Validating Indigenous Farmers’ Practice in the Management of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) in Maize Cropping Systems in Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Study Plant
2.2. Fall Armyworm Colony
2.3. Preparation of Treatments
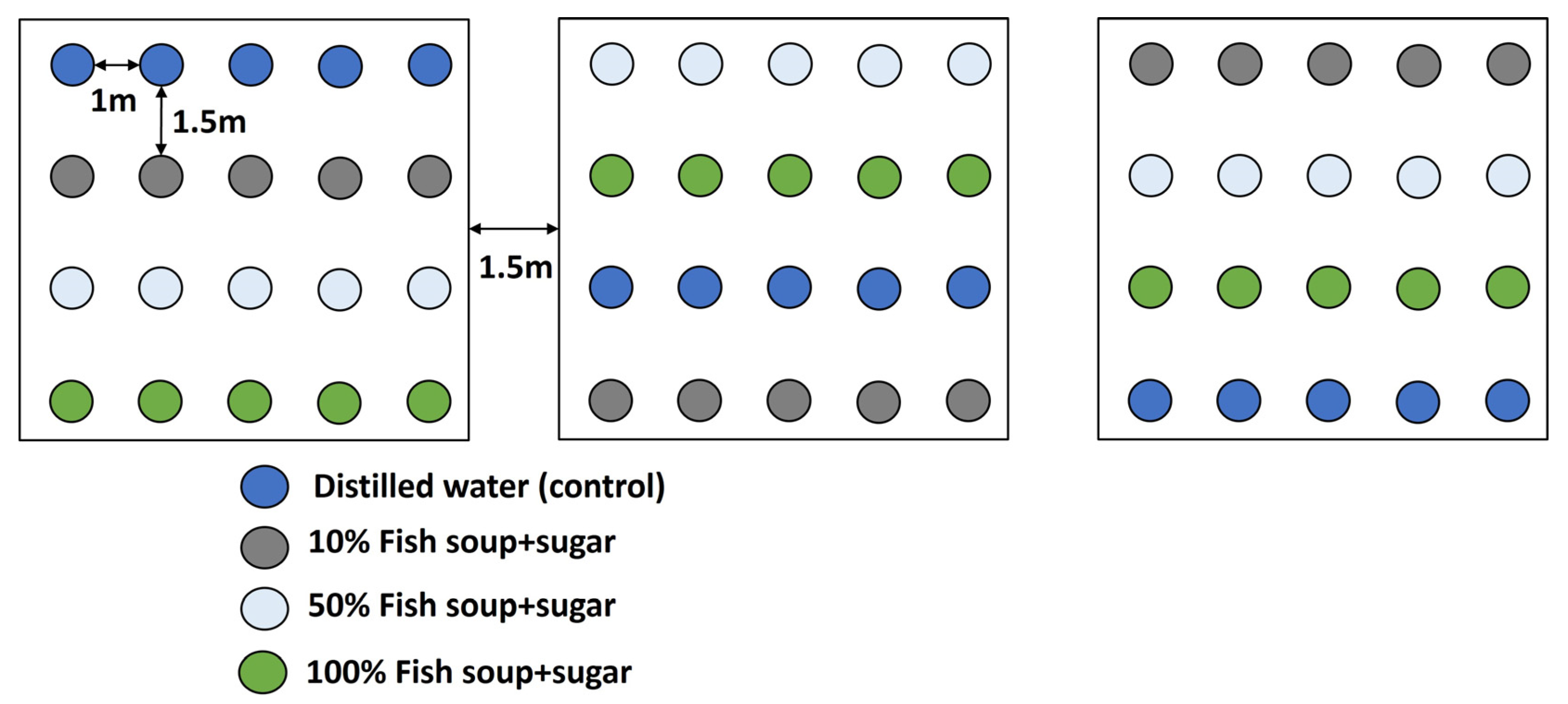
2.4. Experimental Design and Treatment Application
2.5. Assessment of Foliar Damage and Plant Recovery
2.6. Assessment of Abundance and Diversity of Visiting Insects
2.7. Assessment of Plant Growth Parameters
2.8. Proximate Analysis and Mineral Composition of Fish Soup
2.9. Data Analyses
3. Results
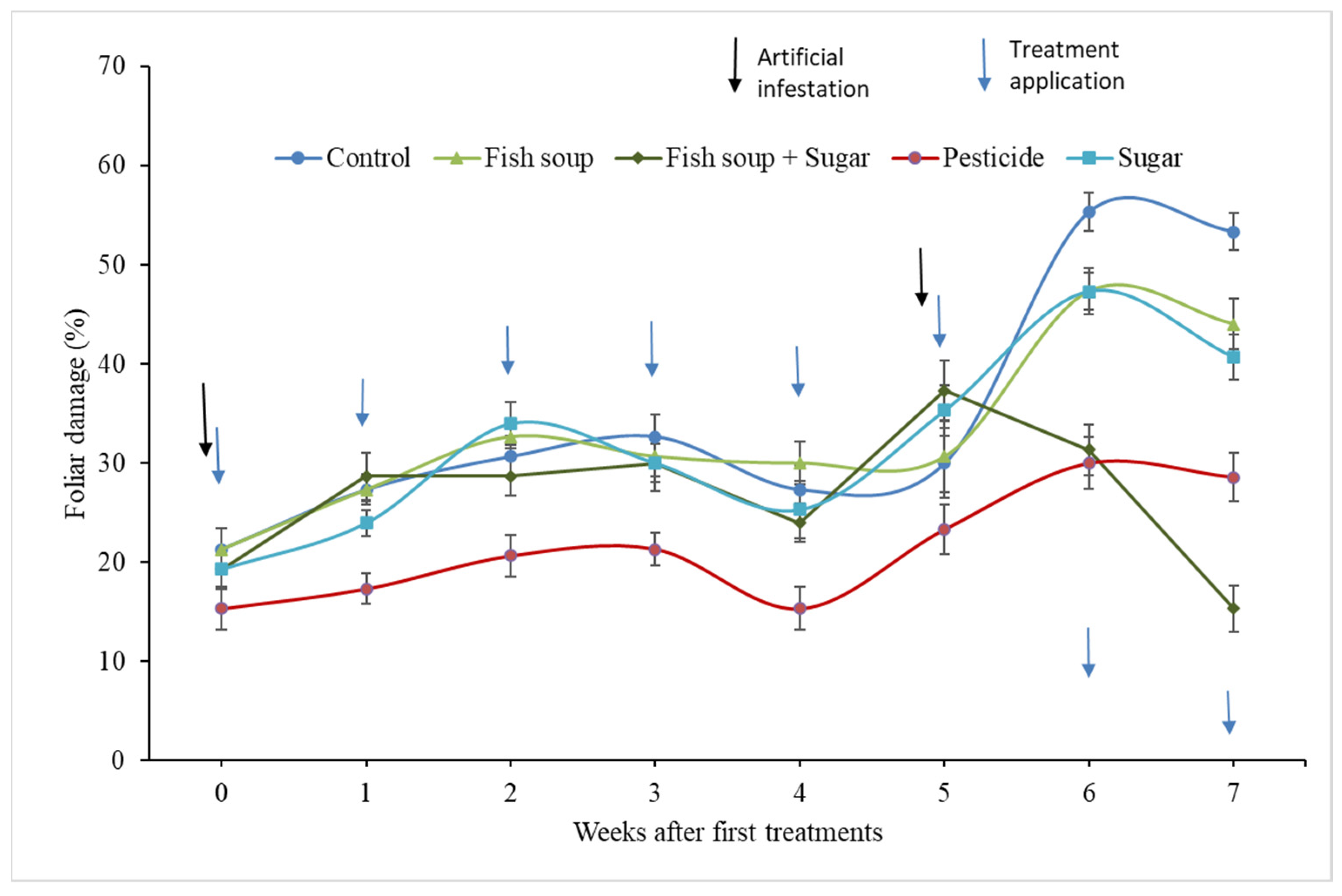
3.1. Effect of Fish Soup and Sugar on Foliar Damage and Recovery of FAW-Infested Maize Plants
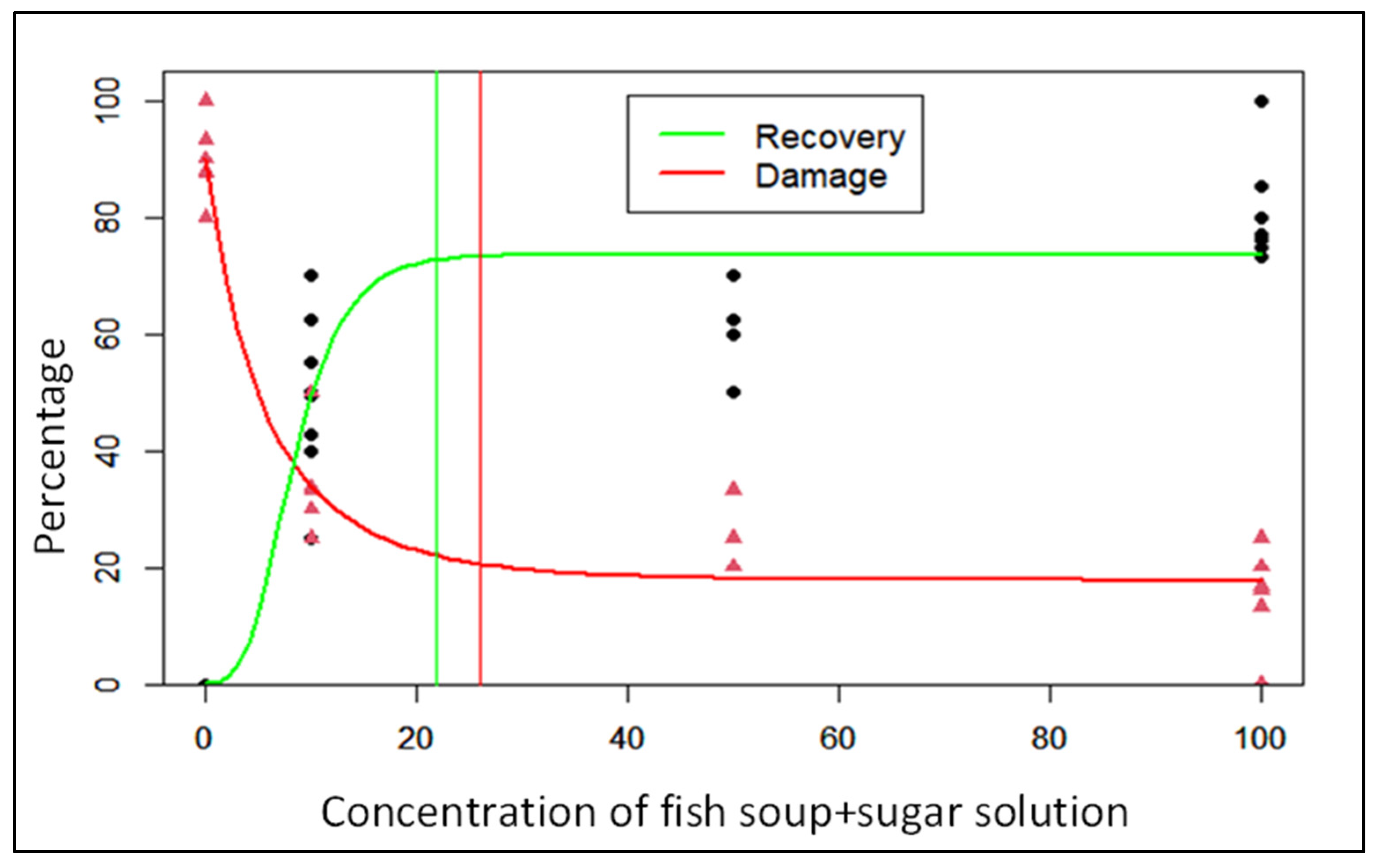
3.2. Estimates of Optimal Doses of Fish Soup and Sugar for the Least Foliar Damage and Peak Recovery
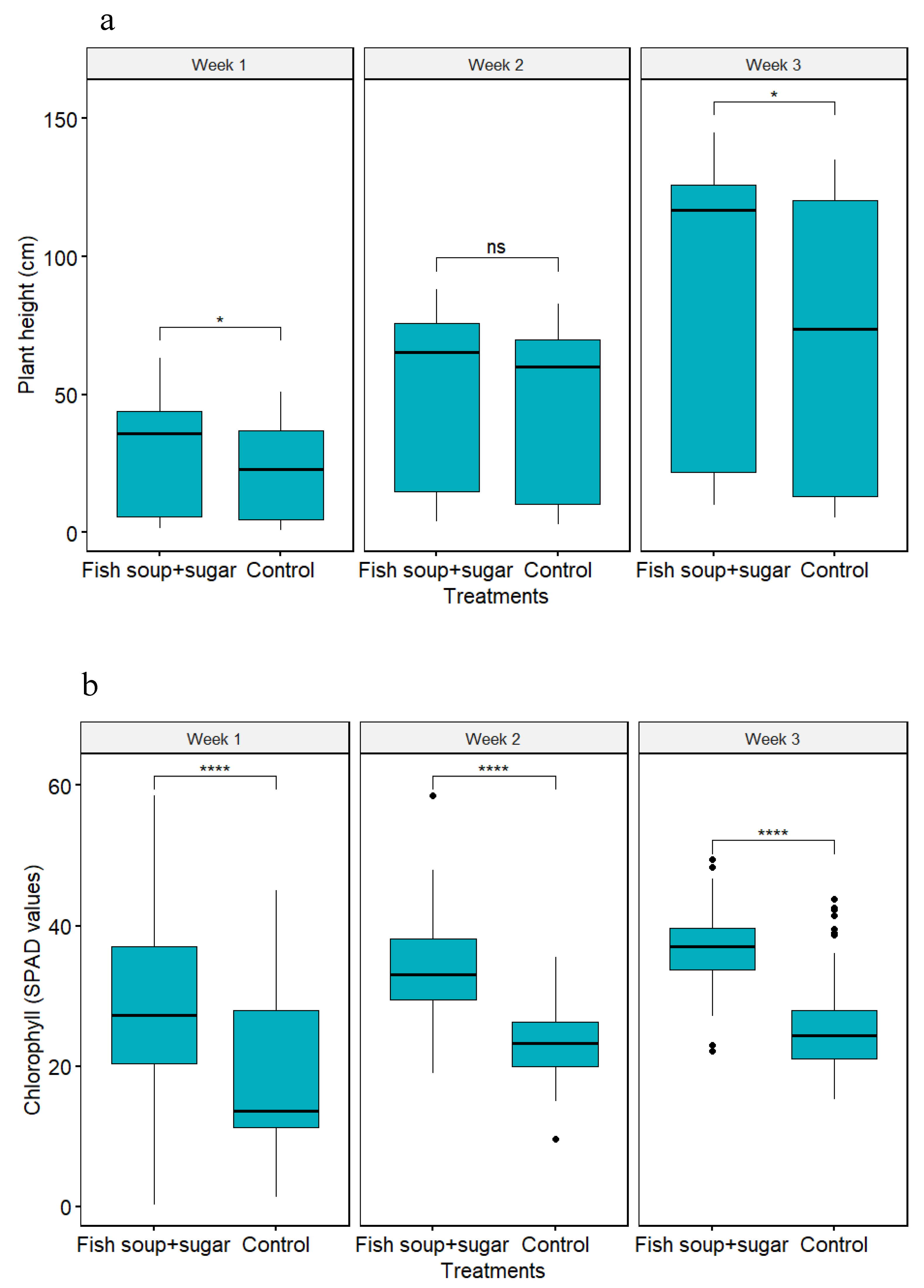
3.3. Effect of Fish Soup and Sugar Solution on Maize Plant Growth Parameters
