Cancer-Therapy-Related Cardiac Dysfunction: Latest Advances in Prevention and Treatment
Abstract
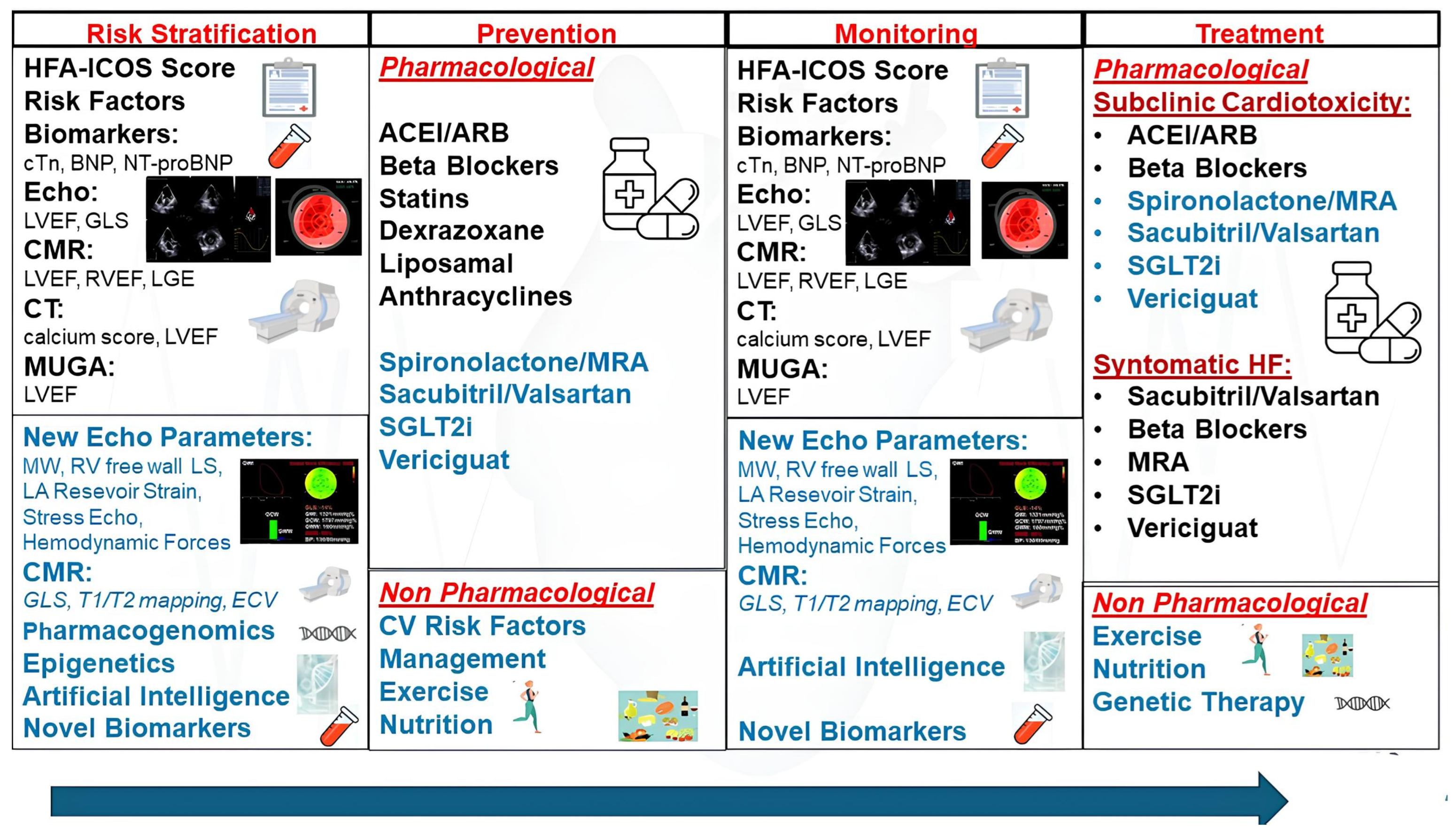
:1. Introduction
2. Classes of Cancer Therapy and Their Mechanisms Causing CTRCD
3. Early Detection and Monitoring of CTRCD
3.1. Serum Cardiac Biomarkers
3.2. Imaging Techniques for Monitoring Cardiac Function
4. Pharmacological Interventions for Prevention of CTRCD
5. Pharmacological Interventions for Treatment of CTRCD
6. Novel Cardioprotective Drugs for Prevention of CTRCD
6.1. Sacubitril/Valsartan
6.2. SGLT2i
6.3. Vericiguat
7. Lifestyle Modifications and Non-Pharmacological Strategies for Prevention of CTRCD
7.1. Exercise and Rehabilitation
7.2. Nutritional Interventions
8. Future Directions and Innovative Technologies for Prevention, Early Detection, and Treatment of CTRCD
8.1. Pharmacogenomics and Epigenetics in Predicting CTRCD
8.2. Artificial Intelligence (AI) for Prediction of CTRCD Risk and Treatment Response
8.3. Novel Biomarkers and Molecular Targets for Early Detection of CTRCD
8.4. Imaging Tecniques for Early Detection of CTRCD
8.5. Gene Therapy in Addressing and Reversing CTRCD
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Bychowski, J.; Sobiczewski, W. Current perspectives of cardio-oncology: Epidemiology, adverse effects, pre-treatment screening and prevention strategies. Cancer Med. 2023, 12, 14545–14555. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.; Chang, H.M. Oncocardiology—Past, present, and future: A review. JAMA Cardiol. 2016, 1, 1066–1072. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluationof adult patients during andafter cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Čelutkienė, J.; Pudil, R.; López-Fernández, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465, Erratum in Eur. Heart J. Cardiovasc. Imaging 2023, 24, e98. [Google Scholar] [CrossRef]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A.; et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef]
- Hutchins, E.; Yang, E.H.; Stein-Merlob, A.F. Inflammation in Chemotherapy-Induced Cardiotoxicity. Curr. Cardiol. Rep. 2024, 26, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Veeder, J.A.; Hothem, L.N.; Cipriani, A.E.; Jensen, B.C.; Rodgers, J.E. Chemotherapy-associated cardiomyopathy: Mechanisms of toxicity and cardioprotective strategies. Pharmacotherapy 2021, 41, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Lenneman, C.G.; Sawyer, D.B. Cardio-oncology: An update on cardiotoxicity of cancer-related treatment. Circ. Res. 2016, 118, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Sahni, G.; Gallardo, A.; Spahillari, A.; Galsky, M.; Eschenhagen, T.; Schaffer, W.; Neilan, T.G.; Ghosh, A.K.; Donisan, T.; et al. Cancer Therapeutic Drug Guide. In Cardio-Oncology Practice Manual: A Companion to Braunwald’s Heart Disease; Herrmann, J., Ed.; Elsevier: Philadelphia, PA, USA, 2023; Volume 4, pp. 452–526. [Google Scholar]
- Saiki, H.; Petersen Yusuf, S.W.; Venkatesulu, B.P.; Mahadevan, L.S.; Krishnan, S. Radiation-induced cardiovascular disease: A clinical perspective. Front. Cardiovasc. Med. 2017, 4, 66. [Google Scholar]
- Michel, L.; Mincu, R.I.; Mahabadi, A.A.; Settelmeier, S.; Al-Rashid, F.; Rassaf, T.; Totzeck, M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: A meta-analysis. Eur. J. Heart Fail. 2020, 22, 350–361. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270, Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412; Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 969. [Google Scholar] [CrossRef]
- Hoffmann, R.; Barletta, G.; von Bardeleben, S.; Vanoverschelde, J.L.; Kasprzak, J.; Greis, C.; Becher, H. Analysis of left ventricular volumes and function: A multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 292–301. [Google Scholar] [CrossRef]
- Porter, T.R.; Mulvagh, S.L.; Abdelmoneim, S.S.; Becher, H.; Belcik, J.T.; Bierig, M.; Choy, J.; Gaibazzi, N.; Gillam, L.D.; Janardhanan, R.; et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J. Am. Soc. Echocardiogr. 2018, 31, 241–274. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain imaging in cardio-oncology. Cardio Oncol. 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Mousavi, N.; Tan, T.C.; Ali, M.; Halpern, E.F.; Wang, L.; Scherrer-Crosbie, M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50%–59% treated with anthracyclines. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 977–984. [Google Scholar] [CrossRef]
- Ali, M.T.; Yucel, E.; Bouras, S.; Wang, L.; Fei, H.W.; Halpern, E.F.; Scherrer-Crosbie, M. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J. Am. Soc. Echocardiogr. 2016, 29, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar] [CrossRef]
- Contaldi, C.; Montesarchio, V.; Catapano, D.; Falco, L.; Caputo, F.; D’Aniello, C.; Masarone, D.; Pacileo, G. Multimodality Cardiovascular Imaging of Cardiotoxicity Due to Cancer Therapy. Life 2023, 13, 2103. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Van den Eynde, J.; Cordrey, K.; Long, R.; Danford, D.A.; Hays, A.G.; Barnes, B.T.; Kutty, S. Deterioration in myocardial work indices precedes changes in global longitudinal strain following anthracycline chemotherapy. Int. J. Cardiol. 2022, 363, 171–178. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Thampinathan, B.; Somerset, E.; Shalmon, T.; Amir, E.; Steve Fan, C.P.; Moon, S.; Abdel-Qadir, H.; Thevakumaran, Y.; Day, J.; et al. Diagnostic and Prognostic Value of Myocardial Work Indices for Identification of Cancer Therapy-Related Cardiotoxicity. JACC Cardiovasc. Imaging 2022, 15, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Buytaert, D.; Beles, M.; Paolisso, P.; Duchenne, J.; Huygh, G.; Langmans, C.; Roelstraete, A.; Verstreken, S.; Goethals, M.; et al. Serial Non-Invasive Myocardial Work Measurements for Patient Risk Stratification and Early Detection of Cancer Therapeutics-Related Cardiac Dysfunction in Breast Cancer Patients: A Single-Centre Observational Study. J. Clin. Med. 2023, 12, 1652. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Kang, R.; Zhao, Y.; Chen, L.; Liu, F.; Wang, X.; Peng, Y.; Zhang, C. Evaluating the effect of PD-1 inhibitors on left ventricular function in lung cancer with noninvasive myocardial work. Quant. Imaging Med. Surg. 2023, 13, 3241–3254. [Google Scholar] [CrossRef]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef]
- El-Sherbeny, W.S.; Sabry, N.M.; El-Saied, S.B.; Elnagar, B. Detection of right ventricular dysfunction by three-dimensional echocardiography and two-dimensional speckle tracking in breast cancer patients receiving anthracycline-based chemotherapy. Cardiooncology 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Cherata, D.A.; Donoiu, I.; Diaconu, R.; Glodeanu, A.; Cârstea, D.; Militaru, C.; Istratoaie, O. Longitudinal strain analysis allows the identification of subclinical deterioration of right ventricular function in patients with cancer therapy-related left ventricular dysfunction. Discoveries 2019, 7, e94. [Google Scholar] [CrossRef]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Clarke, J.L.; Thomas, L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Moreo, A.; Casavecchia, G.; Cadeddu Dessalvi, C.; Bergamini, C.; Zito, C.; Madaudo, C.; Madonna, R.; Cameli, M.; Novo, G. Atrial Strain Assessment for the Early Detection of Cancer Therapy-Related Cardiac Dysfunction in Breast Cancer Women (The STRANO STUDY: Atrial Strain in Cardio-Oncology). J. Clin. Med. 2023, 12, 7127. [Google Scholar] [CrossRef]
- Novo, G.; Santoro, C.; Manno, G.; Di Lisi, D.; Esposito, R.; Mandoli, G.E.; Evola, V.; Pastore, M.C.; Sperlongano, S.; D’Andrea, A.; et al. Usefulness of Stress Echocardiography in the Management of Patients Treated with Anticancer Drugs. J. Am. Soc. Echocardiogr. 2021, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Gargani, L.; Naeije, R.; Rudski, L.; Armstrong, W.F.; Wierzbowska-Drabik, K.; Argiento, P.; Bandera, F.; Cademartiri, F.; Citro, R.; et al. Feasibility of semi-recumbent bicycle exercise Doppler echocardiography for the evaluation of the right heart and pulmonary circulation unit in different clinical conditions: The RIGHT heart international NETwork (RIGHT-NET). Int. J. Cardiovasc. Imaging 2021, 37, 2151–2167. [Google Scholar] [CrossRef]
