired sepsis, a genomic expression analysis of 5 polytrauma, initially non-septic patients who were admitted to the ICU was performed | Following a validation analysis, among the genes found to be dyregulated in ARDS, total blood RNA expression of AQP1 was elevated | [107] | |
| COVID-19 RF | To determine the relationship between inflammation and oxidative stress in COVID-19 patients. AQP1 sera levels were measured in 45 mechanically ventilated critically ill COVID-19 patients and 45 healthy individuals with negative PCR tests for SARS-CoV-2 and no chronic disease | Serum AQP1 levels were higher in the COVID-19 patient group compared to the control group (p < 0.01) | [167] | |
| AQP2 | AKI | To determine whether urine AQP2 can predict AKI in patients with acute heart failure | Urine AQP2 levels were higher in the AKI group; independently associated with AKI; AUC = 0.795 | [209] |
| TBI/Acute and chronic SDH | To investigate the potential of brain AQPs as biomarkers in 41 TBI patients | Strong correlation between AQP2 levels and the volume of chronic SDH and midline shift. In the chronic SDH group, AQP2 plasma concentration negatively correlated with the midline shift measured before surgery (rs = −0.54, p = 0.017) and positively with hematoma volume change between baseline and 30 h post-surgery (rs = 0.627, p = 0.007) | [240] | |
| AQP3 | Sepsis | To investigate whether AQPs are differentially expressed in the blood of septic patients, are related to immune cell count, and impact sepsis survival. Measured AQP3 mRNA expression in whole blood samples of 87 sepsis patients on day 1 and day 8 after sepsis diagnosis | Whole blood AQP3 mRNA expression increased over the duration of sepsis (p < 0.0001), AQP3 expression negatively correlated with neutrophil and leucocyte cell counts, positively correlated with lymphocyte cell count, and negatively correlated with IL-8. ROC showed that patients with AQP3 expression above the cut-off value had a higher chance of survival than those with a lower AQP3 expression on day 8 (82.4% vs. 43.8%, p = 0.017) | [119] |
| Sepsis | To investigate the SNP rs17553719 and the expression of AQP3 in 265 sepsis patients and correlate these measurements with the outcome of sepsis patients and the release of several cytokines | CC genotype exhibited a significant decrease in 30-day survival (38.9%) compared to the CT (66.15%) and TT genotypes (76.3%) (p = 0.003). AQP3 mRNA expression was significantly higher and nearly doubled in the CC compared to the CT (p = 0.0044) and TT genotypes (p = 0.018) on the day of study inclusion. Increased IL-33 concentration in the CC genotype (day 0: p = 0.0026 and day 3: p = 0.008) | [120] | |
| AQP4 | TBI | To determine the time course of AQP4 variation in CSF from 20 patients receiving intensive care after TBI, and to assess the influence of increased ICP on CSF AQP4 in these patients | AQP4 significantly increased in patients with severe brain injury compared to healthy subjects (p< 0.002). AQP4 in CSF remained unchanged in patients with elevated ICP | [238] |
| TBI | To investigate possible associations between genetic variations of the AQP4 gene and the patients’ initial TBI severity, the presence of intracranial hemorrhage, and the long-term clinical outcome after TBI in 363 patients | Significant associations with TBI outcome were detected for rs3763043 [OR (95% CI: 5.15 (1.60–16.5); p = 0.006], rs3875089 [OR (95% CI): 0.18 (0.07–0.50), p = 0.0009], and a common haplotype of AQP4 tag SNPs [OR (95% CI): 2.94, (1.34–6.36); p = 0.0065] | [236] | |
| TBI | To investigate the presence of circulating MPs of brain tissue origin in the systemic and cerebrovenous blood of 15 patients with severe TBI | Concentrations of MPs expressing AQP4 were significantly higher in the TBI group compared with healthy controls (p < 0.001) | [237] | |
| AIS | To study the association between blood pressure and the development of early neurological deterioration in 357 acute ischemic stroke patients with intravenous rt-PA thrombolysis and its possible mechanism | High SBP after thrombolysis correlates with oxidative stress-induced BBB disruption and AQP4 upregulation, linked to early neurological decline | [239] | |
