Plasma microRNAs as Biomarkers for Predicting Radiotherapy Treatment-Induced Cardiotoxicity in Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Objective
2.2. Sample Collection
2.3. miRNA Extraction
2.4. cDNA Synthesis
2.5. Quantitative PCR
2.6. Statistical Analysis
3. Results
3.1. Concentration and Purity of miRNA
3.2. miRNA Expression Changes After Treatment
3.3. Changes in miRNA Expression in Relation to Disease Diagnosis
3.4. Correlation of miRNA Expression and Regular Medication Use
3.5. Correlation of miRNA Expression and Clinical Biomarker of Heart Damage
3.6. Correlation of miRNA Expression and Cardiac Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | miRNA | Experimental Species | Source | Method | Modulation Post-IR (Increased/Decreased) | Type of Disease | Number of Patients/Cell Lines/Rats | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | miRNA-1 | Rat | Left ventricular tissue | RT-qPCR 1 | Decreased | - | 40 | [44] |
| 2 | Rat | Left ventricular tissue | RT-qPCR | Decreased | - | 12 | [59] | |
| 3 | Rat | Ventricular tissue | RT-qPCR | Decreased in left ventricle but not in the right ventricle | - | 10 | [29] | |
| 4 | miRNA-21 | Rat | Left ventricular tissue | RT-qPCR | Increased | - | 40 | [44] |
| 5 | Rat | Left ventricular tissue | RT-qPCR | Increased | - | 12 | [59] | |
| 6 | Rat | Ventricular tissue | RT-qPCR | Increased in both the left and right ventricles | - | 10 | [29] | |
| 7 | miRNA-24 | Human | Serum | qPCR profiling | Increased | Breast cancer | 28 | [60] |
| 8 | miRNA-29a | Human | Plasma | Microarray profiling | Decreased | NSCLC 3 | 5 | [41] |
| 9 | Human | Cell lines | RT-qPCR | Decreased (exosomal expression) | NSCLC | 3 (NCI-H460, A549, NCI-H1299) | [41] | |
| 10 | miRNA-34a | Human | Cell culture | RT-qPCR | Increased | - | 1 (human cardiomyocytes) | [42] |
| 11 | Mice | Plasma/Tissue | NGS 2 | Increased | - | 192 | [61] | |
| 12 | miRNA-222 | Human | Blood | RT-qPCR | Increased | Breast cancer | 136 | [43] |
| 13 | Human | Cell culture | RT-qPCR | Increased at 2 h and reduced at 24 h | - | 1 (human umbilical vein endothelial cells) | [62] |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Cancer Statistics—Specific Cancers—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cancer_statistics_-_specific_cancers#Lung_cancer (accessed on 4 March 2024).