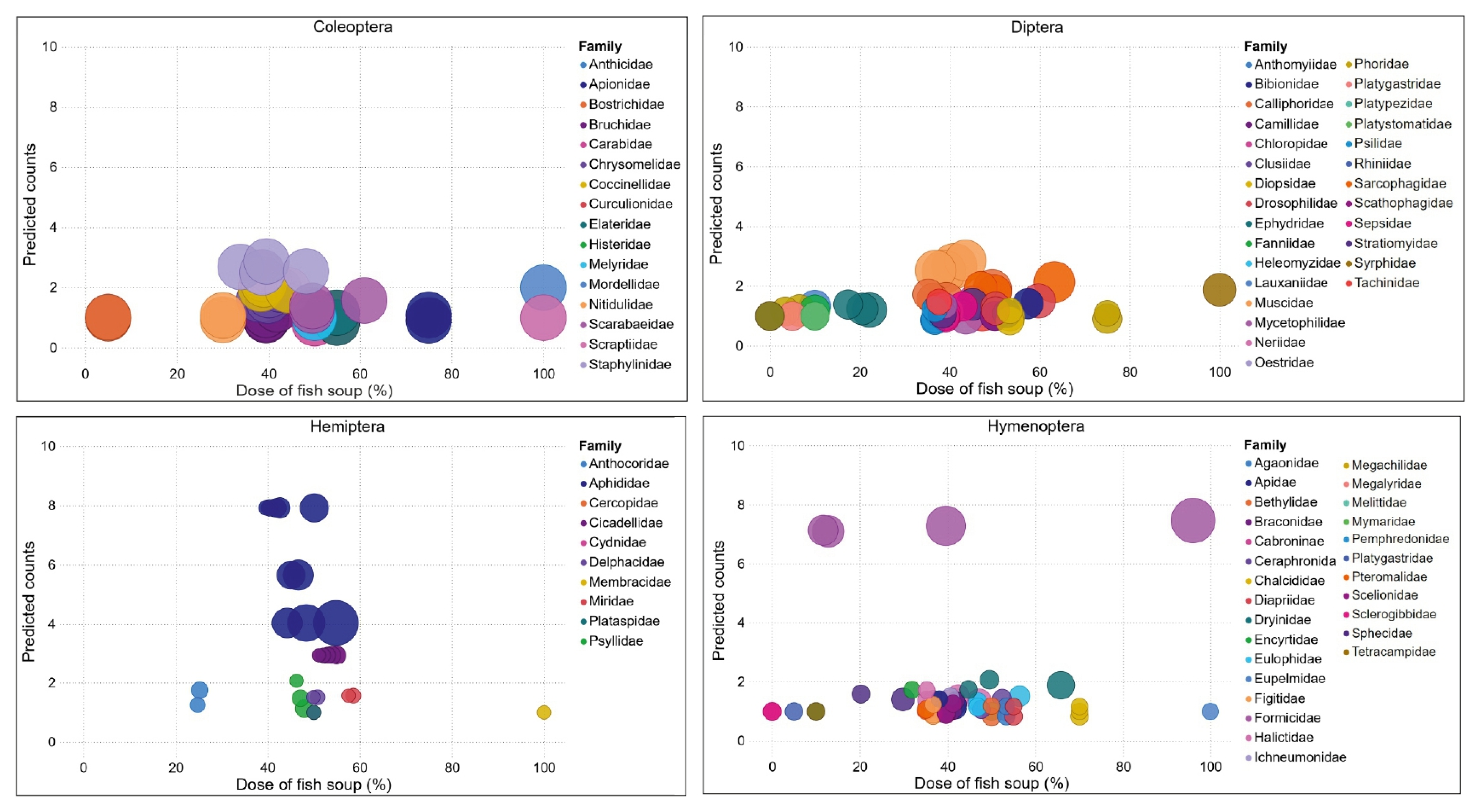
3.4. Diversity of Insects Visiting Fall Armyworm-Infested Maize Plants
3.5. Variability of Visiting Insect Community as a Function of Fish Soup Concentrations Sprayed on FAW-Infested Maize Plants
3.6. Proximate Analysis of Fish Soup, Fish Soup and sugar, and Fish Solid Residue
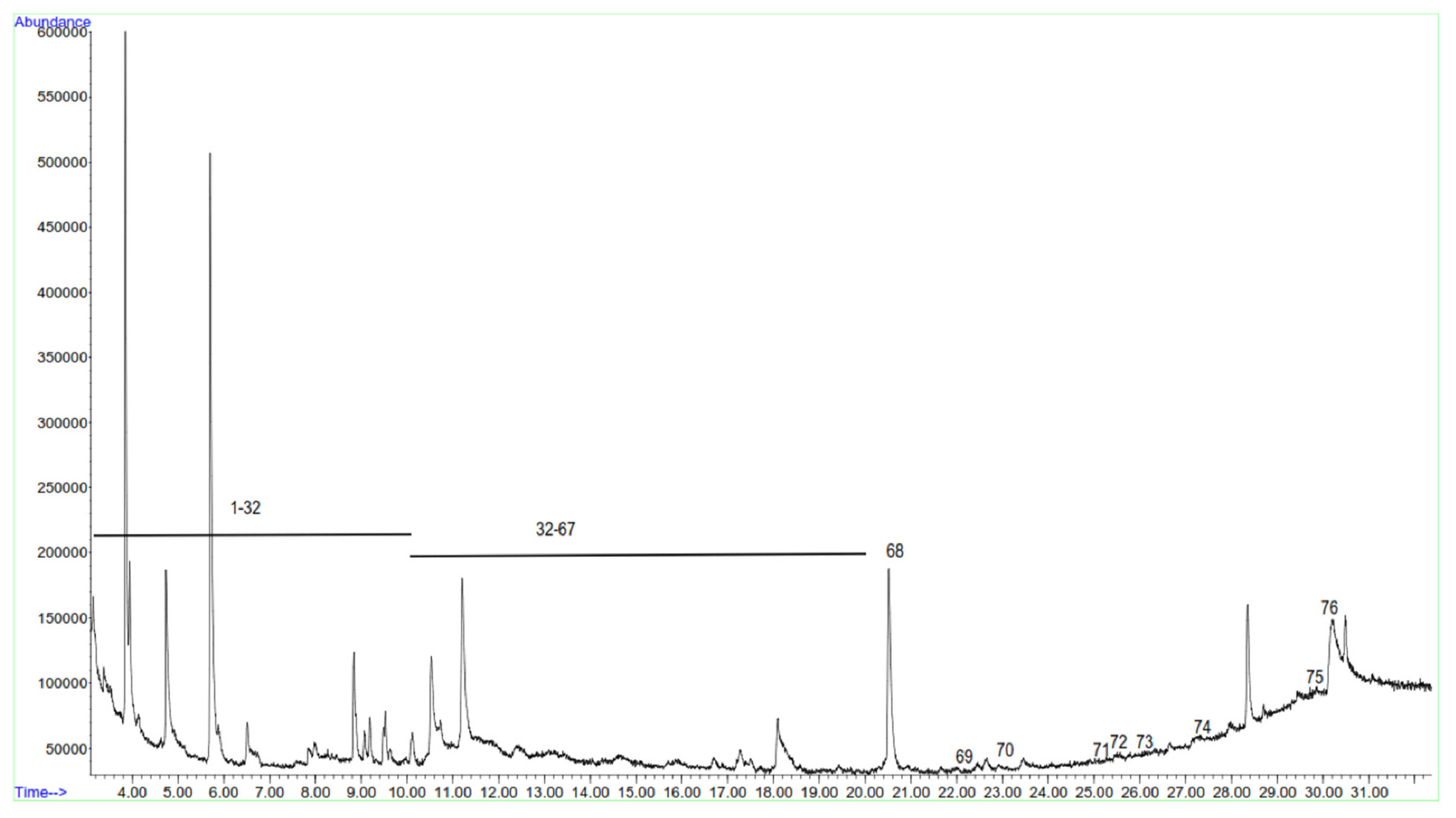
3.7. Volatile Organic Compounds in Fish Soup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasanna, B.; Huesing, J.; Eddy, R.; Peschke, V. Fall Armyworm in Africa: A Guide for Integrated Pest Management; CDMX; CIMMYT: Mexico City, Mexico, 2018. [Google Scholar]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.R.; Roque-Specht, V.F. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Colborn, T. Pesticides–How research has succeeded and failed to translate science into policy: Endocrinological effects on wildlife. Environ. Health Perspect. 