| SAE | To investigate the effects of AQP4 associated with SAE and reveal its underlying mechanism causing cognitive impairment | AQP4 in peripheral blood of patients with SAE is up-regulated | [172] | |
| Acute and chronic SDH | To investigate the potential of brain AQPs as biomarkers in 41 TBI patients | No significant findings for AQP4 in acute SDH | [240] | |
| AQP5 | Sepsis | To test the hypothesis that the AQP5 promoter -1364A/C polymorphism is associated with increased 30-day survival in severe sepsis. Whole blood genotyping of 154 sepsis patients for the AQP5-1364A/C SNP | 30-day survival was significantly associated with AQP5-1364A/C genotypes (p = 0.001). Survival rates were 57% for AA genotypes (n = 90) but 83% for combined AC/CC genotypes (56 vs. 8, respectively). AQP5-1364A/C SNP was a strong and independent prognostic factor for 30-day survival. Homozygous AA subjects were at high risk for death within 30 days [HR (95% CI): 3.59 (1.47–8.80); p = 0.005] compared to AC/CC genotypes | [130] |
| Sepsis | To test if DNA methylation of the AQP5 promoter influences AQP5 expression, is associated with 30-day survival of septic patients, and alters NF-κB binding in whole blood samples of 88 surviving and 47 non-surviving sepsis patients | A greater methylation rate was found at cytosine site nt-937 in the AQP5 promoter linked to NF-kappaB binding in non-survivors compared to survivors (p = 0.002). This was associated with greater AQP5 mRNA expression in non-survivors (p = 0.037). Greater promoter methylation at nt-937 was also associated with an independently increased risk of death within 30 days [HR (95% CI): 3.31 (1.54–6.23); p = 0.002] | [101] | |
| Sepsis | To test if AQP5 expression and the AQP5-1364A/C polymorphism alters neutrophil migration in samples from 4 healthy volunteers | Healthy volunteer neutrophils carrying the A allele of the AQP5-1364A/C SNP show increased migration. Target-oriented migration of neutrophils was seen after 0.5 h in AA-genotype cells but only after 1.5 h in AC/CC-genotype cells, with a threefold lower migrating cell count | [112] | |
| Sepsis | To test if AQP5 promoter methylation differs between genotypes in specific types of immune cells. AQP5 promoter methylation was quantified in cells of 25 septic patients | The C-allele of AQP5-1364 A/C promoter polymorphism was associated with a fivefold increased promoter methylation in neutrophils (p = 0.0055) and a fourfold increase in monocytes (p = 0.0005) and lymphocytes (p = 0.0184) in septic patients and healthy controls. Decreased AQP5 promoter methylation was accompanied by increased AQP5 expression in HL-60 (p = 0.0102) and REH cells (p = 0.0102). The C-allele, which is associated with lower gene expression in sepsis, is accompanied by a higher methylation level of the AQP5 promoter | [132] | |
| ARDS | To examine the outcome associations of the AQP5 promoter -1364A/C polymorphism in whole blood from 136 patients suffering from ARDS | Patients with AA genotype of the AQP5-1364A/C SNP showed increased mortality | [168] | |
| ARDS | To investigate whether the AQP5-1364A/C SNP in whole blood was associated with pulmonary inflammation and survival in 136 ARDS patients | The presence of the AA genotype of the AQP5-1364A/C SNP was associated with aggravated pulmonary inflammation, while the carriers of the C allele showed attenuated pulmonary inflammation and higher 30-day survival | [27] | |
| AKI | To determine whether the AQP5 promoter -1364A/C polymorphism is associated with AKI in patients suffering from pneumonia evoked ARDS | On day 30, homozygous AA genotypes showed an increased prevalence of AKI compared to AC/CC genotypes (57 vs. 24%; p = 0.001). The AA genotype proved to be a strong, independent risk factor for predicting AKI persistence [OR (95% CI): 3.35 (1.2–9.0); p = 0.017] | [168] | |
| AQP9 | Sepsis | To examine the expression of AQP9 in PMNs from 14 SIRS patients and 12 healthy controls and to study the role of AQP9 in morphologic and functional changes of PMNs in SIRS | The PMNs with and without FMLP stimulation from SIRS patients showed significantly higher mean fluorescence intensity of AQP9 than PMNs from healthy subjects. AQP9 expression was significantly higher in PMNs from SIRS patients with FMLP stimulation | [115] |