- Vojtíšek, R. Cardiac toxicity of lung cancer radiotherapy. Rep. Pract. Oncol. Radiother. J. Greatpoland Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 2020, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, A.W.; Fosså, S.D.; Haugnes, H.S.; Nome, R.; Stokke, T.M.; Haugaa, K.H.; Kiserud, C.E.; Edvardsen, T.; Sarvari, S.I. The cardiac impact of cisplatin-based chemotherapy in survivors of testicular cancer: A 30-year follow-up. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Walls, G.M.; McCann, C.; O’Connor, J.; O’Sullivan, A.; Johnston, D.I.; McAleese, J.; McGarry, C.K.; Cole, A.J.; Jain, S.; Butterworth, K.T.; et al. Pulmonary vein dose and risk of atrial fibrillation in patients with non-small cell lung cancer following definitive radiotherapy: An NI-HEART analysis. Radiother. Oncol. 2024, 192, 110085. [Google Scholar] [CrossRef] [PubMed]
- Speirs, C.K.; DeWees, T.A.; Rehman, S.; Molotievschi, A.; Velez, M.A.; Mullen, D.; Fergus, S.; Trovo, M.; Bradley, J.D.; Robinson, C.G. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 293–301. [Google Scholar] [CrossRef]
- Dess, R.T.; Sun, Y.; Matuszak, M.M.; Sun, G.; Soni, P.D.; Bazzi, L.; Murthy, V.L.; Hearn, J.W.D.; Kong, F.M.; Kalemkerian, G.P.; et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1395–1402. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, 6. [Google Scholar] [CrossRef]
- Edouard, A.; Felten, M.L.; Hebert, J.L.; Cosson, C.; Martin, L.; Benhamou, D. Incidence and Significance of Cardiac Troponin I Release in Severe Trauma Patients. Anesthesiology 2004, 101, 1262–1268. [Google Scholar] [CrossRef]
- Abbas, N.A.; John, R.I.; Webb, M.C.; Kempson, M.E.; Potter, A.N.; Price, C.P.; Vickery, S.; Lamb, E.J. Cardiac Troponins and Renal Function in Nondialysis Patients with Chronic Kidney Disease. Clin. Chem. 2005, 51, 2059–2066. [Google Scholar] [CrossRef]
- Eggers, K.M.; Lind, L.; Venge, P.; Lindahl, B. Factors Influencing the 99th Percentile of Cardiac Troponin I Evaluated in Community-Dwelling Individuals at 70 and 75 Years of Age. Clin. Chem. 2013, 59, 1068–1073. [Google Scholar] [CrossRef]