1995, 103, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.S.; Booty, W.G. A multi-level assessment methodology for determining the potential for groundwater contamination by pesticides. Environ. Monit. Assess. 1995, 35, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Assefa, F.; Ayalew, D. Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: A review. Cogent Food Agric. 2019, 5, 1641902. [Google Scholar] [CrossRef]
- Timilsena, P.B.; Niassy, S.; Kimathi, E.; Abdel-Rahman, E.M.; Seidl-Adams, I.; Wamalwa, M.; Tonnang, H.E.; Ekesi, S.; Hughes, D.P.; Rajotte, E.G.; et al. Potential distribution of fall armyworm in Africa and beyond, considering climate change and irrigation patterns. Sci. Rep. 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Senay, S.D.; Pardey, P.G.; Chai, Y.; Doughty, L.; Day, R. Fall armyworm from a maize multi-peril pest risk perspective. Front. Insect Sci. 2022, 2, 971396. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall Armyworm: Impacts and Implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop. Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Belay, D.K.; Huckaba, R.M.; Foster, J.E. Susceptibility of the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), at Santa Isabel, Puerto Rico, to Different Insecticides. Fla. Èntomol. 2012, 95, 476–478. [Google Scholar] [CrossRef]
- Kumela, T.; Simiyu, J.; Sisay, B.; Likhayo, P.; Mendesil, E.; Gohole, L.; Tefera, T. Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int. J. Pest Manag. 2018, 65, 1–9. [Google Scholar] [CrossRef]
- Asare-Nuamah, P. Smallholder farmers’ adaptation strategies for the management of fall armyworm (Spodoptera frugiperda) in rural Ghana. Int. J. Pest Manag. 2022, 68, 8–18. [Google Scholar] [CrossRef]
- Abrahams, P.; Beale, T.; Cock, M.; Corniani, N.; Day, R.; Godwin, J.; Murphy, S.; Richards, G.; Vos, J. Fall Armyworm Status. Impacts and Control Options in Africa: Preliminary Evidence Note 14. 2017. Available online: https://www.cabi.org/Uploads/isc/Dfid%20Faw%20Inception%20Report04may2017final.pdf (accessed on 11 January 2024).