| Sepsis | To investigate whether AQPs are differentially expressed in the blood of septic patients, are related to immune cell count, and impact sepsis survival. Measured AQP3 mRNA expression in whole blood samples of 87 sepsis patients on day 1 and day 8 after sepsis diagnosis | AQP9 mRNA expression was not altered over the duration of sepsis. AQP9 expression positively correlated with neutrophil cell count (p = 0.0017) and negatively with lymphocytes and classical monocytes (p < 0.0257). AQP9 expression weakly correlated negatively with IL-1β, interferon-α2, and IL-33. Kaplan–Meier curve showed increased survival in patients with lower AQP9 expression (68.2% vs. 20.0%, p = 0.003). Elevated levels of AQP9 expression were detrimental to patient survival [HR (95% CI): 5.59 (1.58–19.56); p = 0.008] | [119] | |
| ARDS | To explore key genes and signaling pathways involved in the pathogenesis of ARDS using the mRNA expression profile dataset GSE32707, which includes mRNA expression data of 33 ARDS and 34 control samples | The initial results showed increased whole blood mRNA expression in ARDS patients. However, subsequent validation analysis in a different group of ARDS patients did not confirm these findings | [169] | |
| TBI/Acute and chronic SDH | To investigate the potential of brain AQPs as biomarkers in 41 TBI patients | No significant findings for AQP9 in acute SDH | [240] |
| Compound | Function | Findings | References |
|---|---|---|---|
| RG100204 | AQP9 inhibitor | In a CLP murine sepsis model, it improved outcomes like hypothermia, renal, and cardiac dysfunction by inhibiting the NLRP3 inflammasome pathway In Fao hepatoma cells it reduced the LPS-induced increase in hydrogen peroxide permeability and oxidative stress markers | [275] |
| HTS13286 | Blocks the passage of glycerol and urea through AQP9 | In Fao cells it impaired the secretion of inflammatory cytokines | [148,284] |
| DFP00173 | Selective AQP3 inhibitor | Anti-AQP3 mAb and DFP00173 reduced cell growth, mitochondrial respiration rate, and electron transport chain complex I activity in multiple myeloma | [285,290] |
| Z433927330 | Selective AQP7 inhibitor | In breast cancer mice, no difference in tumor growth in either biweekly- or weekly-treated mice, decreased lung metastasis after weekly treatment with but not in the biweekly treatment cohort, and no difference in overall survival in either weekly or biweekly-treated mice | [285,291] |
| Anti-AQP3 | Inhibits AQP3-facilitated peroxide and glycerol transport | Inhibited AQP3-facilitated peroxide and glycerol transport in liver macrophages and prevented liver injury in experimental animal models | [287] |
| Anti-AQP4 | Targets AQP4 | In an autoimmune inflammatory disesase, neuromyelitis optica (NMO), it blocked cell surface AQP4 binding of polyclonal NMO-IgG in patient sera in cell culture, ex vivo spinal cord and in vivo mouse models of NMO, preventing downstream cytotoxicity and NMO lesions | [286] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettou, C.S.; Issaris, V.; Kokkoris, S.; Poupouzas, G.; Keskinidou, C.; Lotsios, N.S.; Kotanidou, A.; Orfanos, S.E.; Dimopoulou, I.; Vassiliou, A.G. Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness. Life 2024, 14, 1688. https://doi.org/10.3390/life14121688
Vrettou CS, Issaris V, Kokkoris S, Poupouzas G, Keskinidou C, Lotsios NS, Kotanidou A, Orfanos SE, Dimopoulou I, Vassiliou AG. Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness. Life. 2024; 14(12):1688. https://doi.org/10.3390/life14121688
Chicago/Turabian StyleVrettou, Charikleia S., Vasileios Issaris, Stelios Kokkoris, Georgios Poupouzas, Chrysi Keskinidou, Nikolaos S. Lotsios, Anastasia Kotanidou, Stylianos E. Orfanos, Ioanna Dimopoulou, and Alice G. Vassiliou. 2024. "Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness" Life 14, no. 12: 1688. https://doi.org/10.3390/life14121688
APA StyleVrettou, C. S., Issaris, V., Kokkoris, S., Poupouzas, G., Keskinidou, C., Lotsios, N. S., Kotanidou, A., Orfanos, S. E., Dimopoulou, I., & Vassiliou, A. G. (2024). Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness. Life, 14(12), 1688. https://doi.org/10.3390/life14121688