- Wieshammer, S.; Dreyhaupt, J.; Müller, D.; Momm, F.; Jakob, A. Limitations of N-Terminal Pro-B-Type Natriuretic Peptide in the Diagnosis of Heart Disease among Cancer Patients Who Present with Cardiac or Pulmonary Symptoms. Oncology 2016, 90, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N.; Daniels, L.B.; Reilly, M.P.; Lima, J.A.; Wang, T.J.; et al. Inflammation and Circulating Natriuretic Peptide Levels. Circ. Heart Fail. 2020, 13, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, T.; Schlicht, K.; Krause, L.; Hagen, S.; Rohmann, N.; Schulte, D.M.; Türk, K.; Beckmann, A.; Ahrens, M.; Franke, A.; et al. Effect of various weight loss interventions on serum NT-proBNP concentration in severe obese subjects without clinical manifest heart failure. Sci. Rep. 2021, 11, 10096. [Google Scholar] [CrossRef]
- Srisawasdi, P.; Vanavanan, S.; Charoenpanichkit, C.; Kroll, M.H. The Effect of Renal Dysfunction on BNP, NT-proBNP, and Their Ratio. Am. J. Clin. Pathol. 2010, 133, 14–23. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med Biotechnol. 2010, 2, 161–179. [Google Scholar]
- Bostjancic, E.; Zidar, N.; Stajner, D.; Glavac, D. MicroRNA miR-1 is up-regulated in remote myocardium in patients with myocardial infarction. Folia Biol. 2010, 56, 27–31. [Google Scholar] [CrossRef]
- Dong, F.F.; Dong, S.H.; Liang, Y.; Wang, K.; Qin, Y.W.; Zhao, X.X. MiR-34a promotes myocardial infarction in rats by inhibiting the activity of SIRT1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7059–7065. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Wang, F.; Nie, X.; Du, H.; Zhao, Y.; Yin, Z.; Li, H.; Fan, J.; Wen, Z.; Wang, D.W.; et al. The Cell Type–Specific Functions of miR-21 in Cardiovascular Diseases. Front. Genet. 2020, 11, 563166. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Bonauer, A.; Dimmeler, S. RNA Therapeutics in Cardiovascular Disease. Circ. Res. 2018, 123, 205–220. [Google Scholar] [CrossRef]
- Verjans, R.; Peters, T.; Beaumont, F.J.; van Leeuwen, R.; van Herwaarden, T.; Verhesen, W.; Munts, C.; Bijnen, M.; Henkens, M.; Diez, J.; et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload–Induced Heart Failure. Hypertension 2018, 71, 280–288. [Google Scholar] [CrossRef]