- Adamczyk, J.J., Jr.; Leonard, B.R.; Graves, J.B. Toxicity of Selected Insecticides to Fall Armyworms (Lepidoptera: Noctuidae) in Laboratory Bioassay Studies. Fla. Èntomol. 1999, 82, 230–236. [Google Scholar] [CrossRef]
- Togola, A.; Meseka, S.; Menkir, A.; Badu-Apraku, B.; Boukar, O.; Tamò, M.; Djouaka, R. Measurement of Pesticide Residues from Chemical Control of the Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria. Int. J. Environ. Res. Public Health 2018, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Niassy, S.; Murithii, B.; Omuse, E.R.; Kimathi, E.; Tonnang, H.; Ndlela, S.; Mohamed, S.; Ekesi, S. Insight on Fruit Fly IPM Technology Uptake and Barriers to Scaling in Africa. Sustainability 2022, 14, 2954. [Google Scholar] [CrossRef]
- Deguine, J.-P.; Aubertot, J.-N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Srinivasan, R.; Tamò, M.; Subramanian, S. The case for integrated pest management in Africa: Transition from a pesticide-based approach. Curr. Opin. Insect Sci. 2022, 54, 100970. [Google Scholar] [CrossRef]
- Hruska, A.J. Fall armyworm (Spodoptera frugiperda) management by smallholders. CAB Rev. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; van den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- Georgiev, G. Bioecological characteristics of Bracon intercessor Nees (Hymenoptera: Braconidae) as a parasitoid of the poplar clearwing moth, Paranthrene tabaniformis (Rott.) (Lepidoptera: Sesiidae) in Bulgaria. J. Pest Sci. 2005, 78, 161–165. [Google Scholar] [CrossRef]
- FAO. Integrated Management of the Fall Armyworm on Maize a Guide for Farmer Field Schools in Africa. 2018. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 14 May 2023).
- Ibrahim, N.D.; Audu, A.; Dike, M.C.; Lawal, M. Effect of raw diatomaceous earth and plant powders on Callosobruchus sub-innotatus infesting Bambara groundnut seeds. Sci. J. Pure Appl. Sci. 2012, 1, 9–16. [Google Scholar]
- Kemunto, D.; Omuse, E.R.; Mfuti, D.K.; Tamiru, A.; Hailu, G.; Rwiza, I.; Belayneh, Y.T.; Subramanian, S.; Niassy, S. Effect of rabbit urine on the larval behavior, larval mortality, egg hatchability, adult emergence and oviposition preference of the fall armyworm (Spodoptera frugiperda J.E. Smith). Agriculture 2022, 12, 1282. [Google Scholar] [CrossRef]
- Harrison, F.P. Oviposition and Subsequent Infestation of Corn by Fall Armyworm (Lepidoptera: Noctuidae). Fla. Èntomol. 1986, 69, 588–592. [Google Scholar] [CrossRef]
- Tefera, T.; Goftishu, M.; Ba, M.; Muniappan, R. A Guide to Biological Control of Fall Armyworm in Africa Using Egg Parasitoids, 1st ed.; ICRISAT: Nairobi, Kenya, 2019; Volume 4, pp. 16–32. Available online: http://34.250.91.188:8080/xmlui/handle/123456789/1001 (accessed on 11 January 2024).
- Williams, W.P.; Buckley, P.M.; Daves, C.A. Identifying resistance in corn to southwestern corn borer (Lepidoptera: Cram-bidae), fall armyworm (Lepidoptera: Noctuidae), and corn earworm (Lepidoptera; Noctuidae). J. Agric. Urban Entomol. 2006, 23, 87–95. [Google Scholar]
- Muvea, A.M.; Waiganjo, M.M.; Kutima, H.L. Attraction of pest thrips (Thysanoptera: Thripidae) infesting French beans to coloured sticky traps with Lurem-TR and its utility for monitoring thrips populations. Int. J. Trop. Insect Sci. 2014, 34, 197–206. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- ISO 5983-2:2009; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content. Part 2: Block Digestion and Steam Distillation Method. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:5983:-2:ed-2:v1:en (accessed on 2 December 2023).
- ISO 5984:2022; Animal Feeding Stuffs—Determination of Crude Ash. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:5984:ed-3:v1:en (accessed on 2 December 2023).
- ISO 6492:1999; Animal Feeding Stuffs—Determination of Fat Content. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:6492:ed-1:v1:en (accessed on 2 December 2023).