- Ferrara, F.; Gargani, L.; Contaldi, C.; Agoston, G.; Argiento, P.; Armstrong, W.F.; Bandera, F.; Cademartiri, F.; Citro, R.; Cittadini, A.; et al. A multicentric quality-control study of exercise Doppler echocardiography of the right heart and the pulmonary circulation. The RIGHT Heart International NETwork (RIGHT-NET). Cardiovasc. Ultrasound. 2021, 19, 9. [Google Scholar] [CrossRef]
- Civelli, M.; Cardinale, D.; Martinoni, A.; Lamantia, G.; Colombo, N.; Colombo, A.; Gandini, S.; Martinelli, G.; Fiorentini, C.; Cipolla, C.M. Early reduction in left ventricular contractile reserve detected by dobutamine stress echo predicts high-dose chemotherapy-induced cardiac toxicity. Int. J. Cardiol. 2006, 111, 120–126. [Google Scholar] [CrossRef]
- Hamada, H.; Ohkubo, T.; Maeda, M.; Ogawa, S. Evaluation of cardiac reserved function by high-dose dobutamine stress echocardiography in asymptomatic anthracycline-treated survivors of childhood cancer. Pediatr. Int. 2006, 48, 313–320. [Google Scholar] [CrossRef]
- Bountioukos, M.; Doorduijn, J.K.; Roelandt, J.R.; Vourvouri, E.C.; Bax, J.J.; Schinkel, A.F.; Kertai, M.D.; Sonneveld, P.; Poldermans, D. Repetitive dobutamine stress echocardiography for the prediction of anthracycline cardiotoxicity. Eur. J. Echocardiogr. 2003, 4, 300–305. [Google Scholar] [CrossRef]
- Sen, F.; Yildiz, I.; Basaran, M.; Ekenel, M.; Oz, F.; Kilic, L.; Toz, B.; Gurdal, A.; Camlica, H.; Bavbek, S.; et al. Impaired coronary flow reserve in metastatic cancer patients treated with sunitinib. J. BUON 2013, 18, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Capuano, F.; Cocchia, R.; Ranieri, B.; Contaldi, C.; Lacava, G.; Capone, V.; Chianese, S.; Rega, S.; Annunziata, R.; et al. Reference Ranges of Left Ventricular Hemodynamic Forces in Healthy Adults: A Speckle-Tracking Echocardiographic Study. J. Clin. Med. 2021, 10, 5937. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, E.; Marwick, T. Can haemodynamic force analysis be used to identify cancer treatment-related cardiac dysfunction? A comparison with strain and ejection fraction. Eur. Heart J. 2024, 45, ehae666.3197. [Google Scholar] [CrossRef]
- Bellenger, N.G.; Burgess, M.I.; Ray, S.G.; Lahiri, A.; Coats, A.J.; Cleland, J.G.; Pennell, D.J. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur. Heart J. 2000, 21, 1387–1396. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; D’Ascenzi, F.; Mandoli, G.E.; Rizzo, L.; Sisti, N.; Carrucola, C.; Cameli, P.; Bigio, E.; Mondillo, S.; Cameli, M. Traditional and novel imaging of right ventricular function in patients with heart failure and reduced ejection fraction. Curr. Heart Fail. Rep. 2020, 17, 28–33. [Google Scholar] [CrossRef]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.; Shah, D.J.; Jue, J.; White, B.E.; Shenoy, C.; et al. Association of Feature-Tracking Cardiac Magnetic Resonance Imaging Left Ventricular Global Longitudinal Strain with All-Cause Mortality in Patients with Reduced Left Ventricular Ejection Fraction. Circulation 2017, 135, 2313–2315. [Google Scholar] [CrossRef]
- Lunning, M.A.; Kutty, S.; Rome, E.T.; Li, L.; Padiyath, A.; Loberiza, F.; Bociek, R.G.; Bierman, P.J.; Vose, J.M.; Armitage, J.O.; et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am. J. Clin. Oncol. 2015, 38, 377–381. [Google Scholar] [CrossRef]
- Ong, G.; Brezden-Masley, C.; Dhir, V.; Deva, D.P.; Chan, K.K.W.; Chow, C.M.; Thavendiranathan, D.; Haq, R.; Barfett, J.J.; Petrella, T.M.; et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int. J. Cardiol. 2018, 261, 228–233. [Google Scholar] [CrossRef]
- Lambert, J.; Lamacie, M.; Thampinathan, B.; Altaha, M.A.; Esmaeilzadeh, M.; Nolan, M.; Fresno, C.U.; Somerset, E.; Amir, E.; Marwick, T.H.; et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart 2020, 106, 817–823. [Google Scholar] [CrossRef]
- Jordan, J.H.; Castellino, S.M.; Meléndez, G.C.; Klepin, H.D.; Ellis, L.R.; Lamar, Z.; Vasu, S.; Kitzman, D.W.; Ntim, W.O.; Brubaker, P.H.; et al. Left Ventricular Mass Change After Anthracycline Chemotherapy. Circ. Heart Fail. 2018, 11, e004560. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Shalmon, T.; Fan, C.S.; Houbois, C.; Amir, E.; Thevakumaran, Y.; Somerset, E.; Malowany, J.M.; Urzua-Fresno, C.; Yip, P.; et al. Comprehensive Cardiovascular Magnetic Resonance Tissue Characterization and Cardiotoxicity in Women with Breast Cancer. JAMA Cardiol. 2023, 8, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Farhad, H.; Staziaki, P.V.; Addison, D.; Coelho-Filho, O.R.; Shah, R.V.; Mitchell, R.N.; Szilveszter, B.; Abbasi, S.A.; Kwong, R.Y.; Scherrer-Crosbie, M.; et al. Characterization of the Changes in Cardiac Structure and Function in Mice Treated with Anthracyclines Using Serial Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2016, 9, e003584. [Google Scholar] [CrossRef] [PubMed]
- Contaldi, C.; Imbriaco, M.; Alcidi, G.; Ponsiglione, A.; Santoro, C.; Puglia, M.; Barbuto, L.; Cuocolo, A.; Trimarco, B.; Galderisi, M. Assessment of the relationships between left ventricular filling pressures and longitudinal dysfunction with myocardial fibrosis in uncomplicated hypertensive patients. Int. J. Cardiol. 2016, 202, 84–86. [Google Scholar] [CrossRef]
- Tham, E.B.; Haykowsky, M.J.; Chow, K.; Spavor, M.; Kaneko, S.; Khoo, N.S.; Pagano, J.J.; Mackie, A.S.; Thompson, R.B. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: Relationship to exercise capacity, cumulative dose and remodeling. J. Cardiovasc. Magn. Reson. 2013, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Muehlberg, F.; Funk, S.; Zange, L.; von Knobelsdorff-Brenkenhoff, F.; Blaszczyk, E.; Schulz, A.; Ghani, S.; Reichardt, A.; Reichardt, P.; Schulz-Menger, J. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail. 2018, 5, 620–629. [Google Scholar] [CrossRef]
- Plana, J.C.; Thavendiranathan, P.; Bucciarelli-Ducci, C.; Lancellotti, P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc. Imaging 2018, 11, 1173–1186. [Google Scholar] [CrossRef]
- Saunderson, C.E.D.; Plein, S.; Manisty, C.H. Role of cardiovascular magnetic resonance imaging in cardio-oncology. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 383–396. [Google Scholar] [CrossRef]
- Neilan, T.G.; Coelho-Filho, O.R.; Shah, R.V.; Feng, J.H.; Pena-Herrera, D.; Mandry, D.; Pierre-Mongeon, F.; Heydari, B.; Francis, S.A.; Moslehi, J.; et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracyclinebased chemotherapy. Am. J. Cardiol. 2013, 111, 717–722. [Google Scholar] [CrossRef]
- Meléndez, G.C.; Jordan, J.H.; D’Agostino, R.B., Jr.; Vasu, S.; Hamilton, C.A.; Hundley, W.G. Progressive 3-Month Increase in LV Myocardial ECV After Anthracycline-Based Chemotherapy. JACC Cardiovasc. Imaging 2017, 10, 708–709. [Google Scholar] [CrossRef]
- Ferreira de Souza, T.; Quinaglia AC Silva, T.; Osorio Costa, F.; Shah, R.; Neilan, T.G.; Velloso, L.; Nadruz, W.; Brenelli, F.; Sposito, A.C.; Matos-Souza, J.R.; et al. Anthracycline Therapy Is Associated with Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc. Imaging 2018, 11, 1045–1055. [Google Scholar] [CrossRef]
- Maffei, E.; Messalli, G.; Martini, C.; Nieman, K.; Catalano, O.; Rossi, A.; Seitun, S.; Guaricci, A.I.; Tedeschi, C.; Mollet, N.R.; et al. Left and right ventricle assessment with Cardiac CT: Validation study vs. Cardiac MR. Eur. Radiol. 2012, 22, 1041–1049. [Google Scholar] [CrossRef]
- Sharma, A.; Einstein, A.J.; Vallakati, A.; Arbab-Zadeh, A.; Mukherjee, D.; Lichstein, E. Meta-analysis of global left ventricular function comparing multidetector computed tomography with cardiac magnetic resonance imaging. Am. J. Cardiol. 2014, 113, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mattei, J.C.; Yang, E.H.; Ferencik, M.; Baldassarre, L.A.; Dent, S.; Budoff, M.J. Cardiac computed tomography in cardiooncology: JACC: CardioOncology primer. Cardio Oncol. 2021, 3, 635–649. [Google Scholar]