- Wei, W.; Peng, J.; Shen, T. Rosuvastatin Alleviates Ischemia/Reperfusion Injury in Cardiomyocytes by Downregulating Hsa-miR-24-3p to Target Upregulated Uncoupling Protein 2. Cell. Reprogramming 2019, 21, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, S.; Feng, Y.; Shen, J.; Zhao, J. MicroRNA-24-3p inhibition prevents cell growth of vascular smooth muscle cells by targeting Bcl-2-like protein 11. Exp. Ther. Med. 2020, 19, 2467–2474. [Google Scholar] [CrossRef]
- Viczenczova, C.; Kura, B.; Egan Benova, T.; Yin, C.; Kukreja, R.; Slezak, J.; Tribulova, N.; Szeiffova Bacova, B. Irradiation-Induced Cardiac Connexin-43 and miR-21 Responses Are Hampered by Treatment with Atorvastatin and Aspirin. Int. J. Mol. Sci. 2018, 19, 1128. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, X.; Li, S.; Liu, Y.; Cui, Y.; Deng, X. Advances in Biomarkers for Detecting Early Cancer Treatment-Related Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 753313. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.L. The significance of the difference between two means when the population variances are unequal. Biometrika 1938, 29, 350–362. [Google Scholar] [CrossRef]
- Delacre, M.; Lakens, D.; Leys, C. Why Psychologists Should by Default Use Welch’s t-test Instead of Student’s t-test. Int. Rev. Soc. Psychol. 2017, 30, 92–101. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
- Adams, S.P.; Tsang, M.; Wright, J.M. Lipid lowering efficacy of atorvastatin. Cochrane Database Syst. Rev. 2012, 12, CD008226. [Google Scholar] [CrossRef]
- Degirmenci, H.; Açikel, M.; Bakirci, E.M.; Duman, H.; Demirelli, S.; Tas, H.; Simsek, Z.; Karakelleoglu, S.; Aksakal, E.; Erol, M.K. Comparison of effects of nebivolol, carvedilol and irbesartan on left ventricular hypertrophy associated with hypertension. Eur. Rev. Med Pharmacol. Sci. 2014, 18, 630–637. [Google Scholar]
- Chorley, B.N.; Atabakhsh, E.; Doran, G.; Gautier, J.C.; Ellinger-Ziegelbauer, H.; Jackson, D.; Sharapova, T.; Yuen, P.S.; Church, R.J.; Couttet, P.; et al. Methodological considerations for measuring biofluid-based microRNA biomarkers. Crit. Rev. Toxicol. 2021, 51, 264–282. [Google Scholar] [CrossRef]