- ISO 13906:2008; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:13906:ed-1:v1:en (accessed on 2 December 2023).
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:6496:ed-2:v1:en (accessed on 2 December 2023).
- Ritz, C. Toward a unified approach to dose–response modeling in ecotoxicology. Environ. Toxicol. Chem. 2009, 29, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Dettmers, R.; Buehler, D.A.; Bartlett, J.G.; Klaus, N.A. Influence of Point Count Length and Repeated Visits on Habitat Model Performance. J. Wildl. Manag. 1999, 63, 815–823. [Google Scholar] [CrossRef]
- Hutcheson, K. A test for comparing diversities based on the shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; p. 144. [Google Scholar]
- Kiros, S.; Afework, B.; Legese, K. A preliminary study on bird diversity and abundance from Wabe fragmented forests around Gubre subcity and Wolkite town, Southwestern Ethiopia. Int. Int. J. Avian Wildl. Biol. 2018, 3, 333–340. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Version 4.0.5. Computer Software. R Core Team: Vienna, Austria, 2022. Available online: https://www.R-project.org/(accessed on 7 February 2021).
- Bortolotto, O.C. Sugar solution treatment to attract natural enemies and its impact on fall armyworm (Spodoptera frugiperda) in maize fields. Interciencia 2014, 39, 416–421. [Google Scholar]
- Canas, L.A.; O’Neil, R.J. Applications of sugar solutions to maize, and the impact of natural enemies on Fall Armyworm. Int. J. Pest Manag. 1998, 44, 59–64. [Google Scholar] [CrossRef]
- James, D.G. Further Field Evaluation of Synthetic Herbivore-Induced Plan Volatiles as Attractants for Beneficial Insects. J. Chem. Ecol. 2005, 31, 481–495. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Èntomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Pheromones Database: Terpenes, Propanogenins, and Others. Available online: https://lepipheromone.sakura.ne.jp/mepdb_eng.html (accessed on 15 March 2023).
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Sasso, R.; Iodice, L.; Digilio, M.C.; Carretta, A.; Ariati, L.; Guerrieri, E. Host-locating response by the aphid parasitoid Aphidius ervito tomato plant volatiles. J. Plant Interact. 2007, 2, 175–183. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Yusuf, A.A.; Pirk, C.W.W.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Terpenes from herbivore-induced tomato plant volatiles attract Nesidiocoris tenuis (Hemiptera: Miridae), a predator of major tomato pests. Pest Manag. Sci. 2021, 77, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- Milonas, P.G.; Anastasaki, E.; Partsinevelos, G. Oviposition-Induced Volatiles Affect Electrophysiological and Behavioral Responses of Egg Parasitoids. Insects 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Wu, K.; Gao, X.W.; Guo, Y.Y. Field-Testing of Synthetic Herbivore-Induced Plant Volatiles as Attractants for Beneficial Insects. Environ. Èntomol. 2008, 37, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Conboy, N.J.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile Organic Compounds as Insect Repellents and Plant Elicitors: An Integrated Pest Management (IPM) Strategy for Glasshouse Whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Li, Z.; Shi, J.; Meng, L.; Wang, G.; Cheng, J.; Du, Y. Development of lady beetle attractants from floral volatiles and other semiochemicals for the biological control of aphids. J. Asia-Pac. Èntomol. 2020, 23, 1023–1029. [Google Scholar] [CrossRef]
- CABI. Spodoptera Frugiperda (Fall Armyworm) Invasive Species Compendium. 2017. Available online: http://www.cabi.org/isc/datasheet/29810 (accessed on 12 March 2023).
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgari, M.; Ayalew, G.; Tefera, T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Èntomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall Armyworm, Spodoptera frugiperda Infestations in East Africa: Assessment of Damage and Parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.G., Jr.; Stinner, B.R.; Henry, T.J. Biology and Nymphal Stages of Deraeocoris nebulosus (Hemiptera: Miridae), a Predator of Arthropod Pests on Ornamentals. Ann. Èntomol. Soc. Am. 1975, 68, 1063–1068. [Google Scholar] [CrossRef]