- Lopez-Mattei, J.; Yang, E.H.; Baldassarre, L.A.; Agha, A.; Blankstein, R.; Choi, A.D.; Chen, M.Y.; Meyersohn, N.; Daly, R.; Slim, A.; et al. Cardiac computed tomographic imaging in cardio-oncology: An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). Endorsed by the International Cardio-Oncology Society (ICOS). J. Cardiovasc. Comput. Tomogr. 2023, 17, 66–83. [Google Scholar] [CrossRef]
- Sueta, D.; Kidoh, M.; Oda, S.; Egashira, K.; Yamamoto, E.; Kaikita, K.; Matsushita, K.; Yamamoto, Y.; Hirai, T.; Tsujita, K. Usefulness of Cardiac Computed Tomography in the Diagnosis of Anti-Cancer Therapy-Related Cardiac Dysfunction-Consistency with Magnetic Resonance Imaging. Circ. J. 2021, 85, 393–396. [Google Scholar] [CrossRef]
- Tu, C.; Shen, H.; Liu, R.; Wang, X.; Li, X.; Yuan, X.; Chen, Q.; Wang, Y.; Ran, Z.; Lan, X.; et al. Myocardial extracellular volume derived from contrast-enhanced chest computed tomography for longitudinal evaluation of cardiotoxicity in patients with breast cancer treated with anthracyclines. Insights Imaging 2022, 13, 85. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.A.; Othman, A.I.; Amer, M.; El-Missiry, M.A. Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J. Appl. Toxicol. 2001, 21, 469–473. [Google Scholar] [CrossRef]
- Okumura, K.; Jin, D.; Takai, S.; Miyazaki, M. Beneficial effects of angiotensin-converting enzyme inhibition in adriamycin-induced cardiomyopathy in hamsters. Jpn. J. Pharmacol. 2002, 88, 183–188. [Google Scholar] [CrossRef]
- Vaynblat, M.; Shah, H.R.; Bhaskaran, D.; Ramdev, G.; Davis, W.J., 3rd; Cunningham, J.N., Jr.; Chiavarelli, M. Simultaneous angiotensin converting enzyme inhibition moderates ventricular dysfunction caused by doxorubicin. Eur. J. Heart Fail. 2002, 4, 583–586. [Google Scholar] [CrossRef]
- Boucek, R.J., Jr.; Steele, A.; Miracle, A.; Atkinson, J. Effects of angiotensin-converting enzyme inhibitor on delayed-onset doxorubicin-induced cardiotoxicity. Cardiovasc. Toxicol. 2003, 3, 319–329. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 2004, 25, 1454–1470. [Google Scholar] [PubMed]
- Cernecka, H.; Ochodnicka-Mackovicova, K.; Kucerova, D.; Kmecova, J.; Nemcekova, V.; Doka, G.; Kyselovic, J.; Krenek, P.; Ochodnicky, P.; Klimas, J. Enalaprilat increases PPARβ/δ expression, without influence on PPARα and PPARγ, and modulate cardiac function in sub-acute model of daunorubicin-induced cardiomyopathy. Eur. J. Pharmacol. 2013, 714, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Zeglinski, M.; Ludke, A.; Jassal, D.S.; Singal, P.K. Trastuzumab-induced cardiac dysfunction: A ’dual-hit’. Exp. Clin. Cardiol. 2011, 16, 70–74. [Google Scholar]
- Vermeulen, Z.; Hervent, A.S.; Dugaucquier, L.; Vandekerckhove, L.; Rombouts, M.; Beyens, M.; Schrijvers, D.M.; De Meyer, G.R.Y.; Maudsley, S.; De Keulenaer, G.W.; et al. Inhibitory actions of the NRG-1/ErbB4 pathway in macrophages during tissue fibrosis in the heart, skin, and lung. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H934–H945. [Google Scholar] [CrossRef]
- Sandoo, A.; Kitas, G.; Carmichael, A. Breast cancer therapy and cardiovascular risk: Focus on trastuzumab. Vasc. Health Risk Manag. 2015, 11, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Blaes, A.H.; Gaillard, P.; Peterson, B.A.; Yee, D.; Virnig, B. Angiotensin converting enzyme inhibitors may be protective against cardiac complications following anthracycline chemotherapy. Breast Cancer Res. Treat. 2010, 122, 585–590. [Google Scholar] [CrossRef]
- Wittayanukorn, S.; Qian, J.; Westrick, S.C.; Billor, N.; Johnson, B.; Hansen, R.A. Prevention of trastuzumab and anthracyclineinduced cardiotoxicity using angiotensin-converting enzyme inhibitors or beta-blockers in older adults with breast cancer. Am. J. Clin. Oncol. 2018, 41, 909–918. [Google Scholar] [CrossRef]
- Lee, S.; Alsamarrai, A.; Xiao, A.; Wang, T.K.M. Prevention of anthracycline and trastuzumab-induced decline in left ventricular ejection fraction with angiotensin-converting enzyme inhibitors or angiotensin receptor blocker: A narrative systematic review of randomised controlled trials. Intern. Med. J. 2024, 54, 1254–1263. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef]
- Cardinale, D.; Ciceri, F.; Latini, R.; Franzosi, M.G.; Sandri, M.T.; Civelli, M.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Cavina, R.; et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur. J. Cancer 2018, 94, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Długosz-Danecka, M.; Gruszka, A.M.; Szmit, S.; Olszanecka, A.; Ogórka, T.; Sobociński, M.; Jaroszyński, A.; Krawczyk, K.; Skotnicki, A.B.; Jurczak, W. Primary Cardioprotection Reduces Mortality in Lymphoma Patients with Increased Risk of Anthracycline Cardiotoxicity, Treated by R-CHOP Regimen. Chemotherapy 2018, 63, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.; Buzdugan, E.; Ciuleanu, T.E.; Todor, N.; Stoicescu, L. Can the epirubicin cardiotoxicity in cancer patients be prevented by angiotensin converting enzyme inhibitors? J. BUON 2013, 18, 1052–1057. [Google Scholar] [PubMed]
- Nakamae, H.; Tsumura, K.; Terada, Y.; Nakane, T.; Nakamae, M.; Ohta, K.; Yamane, T.; Hino, M. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005, 104, 2492–2498. [Google Scholar] [CrossRef]
- Cadeddu, C.; Piras, A.; Mantovani, G.; Deidda, M.; Dessi, M.; Madeddu, C.; Massa, E.; Mercuro, G. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am. Heart J. 2010, 160, 487.e1–487.e7. [Google Scholar] [CrossRef]
- Dessi, M.; Piras, A.; Madeddu, C.; Cadeddu, C.; Deidda, M.; Massa, E.; Antoni, G.; Mantovani, G.; Mercuro, G. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction. Exp. Ther. Med. 2011, 2, 1003–1009. [Google Scholar] [CrossRef]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, A.; Storas, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef]
- Wang, J.; Pani, B.; Gokhan, I.; Xiong, X.; Kahsai, A.W.; Jiang, H.; Ahn, S.; Lefkowitz, R.J.; Rockman, H.A. β-Arrestin-Biased Allosteric Modulator Potentiates Carvedilol-Stimulated β Adrenergic Receptor Cardioprotection. Mol. Pharmacol. 2021, 100, 568–579. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusano, K.; Nakamura, Y.; Kakishita, M.; Ohta, K.; Nagase, S.; Yamamoto, M.; Miyaji, K.; Saito, H.; Morita, H.; et al. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation 2002, 105, 2867–2871. [Google Scholar] [CrossRef]
- Santos, D.L.; Moreno, A.J.; Leino, R.L.; Froberg, M.K.; Wallace, K.B. Carvedilol protects against doxorubicin-induced mitochondrial cardiomyopathy. Toxicol. Appl. Pharmacol. 2002, 185, 218–227. [Google Scholar] [CrossRef]
- Asanuma, H.; Minamino, T.; Sanada, S.; Takashima, S.; Ogita, H.; Ogai, A.; Asakura, M.; Liao, Y.; Asano, Y.; Shintani, Y.; et al. Beta-adrenoceptor blocker carvedilol provides cardioprotection via an adenosine-dependent mechanism in ischemic canine hearts. Circulation 2004, 109, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.M.; Tilley, D.G.; Chen, J.; Salazar, N.C.; Whalen, E.J.; Violin, J.D.; Rockman, H.A. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. USA 2008, 105, 14555–14560. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.E.; Gul, R.; Blessing, C.P.; Nguyen, J.; Liu, T.; Pulakat, L.; Bastepe, M.; Jackson, E.K.; Andresen, B.T. The β-blocker Nebivolol Is a GRK/β-arrestin biased agonist. PLoS ONE 2013, 8, e71980. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Ramirez, M.C.; Nes, K.; Schuster, A.; Aguayo, R.; Morales, M.; Ramos, C.; Hasson, D.; Sotomayor, C.G.; Henriquez, P.; et al. Prevention of doxorubicin-induced Cardiotoxicity by pharmacological non-hypoxic myocardial preconditioning based on Docosahexaenoic Acid (DHA) and carvedilol direct antioxidant effects: Study protocol for a pilot, randomized, double-blind, controlled trial (CarDHA trial). Trials 2020, 21, 137. [Google Scholar]
- Tashakori Beheshti, A.; Mostafavi Toroghi, H.; Hosseini, G.; Zarifian, A.; Homaei Shandiz, F.; Fazlinezhad, A. Carvedilol administration can prevent doxorubicin-induced cardiotoxicity: A double-blind randomized trial. Cardiology 2016, 134, 47–53. [Google Scholar] [CrossRef]