- Polak, M.; Wieczorek, J.; Botor, M.; Auguścik-Duma, A.; Hoffmann, A.; Wnuk-Wojnar, A.; Gawron, K.; Mizia-Stec, K. Principles and Limitations of miRNA Purification and Analysis in Whole Blood Collected during Ablation Procedure from Patients with Atrial Fibrillation. J. Clin. Med. 2024, 13, 1898. [Google Scholar] [CrossRef]
- Dinh, T.K.T.; Fendler, W.; Chałubińska-Fendler, J.; Acharya, S.S.; O’Leary, C.; Deraska, P.V.; D’Andrea, A.D.; Chowdhury, D.; Kozono, D. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat. Oncol. 2016, 11, 61. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, W.; Hou, M. Macrophage migration inhibitory factor serves a pivotal role in the regulation of radiation-induced cardiac senescencethrough rebalancing the microRNA-34a/sirtuin 1 signaling pathway. Int. J. Mol. Med. 2018, 42, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Esplugas, R.; Arenas, M.; Serra, N.; Bellés, M.; Bonet, M.; Gascón, M.; Vallvé, J.C.; Linares, V. Effect of radiotherapy on the expression of cardiovascular disease-related miRNA-146a, -155, -221 and -222 in blood of women with breast cancer. PLoS ONE 2019, 14, e0217443. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Yin, C.; Frimmel, K.; Krizak, J.; Okruhlicova, L.; Kukreja, R.C.; Slezak, J. Changes of microRNA-1, -15b and -21 levels in irradiated rat hearts after treatment with potentially radioprotective drugs. Physiol. Res. 2016, 65 (Suppl. S1), S129–S137. [Google Scholar] [CrossRef]
- Kang, M.; Xiao, J.; Wang, J.; Zhou, P.; Wei, T.; Zhao, T.; Wang, R. MiR-24 enhances radiosensitivity in nasopharyngeal carcinoma by targeting SP1. Cancer Med. 2016, 5, 1163–1173. [Google Scholar] [CrossRef]
- Galardi, S.; Mercatelli, N.; Giorda, E.; Massalini, S.; Frajese, G.V.; Ciafrè, S.A.; Farace, M.G. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 2007, 282, 23716–23724. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Z.; Dou, Z. miR-24-3p regulates bladder cancer cell proliferation, migration, invasion and autophagy by targeting DEDD. Oncol. Rep. 2017, 37, 1123–1131. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Siemens, H.; Jackstadt, R.; Hünten, S.; Kaller, M.; Menssen, A.; Götz, U.; Hermeking, H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 2011, 10, 4256–4271. [Google Scholar] [CrossRef]