- Hay-Roe, M.M.; Meagher, R.L.; Nagoshi, R.N.; Newman, Y. Distributional patterns of fall armyworm parasitoids in a corn field and a pasture field in Florida. Biol. Control. 2016, 96, 48–56. [Google Scholar] [CrossRef]
- Ruíz-Nájera, R.E.; Molina-Ochoa, J.; Carpenter, J.E.; Espinosa-Moreno, J.A.; Ruíz-Nájera, J.A.; Lezama-Gutiérrez, R.; Foster, J.E. Survey for Hymenopteran and Dipteran Parasitoids of the Fall Armyworm (Lepidoptera: Noctuidae) in Chiapas, Mexico. J. Agric. Urban Èntomol. 2007, 24, 35–42. [Google Scholar] [CrossRef]
- Kenis, M.; du Plessis, H.; Van den Berg, J.; Ba, M.N.; Goergen, G.; Kwadjo, K.E.; Baoua, I.; Tefera, T.; Buddie, A.; Cafà, G.; et al. Telenomus remus, a Candidate Parasitoid for the Biological Control of Spodoptera frugiperda in Africa, is already Present on the Continent. Insects 2019, 10, 92. [Google Scholar] [CrossRef]
- Agboyi, L.K.; Goergen, G.; Beseh, P.; Mensah, S.A.; Clottey, V.A.; Glikpo, R.; Buddie, A.; Cafà, G.; Offord, L.; Day, R.; et al. Parasitoid Complex of Fall Armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 2020, 11, 68. [Google Scholar] [CrossRef]
- Koffi, D.; Kyerematen, R.; Eziah, V.Y.; Agboka, K.; Adom, M.; Goergen, G.; Meagher, R.L. Natural Enemies of the Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in Ghana. Fla. Èntomol. 2020, 103, 85–90. [Google Scholar] [CrossRef]



| Parameters | YAsym (±SE%) | ug | λg | Copt (%) |
|---|---|---|---|---|
| FAW foliar damage | 17.8 ± 1.8 | 0.61 | −5.58 | 25.9 |
| Plant recovery | 73.6 ± 2.4 | 8.07 | 3.54 | 21.8 |
| Insect Order | Treatment Type | Abundance | Richness | Shannon-Weiner Diversity Index | Evenness |
|---|---|---|---|---|---|
| Coleoptera | Control | 93 | 51 | 3.7 | 0.9 |
| Fish soup and sugar (10%) | 71 | 41 | 3.4 | 0.9 | |
| Fish soup and sugar (50%) | 95 | 56 | 3.8 | 0.9 | |
| Fish soup and sugar (100%) | 149 | 79 | 4.1 | 0.9 | |
| Diptera | Control | 60 | 39 | 3.5 | 0.9 |
| Fish soup and sugar (10%) | 164 | 102 | 4.4 | 1.0 | |
| Fish soup and sugar (50%) | 105 | 68 | 4.0 | 0.9 | |
| Fish soup and sugar (100%) | 89 | 48 | 3.6 | 0.9 | |
| Hemiptera | Control | 84 | 17 | 2.2 | 0.8 |
| Fish soup and sugar (10%) | 30 | 14 | 2.5 | 0.9 | |
| Fish soup and sugar (50%) | 50 | 23 | 2.7 | 0.9 | |
| Fish soup and sugar (100%) | 79 | 27 | 3.0 | 0.9 | |
| Hymenoptera | Control | 118 | 55 | 3.7 | 0.9 |
| Fish soup and sugar (10%) | 278 | 93 | 3.9 | 0.9 | |
| Fish soup and sugar (50%) | 210 | 49 | 2.9 | 0.8 | |
| Fish soup and sugar (100%) | 400 | 62 | 3.0 | 0.7 |
| Proximate Composition | Unit | Fish Soup | Fish Soup and sugar | Residue (Solid) | Method |
|---|---|---|---|---|---|
| Energy | MJ/kg | 1.03 | 6.03 | 6.04 | Calculated |
| Protein | % | 5.35 | 4.13 | 66.6 | ISO 5983-2 [32] |
| Total ash | % | 1.00 | 0.79 | 13.00 | ISO 5984 [33] |
| Fat | % | 0.43 | <0.10 | 12.4 | ISO 6492 [34] |
| Fibre | % | <0.02 | 0.16 | 7.09 | ISO 13906 [35] |
| Dry matter | % | 6.59 | 36.8 | 40.7 | ISO 6496 [36] |
| Calcium | % | 0.013 | 0.008 | 3.980 | ICP-MS |
| Potassium | % | 0.24 | 0.16 | 0.54 | ICP-MS |
| Magnesium | % | 0.0067 | 0.0042 | 0.1700 | ICP-MS |
| Phosphorous | % | 0.11 | 0.07 | 2.42 | ICP-MS |
| Sulphur | % | 0.075 | 0.051 | 0.740 | ICP-MS |
| Boron | ppm | 0.029 | −0.014 | 0.72 | ICP-MS |
| Molybdenum | ppm | <0.10 | <0.10 | 0.30 | ICP-MS |
| Iron | ppm | 7.95 | 8.57 | 131 | ICP-MS |
| Copper | ppm | −0.32 | −0.4 | 5.70 | ICP-MS |
| Zinc | ppm | 2.72 | 1.15 | 273 | ICP-MS |
| Manganese | ppm | 0.28 | 0.73 | 26.0 | ICP-MS |
| Sodium | ppm | 463 | 286 | 1450 | ICP-MS |
| Cobalt | ppm | 0.016 | 0.012 | <0.01 | ICP-MS |
| Peak no. | RT | RI | Compound Name | Chemical Class | Abundance * |
|---|---|---|---|---|---|
| 1 | 3.15 b | – | 5-methyl-2-heptanone | Ketone | 0.4 ± 0.11 |
| 2 | 3.18 b | – | 3-thietanol | Thiol | 0.1 ± 0.01 |
| 3 | 3.33 b | – | Acetoin | Ketone | 2.3 ± 0.94 |
| 4 | 3.43 a | – | Ethyl propanoate | Esther | 1.0 ± 0.37 |
| 5 | 3.74 b | 579 | 3,7-dimethyl nonane | Alkane | 0.2 ± 0.01 |
| 6 | 3.85 b | 628 | Isopentyl formate | Esther | 3.1 ±0.56 |
| 7 | 3.89 a | 644 | 3-methyl-1-butanol | Alcohol | 41.4 ± 0.91 |
| 8 | 3.94 a | 664 | 2-methyl-1-butanol | Alcohol | 3.8 ± 2.26 |
| 9 | 4.13 b | 704 | 7-octen-2-one | Ketone | 0.3 ± 0.05 |
| 10 | 4.47 b | 716 | (E)-2-pentenal | Aldehyde | 0.1 ± 0.03 |
| 11 | 4.73 a | 725 | Pentanol | Alcohol | 3.0 ± 1.62 |
| 12 | 4.88 b | 731 | Z-2-penten-1-ol | Alcohol | 0.5 ± 0.17 |