- Attar, A.; Behnagh, A.K.; Hosseini, M.; Amanollahi, F.; Shafiekhani, P.; Kabir, A. Beta-blockers for primary prevention of anthracycline-induced cardiac toxicity: An updated meta-analysis of randomized clinical trials. Cardiovasc. Ther. 2022, 2022, 8367444. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R., Jr.; das Dores Cruz, F.; Gonçalves Brandao, S.M.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Kalil Filho, R.; Hoff, P.M.; et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: The CECCY trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Abuosa, A.M.; Elshiekh, A.H.; Qureshi, K.; Abrar, M.B.; Kholeif, M.A.; Kinsara, A.J.; Andejani, A.; Ahmed, A.H.; Cleland, J.G.F. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J. 2018, 70 (Suppl. S3), S96–S100. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Q.; Yang, Z.G.; Diao, K.Y.; He, Y.; Shi, K.; Shen, M.T.; Fu, H.; Guo, Y.K. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity-a systematic review and meta-analysis of carvedilol. Heart Fail. Rev. 2019, 24, 325–333. [Google Scholar] [CrossRef]
- Bosch, X.; Rovira, M.; Sitges, M.; Domenech, A.; Ortiz-Perez, J.T.; de Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzo, M.; Esteve, J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 2013, 61, 2355–2362. [Google Scholar]
- Wei, Y.; Whaley-Connell, A.T.; Habibi, J.; Rehmer, J.; Rehmer, N.; Patel, K.; Hayden, M.; DeMarco, V.; Ferrario, C.M.; Ibdah, J.A.; et al. Mineralocorticoid receptor antagonism attenuates vascular apoptosis and injury via rescuing protein kinase B activation. Hypertension 2009, 53, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Akpek, M.; Ozdogru, I.; Sahin, O.; Inanc, M.; Dogan, A.; Yazici, C.; Berk, V.; Karaca, H.; Kalay, N.; Oguzhan, A.; et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2015, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A.; Hall, P.; MacPherson, I.R.; Joshi, S.S.; Singh, T.; Maclean, M.; Lewis, S.; Rodriguez, A.; Fletcher, A.; Everett, R.J.; et al. Multicenter, Prospective, Randomized Controlled Trial of High-Sensitivity Cardiac Troponin I-Guided Combination Angiotensin Receptor Blockade and Beta-Blocker Therapy to Prevent Anthracycline Cardiotoxicity: The Cardiac CARE Trial. Circulation 2023, 148, 1680–1690. [Google Scholar] [CrossRef]
- Livi, L.; Barletta, G.; Martella, F.; Saieva, C.; Desideri, I.; Bacci, C.; Del Bene, M.R.; Airoldi, M.; Amoroso, D.; Coltelli, L.; et al. Cardioprotective Strategy for Patients with Nonmetastatic Breast Cancer Who Are Receiving an Anthracycline-Based Chemotherapy: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.S.; Eekhoudt, C.R.; Jassal, D.S. Mechanisms of anthracycline-mediated cardiotoxicity and preventative strategies in women with breast cancer. Mol. Cell Biochem. 2021, 476, 3099–3109. [Google Scholar] [CrossRef]
- Keshavarzian, E.; Sadighpour, T.; Mortazavizadeh, S.M.; Soltani, M.; Motevalipoor, A.F.; Khamas, S.S.; Moazen, M.; Kogani, M.; Amin Hashemipour, S.M.; Hosseinpour, H.; et al. Prophylactic Agents for Preventing Cardiotoxicity Induced Following Anticancer Agents: A Systematic Review and Meta-Analysis of Clinical Trials. Rev. Recent Clin. Trials. 2023, 18, 112–122. [Google Scholar]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef]
- Jiang, R.; Lou, L.; Shi, W.; Chen, Y.; Fu, Z.; Liu, S.; Sok, T.; Li, Z.; Zhang, X.; Yang, J. Statins in Mitigating Anticancer Treatment-Related Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 10177. [Google Scholar] [CrossRef]
- Riad, A.; Bien, S.; Westermann, D.; Becher, P.M.; Loya, K.; Landmesser, U.; Kroemer, H.K.; Schultheiss, H.P.; Tschope, C. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009, 69, 695–699. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, S.; Ge, L.; Zhang, S.; Zhai, C.; Chen, W.; Sui, S. Atorvastatin Improves Doxorubicin-Induced Cardiac Dysfunction by Modulating Hsp70, Akt, and MAPK Signaling Pathways. J. Cardiovasc. Pharmacol. 2019, 73, 223–231. [Google Scholar] [CrossRef]
- Pecoraro, M.; Marzocco, S.; Belvedere, R.; Petrella, A.; Franceschelli, S.; Popolo, A. Simvastatin Reduces Doxorubicin-Induced Cardiotoxicity: Effects beyond Its Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 7573. [Google Scholar] [CrossRef] [PubMed]
- Refaie, M.M.; El-Hussieny, M.; Bayoumi, A.M.; Shehata, S.; Welson, N.N.; Abdelzaher, W.Y. Simvastatin cardioprotection in cyclophosphamide-induced toxicity via the modulation of inflammasome/caspase 1/interleukin1β pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221111440. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Elkhoely, A.A. Targeting proinflammatory cytokines, oxidative stress, TGF-β1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomed. Pharmacother. 2017, 93, 17–26. [Google Scholar] [CrossRef]
- Cho, D.H.; Lim, I.R.; Kim, J.H.; Kim, M.N.; Kim, Y.H.; Park, K.H.; Park, S.M.; Shim, W.J. Protective Effects of Statin and Angiotensin Receptor Blocker in a Rat Model of Doxorubicin- and Trastuzumab-Induced Cardiomyopathy. J. Am. Soc. Echocardiogr. 2020, 33, 1253–1263. [Google Scholar] [CrossRef]
- Muhammad, R.N.; Sallam, N.; El-Abhar, H.S. Activated ROCK/Akt/eNOS and ET-1/ERK pathways in 5-fluorouracil-induced cardiotoxicity: Modulation by simvastatin. Sci. Rep. 2020, 10, 14693. [Google Scholar] [CrossRef] [PubMed]
- Monceau, V.; Pasinetti, N.; Schupp, C.; Pouzoulet, F.; Opolon, P.; Vozenin, M.C. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr. Drug Targets 2010, 11, 1395–1404. [Google Scholar] [CrossRef]
- Ait-Aissa, K.; Leng, L.N.; Lindsey, N.R.; Guo, X.; Juhr, D.; Koval, O.M.; Grumbach, I.M. Mechanisms by which statins protect endothelial cells from radiation-induced injury in the carotid artery. Front. Cardiovasc. Med. 2023, 10, 1133315. [Google Scholar] [CrossRef]
- Lenarczyk, M.; Su, J.; Haworth, S.T.; Komorowski, R.; Fish, B.L.; Migrino, R.Q.; Harmann, L.; Hopewell, J.W.; Kronenberg, A.; Patel, S.; et al. Simvastatin mitigates increases in risk factors for and the occurrence of cardiac disease following 10 Gy total body irradiation. Pharmacol. Res. Perspect. 2015, 3, e00145. [Google Scholar] [CrossRef]
- Efentakis, P.; Choustoulaki, A.; Kwiatkowski, G.; Varela, A.; Kostopoulos, I.V.; Tsekenis, G.; Ntanasis-Stathopoulos, I.; Georgoulis, A.; Vorgias, C.E.; Gakiopoulou, H.; et al. Early microvascular coronary endothelial dysfunction precedes pembrolizumab-induced cardiotoxicity. Preventive role of high dose of atorvastatin. Basic Res. Cardiol. 2024, 120, 263–286. [Google Scholar] [CrossRef]
- Seicean, S.; Seicean, A.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J. Am. Coll. Cardiol. 2012, 60, 2384–2390. [Google Scholar] [CrossRef]
- Chotenimitkhun, R.; D’Agostino, R., Jr.; Lawrence, J.A.; Hamilton, C.A.; Jordan, J.H.; Vasu, S.; Lash, T.L.; Yeboah, J.; Herrington, D.M.; Hundley, W.G. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can. J. Cardiol. 2015, 31, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Acar, Z.; Kale, A.; Turgut, M.; Demircan, S.; Durna, K.; Demir, S.; Meric, M.; Ağaç, M.T. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 988–989. [Google Scholar] [CrossRef]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Houbois, C.; Marwick, T.H.; Kei, T.; Saha, S.; Runeckles, K.; Huang, F.; Shalmon, T.; Thorpe, K.E.; Pezo, R.C.; et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Titus, A.; Cheema, H.A.; Shafiee, A.; Seighali, N.; Shahid, A.; Bhanushali, K.B.; Kumar, A.; Khan, S.U.; Khadke, S.; Thavendiranathan, P.; et al. Statins for Attenuating Cardiotoxicity in Patients Receiving Anthracyclines: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101885. [Google Scholar] [CrossRef]
- Calvillo-Arguelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective Effect of Statins in Patients With HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can. J. Cardiol. 2019, 35, 153–159. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-El-Sooud, K.; Aleya, L.; Bungǎu, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Gietema, J.A.; Milojkovic Kerklaan, B.; van Werkhoven, E.D.; Altena, R.; Honkoop, A.; Los, M.; Smit, W.M.; Nieboer, P.; Smorenburg, C.H.; et al. Angiotensin II-Receptor Inhibition with Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients with Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1030–1037. [Google Scholar] [CrossRef]