- Lu, K.; Wang, J.; Song, Y.; Zhao, S.; Liu, H.; Tang, D.; Pan, B.; Zhao, H.; Zhang, Q. miRNA-24-3p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting p27Kip1. Oncol. Rep. 2015, 34, 995–1002. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Tang, Q.; Sheng, H.; Long, S.; Wu, W. MicroRNA-24 in Cancer: A Double Side Medal with Opposite Properties. Front. Oncol. 2020, 10, 553714. [Google Scholar] [CrossRef]
- Ye, H.; Ling, S.; Castillo, A.C.; Thomas, B.; Long, B.; Qian, J.; Perez-Polo, J.R.; Ye, Y.; Chen, X.; Birnbaum, Y. Nebivolol Induces Distinct Changes in Profibrosis MicroRNA Expression Compared with Atenolol, in Salt-Sensitive Hypertensive Rats. Hypertension 2013, 61, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Fajardo, C.M.; Basso, R.G.; Hirata, M.H.; Hirata, R.D.C. Role of microRNAs 221/222 on statin induced nitric oxide release in human endothelial cells. Arq. Bras. De Cardiol. 2015, 104, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Shao, X.; Zhang, X.; Han, Z.; Yang, C.; Li, X. Circulating microRNA-1 in the diagnosis and predicting prognosis of patients with chest pain: A prospective cohort study. BMC Cardiovasc. Disord. 2019, 19, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.J.; Liu, T.; Zhang, H.; Yang, S.J. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 323–329. [Google Scholar]
- Ntelios, D.; Meditskou, S.; Efthimiadis, G.; Pitsis, A.; Nikolakaki, E.; Girtovitis, F.; Parcharidou, D.; Zegkos, T.; Kouidou, S.; Karvounis, H.; et al. Elevated plasma levels of miR-29a are associated with hemolysis in patients with hypertrophic cardiomyopathy. Clin. Chim. Acta 2017, 471, 321–326. [Google Scholar] [CrossRef]
- Sieweke, J.T.; Pfeffer, T.J.; Biber, S.; Chatterjee, S.; Weissenborn, K.; Grosse, G.M.; Hagemus, J.; Derda, A.A.; Berliner, D.; Lichtinghagen, R.; et al. miR–21 and NT-proBNP Correlate with Echocardiographic Parameters of Atrial Dysfunction and Predict Atrial Fibrillation. J. Clin. Med. 2020, 9, 1118. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, S.; Li, X.; Guo, D.; Wang, Y.; Hu, Y. Serum miR-222 is independently associated with atrial fibrillation in patients with degenerative valvular heart disease. BMC Cardiovasc. Disord. 2021, 21, 98. [Google Scholar] [CrossRef]