- Janbabai, G.; Nabati, M.; Faghihinia, M.; Azizi, S.; Borhani, S.; Yazdani, J. Effect of Enalapril on Preventing Anthracycline-Induced Cardiomyopathy. Cardiovasc. Toxicol. 2017, 17, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; D’Agostino, R., Jr.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, 10.1056. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, T.; Xiong, X.; Liu, N.; Pang, B.; Ruan, Y.; Gao, Y.; Shang, H.; Xing, Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 51, 104577. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhang, Y.; Liu, W.; He, C. Effects of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use on cancer therapy-related cardiac dysfunction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2021, 26, 101–109. [Google Scholar] [CrossRef]
- Caspani, F.; Tralongo, A.C.; Campiotti, L.; Asteggiano, R.; Guasti, L.; Squizzato, A. Prevention of anthracycline-induced cardiotoxicity: A systematic review and meta-analysis. Intern. Emerg. Med. 2021, 16, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, R.; Jiang, J.; Hu, Y.; Li, H.; Wang, Y. ACEI/ARB and beta-blocker therapies for preventing cardiotoxicity of antineoplastic agents in breast cancer: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 1405–1415. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Contaldi, C.; De Vivo, S.; Martucci, M.L.; D’Onofrio, A.; Ammendola, E.; Nigro, G.; Errigo, V.; Pacileo, G.; Masarone, D. Effects of Cardiac Contractility Modulation Therapy on Right Ventricular Function: An Echocardiographic Study. Appl. Sci. 2022, 12, 7917. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 9, 1757–1780. [Google Scholar] [CrossRef]
- Martín-Garcia, A.; López-Fernández, T.; Mitroi, C.; Chaparro-Muñoz, M.; Moliner, P.; Martin-Garcia, A.C.; Martinez-Monzonis, A.; Castro, A.; Lopez-Sendon, J.L.; Sanchez, P.L. Effectiveness of sacubitril-valsartan in cancer patients with heart failure. ESC Heart Fail. 2020, 7, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Gregorietti, V.; Fernandez, T.L.; Costa, D.; Chahla, E.O.; Daniele, A.J. Use of Sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy. Cardiooncology 2020, 6, 24. [Google Scholar] [CrossRef]
- Daniele, A.J.; Gregorietti, V.; Costa, D.; Lopez-Fernandez, T. Use of emgliflozine in cardiotoxicity treatment. EMPACARD-treatment registry. Six-months follow-up. Eur. Heart J. 2022, 43, ehac544–ehac2590. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Costa, I.B.S.D.S.D.; Lopes, M.A.C.Q.; Hoff, P.M.G.; Diz, M.D.P.E.; Fonseca, S.M.R.; Bittar, C.S.; Rehder, M.H.H.D.S.; Rizk, S.I.; Almeida, D.R.; et al. Brazilian Cardio-oncology Guideline—2020. Arq. Bras. Cardiol. 2020, 115, 1006–1043. [Google Scholar] [CrossRef]
- Oda, H.; Hayashi, Y.; Oyanagi, N.; Tanaka, K.; Ozaki, K.; Kashiwa, A.; Hosaka, Y.; Tsuchida, K.; Takahashi, K. Add-on multidrug treatment based on quadruple therapy successfully treated worsening heart failure caused by anthracycline-induced cardiomyopathy in a survivor of cancer as a young adult: A case report. BMC Cardiovasc. Disord. 2024, 24, 505. [Google Scholar] [CrossRef]
- Shi, H.; Lu, H.; Zheng, Y.; Pu, P.; Wei, L.; Hu, D.; Tang, H.; Wang, L. Bioinformatics and experimental studies jointly reveal that Sacubitril Valsartan improves myocardial oxidative stress and inflammation by regulating the MAPK signaling pathway to treat chemotherapy related cardiotoxicity. Biochem. Biophys. Res. Commun. 2024, 690, 149244. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Yan, S.; Lin, L.; Qiu, X.; Lin, X.; Wang, W. Sacubitril/valsartan attenuated myocardial inflammation, fibrosis, apoptosis and promoted autophagy in doxorubicin-induced cardiotoxicity mice via regulating the AMPKα-mTORC1 signaling pathway. Mol. Cell. Biochem. 2024, 480, 1898–1908. [Google Scholar] [CrossRef]
- Mecinaj, A.; Vinje-Jakobsen, V.; Ngo, D.T.M.; Sverdlov, A.L.; Myhre, P.L. The SARAH trial: More evidence on the role of neurohormonal blockers in prevention of anthracycline-induced cardiotoxicity. Heart Fail Rev. 2025. [Google Scholar] [CrossRef]
- Tajstra, M.; Dyrbuś, M.; Rutkowski, T.; Składowski, K.; Sosnowska-Pasiarska, B.; Góźdź, S.; Radecka, B.; Staszewski, M.; Majsnerowska, A.; Myrda, K.; et al. Sacubitril/valsartan for cardioprotection in breast cancer (MAINSTREAM): Design and rationle of the randomized trial. ESC Heart Fail. 2023, 10, 3174–3183. [Google Scholar] [CrossRef]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 159–182. [Google Scholar] [CrossRef]
- Chang, W.T.; Shih, J.Y.; Lin, Y.W.; Chen, Z.C.; Kan, W.C.; Lin, T.H.; Hong, C.S. Dapagliflozin protects against doxorubicin-induced cardiotoxicity by restoring STAT3. Arch. Toxicol. 2022, 96, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.L.; Chu, P.M.; Cheng, H.C.; Huang, Y.T.; Chou, W.C.; Tsai, K.L.; Chan, S.H. Dapagliflozin Mitigates Doxorubicin-Caused Myocardium Damage by Regulating AKT-Mediated Oxidative Stress, Cardiac Remodeling, and Inflammation. Int. J. Mol. Sci. 2022, 23, 10146. [Google Scholar] [CrossRef]
- Madonna, R.; Barachini, S.; Moscato, S.; Ippolito, C.; Mattii, L.; Lenzi, C.; Balistreri, C.R.; Zucchi, R.; De Caterina, R. Sodium-glucose cotransporter type 2 inhibitors prevent ponatinib-induced endothelial senescence and disfunction: A potential rescue strategy. Vascul. Pharmacol. 2022, 142, 106949. [Google Scholar] [CrossRef]
- Bhatti, A.W.; Patel, R.; Dani, S.S.; Khadke, S.; Makwana, B.; Lessey, C.; Shah, J.; Al-Husami, Z.; Yang, E.H.; Thavendiranathan, T.; et al. SGLT2i and primary prevention of cancer therapy–related cardiac dysfunction in patients with diabetes. JACC CardioOncol. 2024, 6, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Gongora, C.A.; Drobni, Z.D.; Quinaglia Araujo Costa Silva, T.; Zafar, A.; Gong, J.; Zlotoff, D.A.; Gilman, H.K.; Hartmann, S.E.; Sama, S.; Nikolaidou, S.; et al. Sodium-Glucose Co-Transporter-2 Inhibitors and Cardiac Outcomes Among Patients Treated with Anthracyclines. JACC Heart Fail. 2022, 10, 559–567. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Carrasco, R.; Austin, P.C.; Chen, Y.; Zhou, L.; Fang, J.; Su, H.M.H.; Lega, I.C.; Kaul, P.; Neilan, T.G.; et al. The Association of Sodium-Glucose Cotransporter 2 Inhibitors with Cardiovascular Outcomes in Anthracycline-Treated Patients with Cancer. JACC CardioOncol. 2023, 5, 318–328. [Google Scholar] [CrossRef]
- Avula, V.; Sharma, G.; Kosiborod, M.N.; Vaduganathan, M.; Neilan, T.G.; Lopez, T.; Dent, S.; Baldassarre, L.; Scherrer-Crosbie, M.; Barac, A.; et al. SGLT2 Inhibitor Use and Risk of Clinical Events in Patients with Cancer Therapy-Related Cardiac Dysfunction. JACC Heart Fail. 2024, 12, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.J.; Gregorietti, V.; Costa, D.; López-Fernández, T. Use of EMPAgliflozin in the prevention of CARDiotoxicity: The EMPACARD—PILOT trial. Cardiooncology 2024, 10, 58. [Google Scholar] [CrossRef]
- Quagliariello, V.; Berretta, M.; Bisceglia, I.; Giacobbe, I.; Iovine, M.; Giordano, V.; Arianna, R.; Barbato, M.; Izzo, F.; Maurea, C.; et al. The sGCa Vericiguat Exhibit Cardioprotective and Anti-Sarcopenic Effects through NLRP-3 Pathways: Potential Benefits for Anthracycline-Treated Cancer Patients. Cancers 2024, 16, 1487. [Google Scholar] [CrossRef]
- Mir, A.; Badi, Y.; Bugazia, S.; Nourelden, A.Z.; Fathallah, A.H.; Ragab, K.M.; Alsillak, M.; Elsayed, S.M.; Hagrass, A.I.; Bawek, S.; et al. Efficacy and safety of cardioprotective drugs in chemotherapy-induced cardiotoxicity: An updated systematic review & network meta-analysis. Cardiooncology 2023, 9, 10. [Google Scholar]