- Viczenczova, C.; Bacova, B.S.; Benova, T.E.; Kura, B.; Yin, C.; Weismann, P.; Kukreja, R.; Slezak, J.; Tribulova, N. Myocardial connexin-43 and PKC signalling are involved in adaptation of the heart to irradiation-induced injury: Implication of miR-1 and miR-21. Gen. Physiol. Biophys. 2016, 35, 215–222. [Google Scholar] [CrossRef]
- Chałubińska-Fendler, J.; Nowicka, Z.; Dróżdż, I.; Graczyk, Ł.; Piotrowski, G.; Tomasik, B.; Spych, M.; Fijuth, J.; Papis-Ubych, A.; Kędzierawski, P.; et al. Radiation-induced circulating microRNAs linked to echocardiography parameters after radiotherapy. Front. Oncol. 2023, 13, 1150979. [Google Scholar] [CrossRef]
- Rogers, C.J.; Lukaszewicz, A.I.; Yamada-Hanff, J.; Micewicz, E.D.; Ratikan, J.A.; Starbird, M.A.; Miller, T.A.; Nguyen, C.; Lee, J.T.; Olafsen, T.; et al. Identification of miRNA signatures associated with radiation-induced late lung injury in mice. PLoS ONE 2020, 15, e0232411. [Google Scholar] [CrossRef]
- Esplugas, R.; Bellés, M.; Serra, N.; Arenas, M.; Hernández, V.; Vallvé, J.C.; Linares, V. Effect of Radiation on the Expression of CVD-related miRNAs, Inflammation and Endothelial Dysfunction of HUVECs. Anticancer. Res. 2019, 39, 771–780. [Google Scholar] [CrossRef] [PubMed]

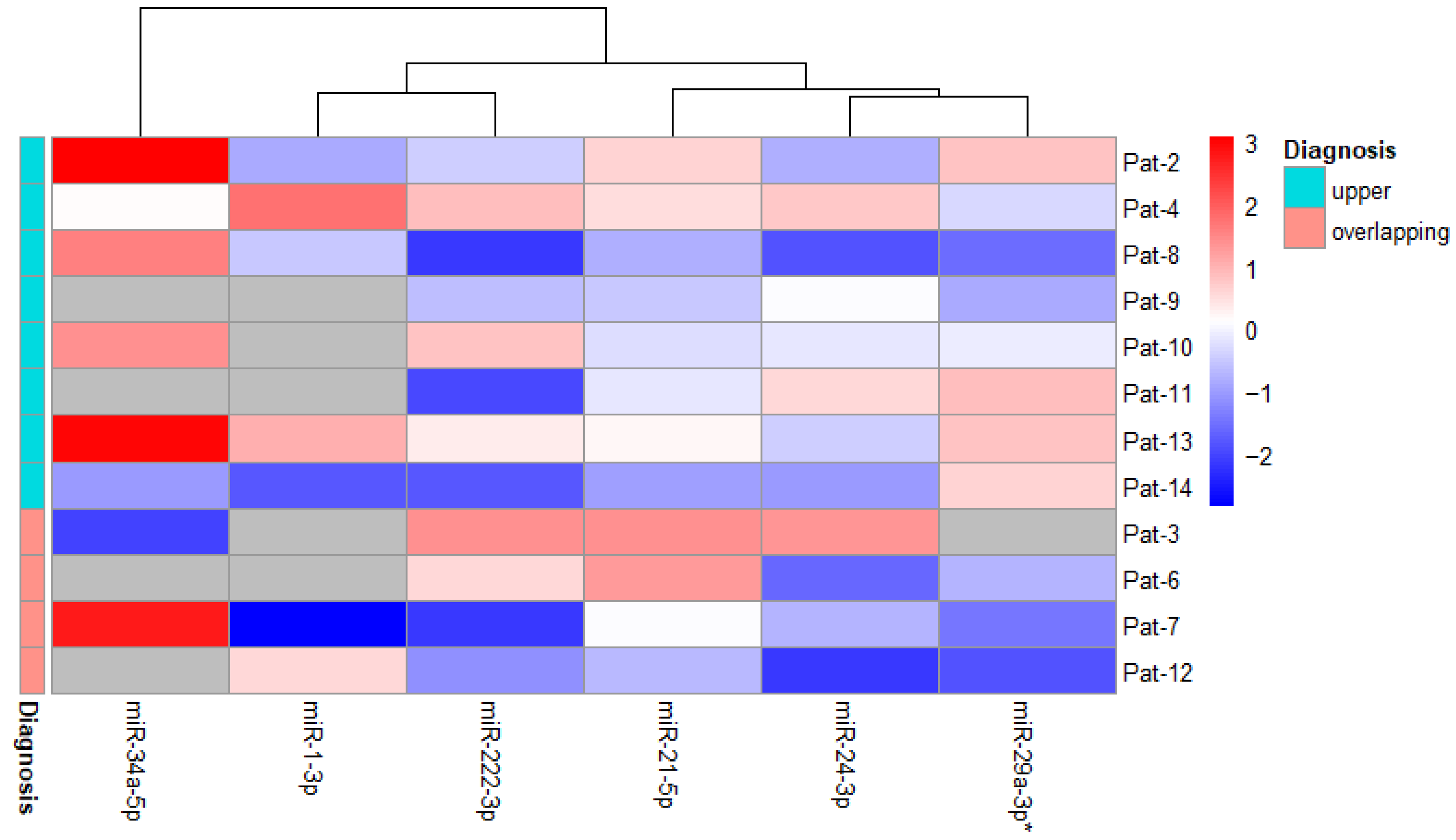
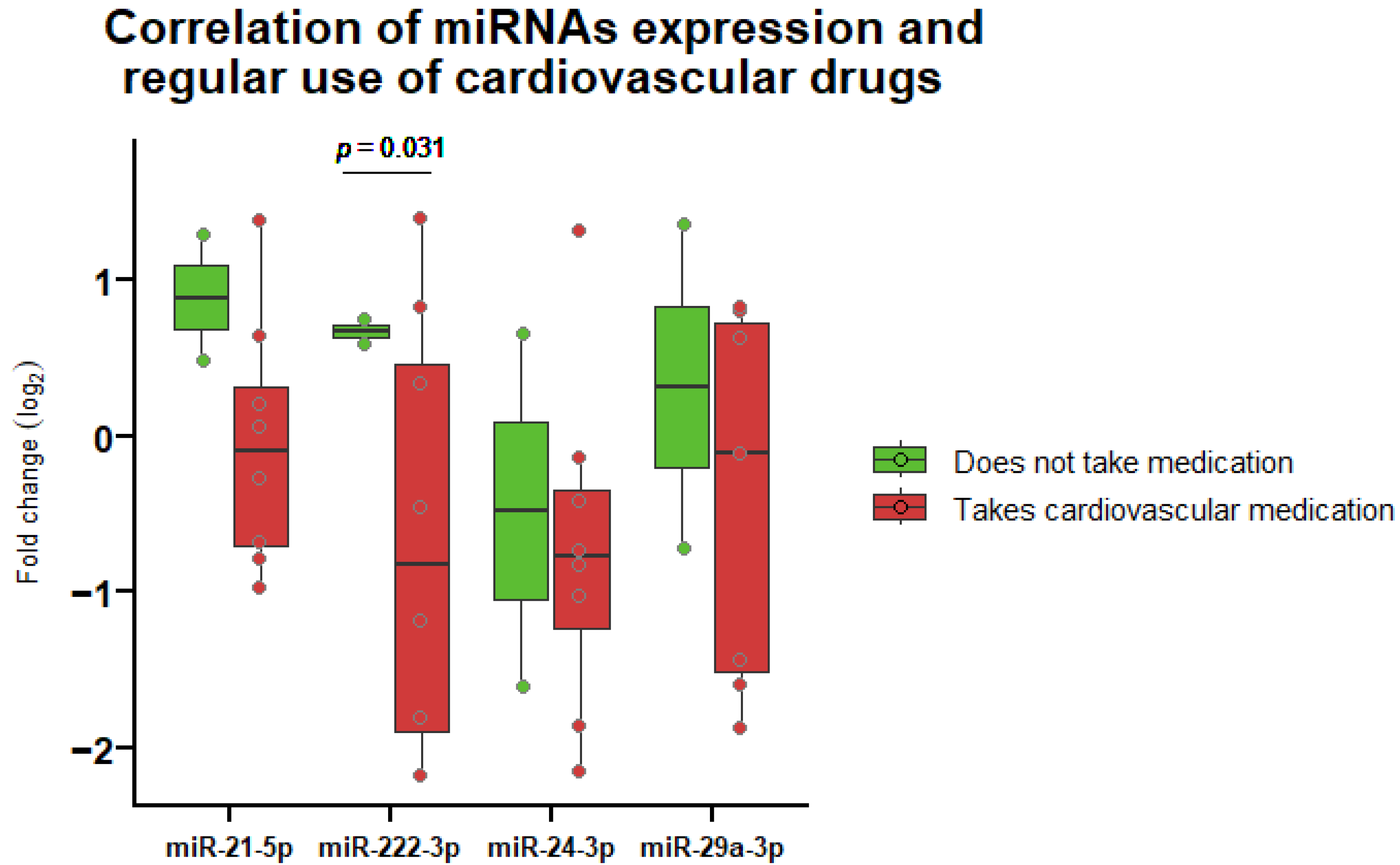

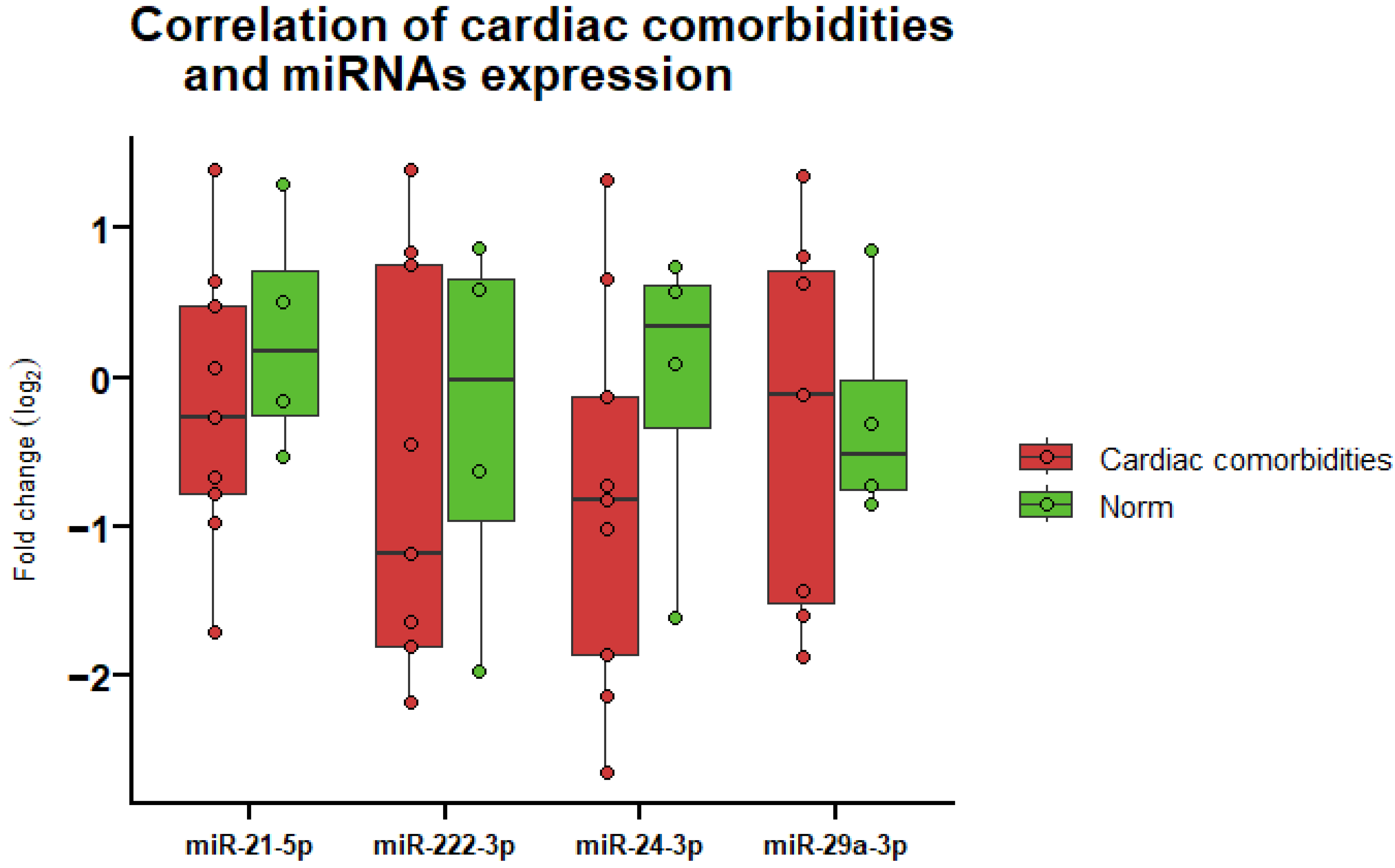


| Patient ID | Sex | Age | NT-proBNP | Tn I | Cardiac Comorbidity | Diagnosis | Regular Use of Cardiovascular Medication |
|---|---|---|---|---|---|---|---|
| Pat-1 | Female | 71 | ↑1 | Norm | + 2 | Middle 4 | Other 7 |
| Pat-2 | Female | 81 | ↑ | Norm | + | Upper 5 | + |
| Pat-3 | Male | 68 | No data | No data | + | Overlapping 6 | + |
| Pat-4 | Male | 62 | Norm | Norm | −3 | Upper | Other |
| Pat-5 | Male | 68 | Norm | Norm | + | Middle | − |
| Pat-6 | Male | 65 | Norm | Norm | − | Overlapping | − |
| Pat-7 | Male | 77 | ↑ | Norm | + | Overlapping | + |
| Pat-8 | Male | 72 | ↑ | ↑ | + | Upper | + |
| Pat-9 | Male | 67 | Norm | Norm | − | Upper | Other |
| Pat-10 | Female | 76 | ↑ | Norm | + | Upper | + |
| Pat-11 | Male | 75 | No data | No data | − | Upper | Other |
| Pat-12 | Male | 78 | ↑ | Norm | + | Overlapping | + |
| Pat-13 | Male | 70 | No data | No data | No data | Upper | + |
| Pat-14 | Male | 73 | No data | No data | + | Upper | + |