- Quagliariello, V.; Iovine, M.; Giacobbe, I.; Giordano, V.; Arianna, R.; Barbato, M.; Izzo, F.; Maurea, F.; Bisceglia, I.; Paccone, A.; et al. Soluble guanylate cyclase activator vericiguat prevents anthracycline–mediated cardiotoxicity and sarcopenia through no–sgc–cgmp–nlrp3 pathway: Potential application in cancer patients. Eur. Heart J. Suppl. 2024, 26, ii31. [Google Scholar] [CrossRef]
- Dozic, S.; Howden, E.J.; Bell, J.R.; Mellor, K.M.; Delbridge, L.M.D.; Weeks, K.L. Cellular Mechanisms Mediating Exercise-Induced Protection against Cardiotoxic Anthracycline Cancer Therapy. Cells 2023, 12, 1312. [Google Scholar] [CrossRef]
- Chung, W.P.; Yang, H.L.; Hsu, Y.T.; Hung, C.H.; Liu, P.Y.; Liu, Y.W.; Chan, S.H.; Tsai, K.L. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2022, 65, 101485. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Eves, N.D.; Shave, R.E.; Bland, K.A.; Bovard, J.; Gelmon, K.A.; Virani, S.A.; McKenzie, D.C.; Stöhr, E.J.; Waburton, D.E.R.; et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: A RCT. Breast Cancer Res. Treat. 2018, 167, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Bennett, H.; Bezak, E.; Perry, R. The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: Systematic review. Eur. J. Prev. Cardiol. 2022, 29, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Wernhart, S.; Rassaf, T. Relevance of Cardiovascular Exercise in Cancer and Cancer Therapy-Related Cardiac Dysfunction. Curr. Heart Fail. Rep. 2024, 21, 238–251. [Google Scholar] [CrossRef]
- Foulkes, S.J.; Howden, E.J.; Haykowsky, M.J.; Antill, Y.; Salim, A.; Nightingale, S.S.; Loi, S.; Claus, P.; Janssens, K.; Mitchell, A.M.; et al. Exercise for the Prevention of Anthracycline-Induced Functional Disability and Cardiac Dysfunction: The BREXIT Study. Circulation 2023, 147, 532–545. [Google Scholar] [CrossRef]
- Chen, K.; Guan, H.; Sun, M.; Zhang, Y.; Zhong, W.; Guo, X.; Zuo, A.; Zhuang, H. Effects of Physical Activity on Cardiotoxicity and Cardio respiratory Function in Cancer Survivors Undergoing Chemotherapy: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2024, 23, 15347354241291176. [Google Scholar] [CrossRef]
- Díaz-Balboa, E.; Peña-Gil, C.; Rodríguez-Romero, B.; Cuesta-Vargas, A.I.; Lado-Baleato, O.; Martínez-Monzonís, A.; Pedreira-Pérez, M.; Palacios-Ozores, P.; López-López, R.; González-Juanatey, J.R.; et al. Exercise-based cardio-oncology rehabilitation for cardiotoxicity prevention during breast cancer chemotherapy: The ONCORE randomized controlled trial. Prog. Cardiovasc. Dis. 2024, 85, 74–81. [Google Scholar] [CrossRef]
- Hall, S.E.; Smuder, A.J.; Hayward, R. Effects of Calorie Restriction and Voluntary Exercise on Doxorubicin-Induced Cardiotoxicity. Integr. Cancer Ther. 2019, 18, 1534735419843999. [Google Scholar] [CrossRef]
- Stephenson, E.; Mclaughlin, M.; Bray, J.W.; Saxton, J.M.; Vince, R.V. Nutrition Modulation of Cardiotoxicity in Breast Cancer: A Scoping Review. Nutrients 2024, 16, 3777. [Google Scholar] [CrossRef]
- Muckiene, G.; Vaitiekus, D.; Zaliaduonyte, D.; Bartnykaite, A.; Plisiene, J.; Zabiela, V.; Juozaityte, E.; Jurkevicius, R. The Impact of Polymorphisms in ATP-Binding Cassette Transporter Genes on Anthracycline-Induced Early Cardiotoxicity in Patients with Breast Cancer. J. Cardiovasc. Dev. Dis. 2023, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Fonoudi, H.; Jouni, M.; Cejas, R.B.; Magdy, T.; Blancard, M.; Ge, N.; Shah, D.A.; Lyra-Leite, D.M.; Neupane, A.; Gharib, M.; et al. Functional Validation of Doxorubicin-Induced Cardiotoxicity-Related Genes. JACC CardioOncol. 2024, 6, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Krajinovic, M.; Elbared, J.; Drouin, S.; Bertout, L.; Rezgui, A.; Ansari, M.; Raboisson, M.J.; Lipshultz, S.E.; Silverman, L.B.; Sallan, S.E.; et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2016, 16, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Ross, C.J.; Rassekh, S.R.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr. Blood Cancer. 2013, 60, 1375–1381. [Google Scholar] [CrossRef]
- Hurkmans, E.G.E.; Brand, A.C.A.M.; Verdonschot, J.A.J.; Te Loo, D.M.W.M.; Coenen, M.J.H. Pharmacogenetics of chemotherapy treatment response and -toxicities in patients with osteosarcoma: A systematic review. BMC Cancer 2022, 22, 1326. [Google Scholar] [CrossRef]
- Mohan, U.P.; PB, T.P.; Iqbal, S.T.A.; Arunachalam, S. Mechanisms of doxorubicin-mediated reproductive toxicity—A review. Reprod. Toxicol. 2021, 102, 80–89. [Google Scholar] [CrossRef]
- Blanco, J.G.; Sun, C.L.; Landier, W.; Chen, L.; Esparza-Duran, D.; Leisenring, W.; Mays, A.; Friedman, D.L.; Ginsberg, J.P.; Hudson, M.M.; et al. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1415–1421. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Cao, M.L.; Liu, Y.W.; He, Y.Q.; Yang, C.X.; Gao, F. CD44 mediates oligosaccharides of hyaluronan-induced proliferation, tube formation and signal transduction in endothelial cells. Exp. Biol. Med. 2011, 236, 84–90. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, B.W.; Kim, J.; Lee, S.; You, H.; Lee, J.; Lee, S.; Park, J.H.; Kim, J.; Sim, W.; et al. Hyaluronic acid stimulation of stem cells for cardiac repair: A cell-free strategy for myocardial infarct. J. Nanobiotechnology 2024, 22, 149. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Sun, C.L.; Armenian, S.H.; Hakonarson, H.; Hageman, L.; Ding, Y.; Landier, W.; Blanco, J.G.; Chen, L.; et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: A report from the children’s oncology group. J. Clin. Oncol. 2014, 32, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Beauclair, S.; Formento, P.; Fischel, J.L.; Lescaut, W.; Largillier, R.; Chamorey, E.; Hofman, P.; Ferrero, J.M.; Pagès, G.; Milano, G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann. Oncol. 2007, 18, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Zemmour, H.; Planer, D.; Magenheim, J.; Moss, J.; Neiman, D.; Gilon, D.; Korach, A.; Glaser, B.; Shemer, R.; Landesberg, G.; et al. Non-invasive detection of human cardiomyocyte death using methylation patterns of circulating DNA. Nat. Commun. 2018, 9, 1443. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Hu, G.; Chen, X.; Liu, H.; Li, C.; Guo, X.; Huang, C.; Sun, F.; Li, T.; et al. Rectifying METTL4-Mediated N6-Methyladenine Excess in Mitochondrial DNA Alleviates Heart Failure. Circulation 2024, 150, 1441–1458. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.A.; Todorova, V.K.; Stone, A.; Carter, W.; Plotkin, M.D.; Hsu, P.C.; Wei, J.Y.; Su, J.L.; Makhoul, I. Genome-Wide DNA Methylation Signatures Predict the Early Asymptomatic Doxorubicin-Induced Cardiotoxicity in Breast Cancer. Cancers 2021, 13, 6291. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Hussain, M.; Brown, S.A.; Budd, T.; Tang, W.H.W.; Abraham, J.; Xu, B.; Shah, C.; Moudgil, R.; et al. Machine Learning-Based Risk Assessment for Cancer Therapy-Related Cardiac Dysfunction in 4300 Longitudinal Oncology Patients. J. Am. Heart Assoc. 2020, 9, e019628. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Chou, C.; Ngorsuraches, S.; Qian, J. Using Machine Learning Approaches to Predict Short-Term Risk of Cardiotoxicity Among Patients with Colorectal Cancer After Starting Fluoropyrimidine-Based Chemotherapy. Cardiovasc. Toxicol. 2022, 22, 130–140. [Google Scholar] [CrossRef]
- Kuwahara, A.; Iwasaki, Y.; Kobayashi, M.; Takagi, R.; Yamada, S.; Kubo, T.; Satomi, K.; Tanaka, N. Artificial intelligence-derived left ventricular strain in echocardiography in patients treated with chemotherapy. Int. J. Cardiovasc. Imaging 2024, 40, 1903–1910. [Google Scholar] [CrossRef]
- Sangha, V.; Nargesi, A.A.; Dhingra, L.S.; Khunte, A.; Mortazavi, B.J.; Ribeiro, A.H.; Banina, E.; Adeola, O.; Garg, N.; Brandt, C.A.; et al. Detection of Left Ventricular Systolic Dysfunction from Electrocardiographic Images. Circulation 2023, 148, 765–777. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Sangha, V.; Dhingra, L.S.; Aminorroaya, A.; Coppi, A.; Krumholz, H.M.; Baldassarre, L.A.; Khera, R. Artificial Intelligence-Enhanced Risk Stratification of Cancer Therapeutics-Related Cardiac Dysfunction Using Electrocardiographic Images. Circ. Cardiovasc. Qual. Outcomes 2024, 18, e011504. [Google Scholar] [CrossRef]