| Panel | AUC | 95% CI | Sensitivity, % | Specificity, % | PPV, % | NPV, % | p Value |
|---|---|---|---|---|---|---|---|
| miR-21-5p | 0.67 | 0.35, 0.98 | 44.44 | 100.00 | 100.00 | 44.44 | 0.339 |
| miR-24-3p | 0.69 | 0.36, 1.00 | 77.78 | 75.00 | 87.50 | 60.00 | 0.289 |
| miR-29a-3p | 0.46 | 0.07, 0.85 | 57.14 | 75.00 | 80.00 | 50.00 | 0.865 |
| miR-222-3p | 0.58 | 0.23, 0.94 | 55.56 | 75.00 | 83.33 | 42.86 | 0.666 |
| Four miRs panel | 0.79 | 0.48, 1.00 | 71.43 | 100.00 | 100.00 | 66.67 | 0.106 |
| Panel | AUC | 95% CI | Sensitivity, % | Specificity, % | PPV, % | NPV, % | p Value |
|---|---|---|---|---|---|---|---|
| NT-proBNP | 0.93 | 0.79, 1.00 | 85.71 | 100.00 | 100.00 | 75.00 | <0.001 |
| NT-proBNP + miR-21-5p | 0.91 | 0.70, 1.00 | 85.71 | 100.00 | 100.00 | 75.00 | 0.004 |
| NT-proBNP + miR-24-3p | 0.95 | 0.82, 1.00 | 85.71 | 100.00 | 100.00 | 75.00 | <0.001 |
| NT-proBNP + miR-29a-3p | 1.00 | 1.00, 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | <0.001 |
| NT-proBNP + miR-222-3p | 0.95 | 0.82, 1.00 | 85.71 | 100.00 | 100.00 | 75.00 | <0.001 |
| NT-proBNP + four miRs panel | 1.00 | 1.00, 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazlauskaitė, P.; Vaicekauskaitė, I.; Venius, J.; Sabaliauskaitė, R.; Steponavičienė, R. Plasma microRNAs as Biomarkers for Predicting Radiotherapy Treatment-Induced Cardiotoxicity in Lung Cancer. Life 2024, 14, 1619. https://doi.org/10.3390/life14121619
Kazlauskaitė P, Vaicekauskaitė I, Venius J, Sabaliauskaitė R, Steponavičienė R. Plasma microRNAs as Biomarkers for Predicting Radiotherapy Treatment-Induced Cardiotoxicity in Lung Cancer. Life. 2024; 14(12):1619. https://doi.org/10.3390/life14121619
Chicago/Turabian StyleKazlauskaitė, Paulina, Ieva Vaicekauskaitė, Jonas Venius, Rasa Sabaliauskaitė, and Rita Steponavičienė. 2024. "Plasma microRNAs as Biomarkers for Predicting Radiotherapy Treatment-Induced Cardiotoxicity in Lung Cancer" Life 14, no. 12: 1619. https://doi.org/10.3390/life14121619
APA StyleKazlauskaitė, P., Vaicekauskaitė, I., Venius, J., Sabaliauskaitė, R., & Steponavičienė, R. (2024). Plasma microRNAs as Biomarkers for Predicting Radiotherapy Treatment-Induced Cardiotoxicity in Lung Cancer. Life, 14(12), 1619. https://doi.org/10.3390/life14121619