- Brown, C.; Mantzaris, M.; Nicolaou, E.; Karanasiou, G.; Papageorgiou, E.; Curigliano, G.; Cardinale, D.; Filippatos, G.; Memos, N.; Naka, K.K.; et al. A systematic review of miRNAs as biomarkers for chemotherapy-induced cardiotoxicity in breast cancer patients reveals potentially clinically informative panels as well as key challenges in miRNA research. Cardiooncology 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Reinal, I.; Peiró-Molina, E.; Buigues, M.; Tejedor, S.; Hernándiz, A.; Selva, M.; Hervás, D.; Cañada, A.J.; Dorronsoro, A.; et al. MicroRNA-4732-3p Is Dysregulated in Breast Cancer Patients with Cardiotoxicity, and Its Therapeutic Delivery Protects the Heart from Doxorubicin-Induced Oxidative Stress in Rats. Antioxidants 2022, 11, 1955. [Google Scholar] [CrossRef]
- Pillai, S.S.; Pereira, D.G.; Bonsu, G.; Chaudhry, H.; Puri, N.; Lakhani, H.V.; Tirona, M.T.; Sodhi, K.; Thompson, E. Biomarker panel for early screening of trastuzumab -induced cardiotoxicity among breast cancer patients in west virginia. Front. Pharmacol. 2022, 13, 953178. [Google Scholar] [CrossRef]
- Oren, A.; Taylor, J.M. The subcellular localization of defensins and myeloperoxidase in human neutrophils: Immunocytochemical evidence for azurophil granule heterogeneity. J. Lab. Clin. Med. 1995, 125, 340–347. [Google Scholar] [PubMed]
- Lakhani, H.V.; Pillai, S.S.; Zehra, M.; Dao, B.; Tirona, M.T.; Thompson, E.; Sodhi, K. Detecting early onset of anthracyclines-induced cardiotoxicity using a novel panel of biomarkers in West-Virginian population with breast cancer. Sci. Rep. 2021, 11, 7954. [Google Scholar] [CrossRef]
- Alves, M.T.; Simões, R.; Pestana, R.M.C.; de Oliveira, A.N.; Oliveira, H.H.M.; Soares, C.E.; Sabino, A.P.; Silva, L.M.; Gomes, K.B. Interleukin-10 Levels are Associated with Doxorubicin-Related Cardiotoxicity in Breast Cancer Patients in a One-Year Follow-Up Study. Immunol. Investig. 2022, 51, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Baruch, R.; Zahler, D.; Zornitzki, L.; Arbel, Y.; Rozenbaum, Z.; Arnold, J.H.; Raphael, A.; Khoury, S.; Banai, S.; Topilsky, Y.; et al. High neutrophil-to-lymphocyte ratio as an early sign of cardiotoxicity in breast cancer patients treated with anthracycline. Clin. Cardiol. 2023, 46, 328–335. [Google Scholar] [CrossRef]
- Asnani, A.; Shi, X.; Farrell, L.; Lall, R.; Sebag, I.A.; Plana, J.C.; Gerszten, R.E.; Scherrer-Crosbie, M. Changes in Citric Acid Cycle and Nucleoside Metabolism Are Associated with Anthracycline Cardiotoxicity in Patients with Breast Cancer. J. Cardiovasc. Transl. Res. 2020, 13, 349–356. [Google Scholar] [CrossRef]
- Becker, M.M.C.; Arruda, G.F.A.; Berenguer, D.R.F.; Buril, R.O.; Cardinale, D.; Brandão, S.C.S. Anthracycline cardiotoxicity: Current methods of diagnosis and possible role of 18F-FDG PET/CT as a new biomarker. Cardiooncology 2023, 9, 17. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Song, Z.P.; Chen, Y.G.; Zhang, D.D.; Wang, C.Q. Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. Int. Immunopharmacol. 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Du, J.; Meng, H.; Liu, F.; Yang, N.; Deng, S.; Wan, H.; Ye, D.; Song, E.; Zeng, H. Targeting autophagy with SAR405 alleviates doxorubicin-induced cardiotoxicity. Cell Biol. Toxicol. 2023, 39, 3255–3267. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Therapeutic Indications | Mechanisms of Cardiotoxicity | CRTCD/LV Systolic Dysfunction | References |
|---|---|---|---|---|
| Anthracyclines Doxorubicin Daunorubicin Epirubicin Idarubicin | Breast cancer, Gastric tumor, Leukemias, Lymphomas, Lung cancer, Ovarian tumor, Sarcomas | ↑ Toxic ROS and ↑ Oxidative stress ↓ Endogenous antioxidant enzyme Iron complex accumulation Topoisomerase IIb inhibition Mitocondrial dysfunction DNA damage Cellular apoptosis Dysregulation of calcium homeostatis | +++ | [2,4,9,11,12,13,14] |
| HER2-targeted therapies Trastuzumab Pertuzumab | Breast cancer, Gastric cancer, Esophageal cancer | Direct blockage to HER2 protective effect from cardiotoxin Inibition of Neurugulin 1 ↑ Oxidative stress | +++ | [2,4,9,11,12,13,14] |
| EGFR/HER2 Lapatinib Osimertinib | Breast cancer, NSCLC | ↑ Oxidative stress | ++ | [3,9,14] |
| Antimetabolities Fluorouracil (5-FU) Capecitabina | Breast cancer, Colorectal cancer, Pancreatic cancer | Endothelial cell damage Coronary artery spasm Intensified sympathetic nervous system activation Potential direct toxic impact on heart muscle cells | + | [9,13,14] |
| VEGF Receptor Monoclonal Antibodies Bevacizumab | Colorectal cancer, Glioblastoma, Breast cancer, NSCLC, RCC | ↑ Oxidative stress Mitocondrial dysfunction | ++ | [3,9,13,14] |
| VEGF Tyrosine Kinase Inhibitors (TKI) Axitinib Cabozantinib Lenvatinib Pazopanib Regorafenib Sorafenib Sunitinib Vandetanib | RCC RCC, HCC-differentiated thyroid cancer, HCC, Endometrial cancer, RCC RCC, Soft tissue sarcoma, Colorectal cancers, RCC, HCC, GIST Pancreatic Neuroendocrine tumors, Melanoma, GIST, RCC Medullary thyroid cancer, Breast/lung cancers, CML, CEL, ALL, Mesothelioma | ↑ Oxidative stress Mitocondrial dysfunction | ++ + ++ + + ++ + + | [3,9,13,14] |
| BCR-ABL Inhibitors Imatinib Nilotinib Dasatinib Bosutinib Ponatinib | GIST, CML, ALL GIST, CML CML, ALL CML CML, ALL | Severe mitochondrial impairment Reduction in ATP levels Inhibition of ABL and/or ARG leads to altered cardiomyocyte function | + - ++ ++ ++ | [3,9,14] |
| Bruton TKI Ibrutinib Acalabrutinib | CLL, Mantle cell lymphoma, Waldenström macroglobulinemia, Marginal zone lymphomas | + | [3,9,13,14] | |
| Proteasome Inhibtors (PI) Carfilzomib Bortezomib | Multiple myeloma | ↑ Oxidative stress | ++ + | [3,9,13,14] |
| Immune checkpoint inhibitors (ICI) CTLA-4 inhibitor Ipilimumab PD-1 inhibitors Nivolumab Pembrolizumab | Colorectal cancer, HCC, Melanoma, NSCLC, RCC As above, plus Esophageal cancer, Head and neck Hodgkin lymphoma, Small-cell lung cancer Urothelial carcinoma As above, plus Cervical cancer, Cutaneous squamous cell Carcinoma, Endometrial carcinoma, Gastric cancer, Merkel cell carcinoma, Primary mediastinal large, B-cell Lymphoma | Unknown Generation of autoantibodies and production of proinflammatory cytokines | ++ | [2,3,9,14] |
| CAR T-Cell Therapy | ALL, Diffuse large B-cell lymphoma | Cytokine release | ++ | [3,9,14] |
| Alkylanting Agents Cyclophosphamide Ifosfamide Melphalan Platinum Dugs Cisplatin Carboplatin Oxaliplatin | Leukemias, Lymphomas, Various solid tumors CML, Germ cell tumors, Ovarian cancer, Lymphomas, Head/neck tumors, Lung cancer, Sarcomas | Endothelial capillary damage Anaphylaxis and hypersensitivity reaction Endothelial injury/ apoptosis | + + ++ + | [3,9,13,14] |
| Taxans (Microtubule Inhibitors) Paclitaxel Docetaxel | Breast cancer, Ovarian cancer, NSCLC, Kaposi’s sarcoma, Prostate cancer, Gastric Adenocarcinoma | Reduced calcium in cardiomyocytes, resulting in decreased contraction | ++ | [3,9,13,14] |
| Hormonal Therapy Apalutamide Bicalutamide Darolutamide Nilutamide | Prostate cancer | Induction of a metabolic syndrome condition (dyslipidemia, increased body fat, and insulin resistance) Promotion of atherosclerosis development | ++ | [14] |
| Immunomodulatory Drugs Lenalidomide Pomalidomide Thalidomide | Multiple myeloma, MDS, Mantle cell lymphoma Multiple myeloma, Kaposi sarcoma Multiple myeloma | Unknown | ++ + ++ | [9,14] |
| MEK Inhibitors Binimetinib Cobimetinib Trametinib Selumetinib | Melanoma Melanoma Melanoma, NSCLC, Anaplastic thyroid cancer Neurofibromatosis Type 1 | Inhibition of MAPK signaling: ↑ Oxidative stress Cellular apostosis | ++ +++ ++ +++ | [3,9,14] |
| BRAF (rapidly accelerated fibrosarcoma B TYPE): Vemurafenib Dabrafenib | Melanome NSCLC | Inhibition of MAPK signaling: ↑ Oxidative stress Cellular apostosis | +++ | [3,9,14] |
| Haematopoietic Stem Cell Transplant (HSCT) | Hematological malignancies | + | [3,9,14] | |
| RT | Breast, Lung, Oesophagus, Thyroid, Prostate cancer, Mediastinal lymphoma, Head and neck tumors | Damages mitochondria Activates NADPH oxidase to generate ROS, which in turn exacerbate mitochondrial damage | ++ | [2,9,13,15] |
| Study | Chemotherapy | Cancer Type | Intervention | Follow-Up | N. Patients | Primary Endpoint and Results | Secondary Endpoint and Results |
|---|---|---|---|---|---|---|---|
| CECCY Avilla et al. [98] 2018 | Anthracyclines | Breast Cancer | Carvedilol vs. placebo | 6 months | 192 | Early onset drop in LVEF ≥10% by echo Carvedilol had no impact | Changes in TnI, BNP, and diastolic dysfunction Significant reduction in TnI and diastolic dysfunction |
| Tashakory et al. [96] 2016 | Anthracyclines | Brest Cancer | Carvedilol vs. placebo | 1 week | 70 | Early onset drop in LVEF ≥10% and drop in GLS by echo Carvedilol had no impact | |
| SAFE Livi et al. [105] 2021 | Anthracyclines ± Trastuzumab | Breast Cancer | Bisoprolol vs. placebo Ramipril vs. placebo | 12 months | 174 | Early onset drop in LVEF ≥10% and drop in GLS by echo Significant minor reduction in LVEF and GLS in bisoprolol group vs. placebo group No effect of Ramipril | |
| ICOS-ONE Cardinale et al. [82] 2018 | Anthracyclines | Breast cancer, Sarcoma, Hematological malignancies | Enalapril primary prevention vs. troponin-guided prevention with enalapril | 12 months | 273 | Incidence of troponin elevation No differences between primary prevention with enalapril- and troponin-guided prevention | |
| PRADA Gulati et al. [88] 2016 | Anthracyclines ± Trastuzumab | Breast cancer | Candesartan, Metoprolol succinate, or matching placebo | 10–61 weeks | 126 | Change in LVEF > 5% by CMR Metoprolol had no effect Candesartan protected against early LVEF decline vs. Metoprolol and placebo group | |
| Boekhout et al. [131] 2016 | Trastuzumab | Breast Cancer | Candesartan vs. placebo | 26 weeks | 206 | Decline in LVEF > 15% or a decrease in the LVEF <45% Candesartan had no effect | |
| Akpek et al. [103] 2015 | Anthracyclines | Breast Cancer | Spironolactone vs. placebo | 3 weeks | 83 | Change in LVEF ≥ 10% by echo and increase in TnI LVEF decrease and increase in TnI significantly lower with Spironolactone; no impairment in diastolic function | |
| Acar et al. [123] 2011 | Anthracyclines | Lymphoma, MM, Leukemia | Atorvastatin vs. placebo | 6 months | 40 | Absolute change in LVEF by echo Atorvastatin was effective in maintaining LVEF vs. placebo | |
| OVERCOME Bosch et al. [101] 2013 | Anthracyclines | Hematological malignances | Carvedilol + enalapril | 6 months | 90 | Absolute change in LVEF by echo and CMR Both carvedilol and enalapril prevented LVEF drop by echo and by CMR | Patients in carvedilol + enalapril group had lower incidence of combined event of death or HF death, HF or a final LVEF < 45% |
| Janbabai et al. [132] 2017 | Anthracyclines | Breast cancer, Hematological malignances | Enalapril | 6 months | 69 | Absolute change in LVEF, E/e’ ratio, TnI elevation Enalapril prevented: decline in LVEF, elevation of E/e’ ratio, and elevation of TnI | |
| MANTICORE Tashakori et al. [96] 2016 | Trastuzumab | Breast cancer | Bisoprolol, perindopril, or placebo | 12 months | 94 | LV remodeling: change in LVEDVi by CMR Bisoprolol and perindopril had no effect | |
| STOPCA Neilan et al. [124] 2023 | Anthracyclines | Lymphoma | Atorvastatin vs. placebo | 12 months | 300 | Proportion of participants with an absolute decline in LVEF of ≥10% from that prior to chemotherapy to a final value of <55% Atorvastatin reduced the incidence of cardiac dysfunction | Proportion of participants with an absolute decline in LVEF of ≥5% from that prior to chemotherapy to a final value of <55% Atorvastatin reduced the incidence of cardiac dysfunction |
| PREVENT Hundley et al. [133] 2022 | Anthracyclines | Breast cancer, Lymphoma | Atorvastatin vs. placebo | 24 months | 279 | Absolute change in LVEF by CMR No difference between the two groups | |
| CARDIAC-CARE Henriksen et al. [104] 2023 | Anthracyclines | Breast cancer, Lymphoma | Carvedilol + candesartan | 6 months | 175 | Adjusted change in LVEF by CMR No effect in patients with high-risk on-treatment cardiac troponin I concentrations |
| Meta-Analyses | Chemotherapy | Number of Patients | Intervention | Results |
|---|---|---|---|---|
| Huang et al. [100] 6 RCTs 2019 | Anthracyclines | 495 | Beta-blockers (carvedilol) vs. placebo | LVEF was not significantly distinct between the two groups (MD: 1.74; 95% CI −0.18 to 3.66; p = 0.08) Clinically overt cardiotoxicity was lower in the carvedilol group (Peto OR, 0.42; 95% CI 0.20–0.89; p = 0.02). |
| Li et al. [134] 22 RCTs 2020 | Anthracyclines, Trastuzumab, Cyclophosphamide, Taxanes, Platinum agents, 5-FU, and others | 1916 | ACEI vs. placebo ACEI vs. control Statins vs. control Beta-blockers vs. placebo | Significant reduction in decline in LVEF: Spironolactone (MD: 12.77, 95% IC: 1.76–23.79) Candesartan and carvedilol (MD 12.40, 95% IC: 0.99–23.81) Enalapril (MD: 7.35, 95% IC: 1.16–13.54) Statin (MD 8.36, 95% IC: 0.36–16.36) compared with placebo |
| Fang et al. [135] 9 RCTs 2021 | Anthracyclines ± Trastuzumab | 1095 | ACEI/ARB vs. placebo | Significantly lower reduction in LVEF in ACEI/ARB receivers (MD: 4.24%, 95% IC: 1.53–6.95; p = 0.002) No significant reduction in the risk of cardiotoxicity events (RR: 0.63, p = 0.22) Significant increase in hypotension (RR: 3.94, p = 0.008) |
| Caspani et al. [136] 12 RCTs 2021 | Anthracyclines | 2177 | RAAS blockers, beta-blockers, and aldosterone antagonists | Significantly lower reduction in LVEF in the intervention arm (MD: 3.57, 95% CI 1.04, 6.09) No significant reduction in HF (OR 0.31, 95% CI 0.06, 1.59; 5 studies) in cardioprotected arm No significant increase in hypotension (OR 3.91, 95% CI 0.42, 36.46, 3 studies) in cardioprotected arm |
| Attar et al. [97] 17 RCTs 2022 | Anthracyclines | 1291 | Beta-blockers (carvedilol, bisoprolol, Nebivolol, and Metoprolol) | Significantly lower reduction in LVEF in beta-blocker receivers (MD: 3.44%, 95% CI: 1.41–5.46, p = 0.001, I2 = 94.0%) Among the 8 studies reporting the incidence of CTRCD, there was no significant reduction in CTRCD incidence in beta-blocker receivers (RR: 0.76; 95% CI: 0.53–1.09; I2 = 24.4%; p = 0.235) |
| Keshavarzian et al. [107] 728 studies 2023 | Anthracyclines | 2674 | Dexrazoxane, beta-blockers, ACEI | Increase in LVEF in the intervention group by 0.40 after 6 months (SMD: 0.40, 95% CI 0.27 to 0.54; p <0.05) |
| Titus et al. [126] A total of 3 observational studies, 4 RCTs 2023 | Anthracyclines | 2511 | Statins | Significantly lower incidence of cardiotoxicity in patient who used statins compared to non-users (OR 0.46, 95% CI 0.33–0.63; I2: 0%) No significant difference in the decline in LVEF from the baseline (MD: 4.15, 95% CI Ȓ0.69 to 8.99, I2: 97%) |
| Gao et al. [137] 15 RCTs 2023 | Anthracyclines ± Trastuzumab | 1977 | ACEI/ARB and/or beta-blockers vs. placebo | Significant reduction in decline in LVEF in ACEI/ARB and beta-blocker groups (X2: 184.75, I2: 88.6%; p = 0.000; MD: 0.556, 95% IC: 0.299–0.813) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contaldi, C.; D’Aniello, C.; Panico, D.; Zito, A.; Calabrò, P.; Di Lorenzo, E.; Golino, P.; Montesarchio, V. Cancer-Therapy-Related Cardiac Dysfunction: Latest Advances in Prevention and Treatment. Life 2025, 15, 471. https://doi.org/10.3390/life15030471
Contaldi C, D’Aniello C, Panico D, Zito A, Calabrò P, Di Lorenzo E, Golino P, Montesarchio V. Cancer-Therapy-Related Cardiac Dysfunction: Latest Advances in Prevention and Treatment. Life. 2025; 15(3):471. https://doi.org/10.3390/life15030471
Chicago/Turabian StyleContaldi, Carla, Carmine D’Aniello, Domenico Panico, Andrea Zito, Paolo Calabrò, Emilio Di Lorenzo, Paolo Golino, and Vincenzo Montesarchio. 2025. "Cancer-Therapy-Related Cardiac Dysfunction: Latest Advances in Prevention and Treatment" Life 15, no. 3: 471. https://doi.org/10.3390/life15030471
APA StyleContaldi, C., D’Aniello, C., Panico, D., Zito, A., Calabrò, P., Di Lorenzo, E., Golino, P., & Montesarchio, V. (2025). Cancer-Therapy-Related Cardiac Dysfunction: Latest Advances in Prevention and Treatment. Life, 15(3), 471. https://doi.org/10.3390/life15030471