| 13 | 5.35 b | 748 | 3-hexanone | Ketone | 0.1 ± 0.04 |
| 14 | 5.70 a | 761 | Hexanal | Aldehyde | 6.9 ± 1.54 |
| 15 | 5.88 b | 767 | 6-methyl-2-heptanol | Alcohol | 0.5 ± 0.07 |
| 16 | 6.75 b | 799 | 1,8-nonadien-3-ol | Alcohol | 0.1 ± 0.04 |
| 17 | 7.42 a | 825 | (Z)-3-hexen-1-ol | Alcohol | 0.6 ± 0.09 |
| 18 | 7.98 b | 847 | 3-esthyl-2-methyl-2-pentene | Alkene | 0.4 ± 0.11 |
| 19 | 8.32 a | 860 | 2-heptanone | Ketone | 0.2 ± 0.01 |
| 20 | 8.55 b | 869 | 5-hepten-2-one | Ketone | 0.2 ± 0.02 |
| 21 | 8.56 a | 869 | Heptanal | Aldehyde | 0.2 ± 0.02 |
| 22 | 8.84 b | 880 | 1,2,3,4,5-pentamethyl cyclopentane | Cycloalkane | 0.9 ± 0.15 |
| 23 | 8.97 b | 885 | m-mentha-4,8-diene, (1s,3s)-(+)- | Monoterpene | 0.3 ± 0.06 |
| 24 | 9.07 a | 889 | α-thujene | Monoterpene | 0.4 ± 0.12 |
| 25 | 9.19 a | 894 | α-pinene | Monoterpene | 0.7 ± 0.16 |
| 26 | 9.32 b | 899 | 2-(3-hydroxy-propyl)-cyclohexanol | Alcohol | 0.1 ± 0.01 |
| 27 | 9.48 b | 906 | ethyl 3-methylbutylbutanoate | Esther | 0.2 ± 0.09 |
| 28 | 9.63 b | 913 | 3,3-dimethyl-2-pentanol | Alcohol | 0.4 ± 0.06 |
| 29 | 9.63 b | 913 | cyclohexane, (1,2,2-trimethylbutyl)- | Cycloalkane | 0.3 ± 0.09 |
| 30 | 9.83 b | 922 | 11-oxa-dispiro [4.0.4.1] undecan-1-ol | Spiro compound | 0.2 ± 0.02 |
| 31 | 9.86 b | 923 | β-hydroxyImidazole-5-propionic acid | Imidazole derivative | 0.3 ± 0.04 |
| 32 | 10.08 a | 933 | δ-2-carene | Monoterpene | 1.0 ± 0.50 |
| 33 | 10.21 b | 939 | 3,5,5-trimethyl-2-hexene | Alkene | 0.8 ± 0.19 |
| 34 | 10.31 a | 943 | 1-octen-3-ol | Alcohol | 0.9 ± 0.34 |
| 35 | 10.49 a | 951 | Myrcene | Monoterpene | 2.2 ± 0.39 |
| 36 | 10.73 a | 962 | γ-terpinene | Monoterpene | 0.7 ± 0.26 |
| 37 | 11.13 a | 980 | p-cymene | Monoterpene | 0.6 ± 0.24 |
| 38 | 11.21 a | 984 | β-phellandrene | Monoterpene | 3.2 ± 0.71 |
| 39 | 11.41 b | 993 | (E)-1,2-cyclopropanedicarboxylic acid | Cyclopropane derivative | 0.4 ± 0.16 |
| 40 | 11.65 a | 1005 | (Z)-β-ocimene | Monoterpene | 0.1 ± 0.08 |
| 41 | 11.77 a | 1012 | Benzeneacetaldehyde | Aldehyde | 0.4 ± 0.10 |
| 42 | 12.27 a | 1042 | Dimethyl sulfoxide | Sulfoxide | 0.2 ± 0.10 |
| 43 | 12.32 a | 1045 | Terpinolene | Monoterpene | 0.6 ± 0.23 |
| 44 | 12.53 b | 1058 | 1-cyclohexene-1-methanol | Alcohol | 0.4 ± 0.07 |
| 45 | 12.65 b | 1065 | 2,6-dimethyl cyclohexanol | Alcohol | 0.5 ± 0.17 |
| 46 | 13.31 b | 1105 | 3,4-dimethyl-2-cyclopenten-1-one | Ketone | 0.4 ± 0.16 |
| 47 | 13.85 b | 1136 | 2-butyl furan | Heterocyclic compound | 0.2 ± 0.04 |
| 48 | 14.07 b | 1149 | m-aminobenzamidine | Amine | 0.4 ± 0.15 |
| 49 | 14.10 b | 1151 | 2,5-diethylphenol | Phenol | 0.4 ± 0.17 |
| 50 | 14.18 b | 1156 | 1-methylenespiro [2.4] heptan-4-one | Spiro compound | 0.1 ± 0.02 |
| 51 | 14.50 b | 1175 | Methyl 2-methylpentanoate | Esther | 1.0 ± 0.38 |
| 52 | 15.65 a | 1248 | Terpinen-4-ol | Monoterpene alcohol | 0.3 ± 0.12 |
| 53 | 16.30 a | 1291 | α-copaene | Sesquiterpene | 0.2 ± 0.09 |
| 54 | 16.68 b | 1318 | α-cubebene | Sesquiterpene | 0.4 ± 0.11 |
| 55 | 17.26 b | 1360 | longifolene | Sesquiterpene | 0.5 ± 0.07 |
| 56 | 17.39 b | 1369 | (Z)-muurola-3,5-diene | Diene | 0.3 ± 0.08 |
| 57 | 17.48 b | 1376 | α-guaiene | Sesquiterpene | 0.5 ± 0.09 |
| 58 | 17.71 a | 1392 | α-humulene | Sesquiterpene | 0.2 ± 0.03 |
| 59 | 17.97 a | 1412 | ɣ-muurolene | Sesquiterpene | 0.1 ± 0.05 |
| 60 | 18.08 b | 1420 | Aromadendrene | Sesquiterpene | 1.2 ± 0.82 |
| 61 | 18.09 a | 1421 | β-longipinene | Sesquiterpene | 1.1 ± 0.51 |
| 62 | 18.26 b | 1434 | α-muurolene | Sesquiterpene | 0.2 ± 0.11 |
| 63 | 18.36 a | 1442 | 2,5-bis(1,1-dimethylethyl) phenol | Phenol | 0.3 ± 0.12 |
| 64 | 19.12 b | 1400 | 5-nitrothiophene-2-aldehyde | Aldehyde | 0.1 ± 0.03 |
| 65 | 19.39 b | 1422 | Sulphurous acid, pentyl undecyl ester | Esther | 0.2 ± 0.05 |
| 66 | 20.18 b | 1488 | (E)-longipinane | Sesquiterpene | 2.5 ± 1.85 |
| 67 | 20.43 b | 1603 | 1-pentadecene | Alkane | 0.2 ± 0.05 |
| 68 | 20.51 a | 1600 | hexadecane | Alkane | 2.9 ± 0.23 |
| 69 | 22.43 b | 1669 | 7-methoxy-1H-indole | Indole | 0.1 ± 0.02 |
| 70 | 23.42 b | 1905 | 2-methyl benzothiazole | Heterocyclic compound | 0.0 ± 0.01 |
| 71 | 25.26 b | 2103 | 1,3-adamantanediacetamide | Amide | 0.1 ± 0.04 |
| 72 | 25.76 b | 2158 | 9(E)-octadecenoic acid | Fatty acid | 0.1 ± 0.02 |
| 73 | 26.32 b | 2220 | 2-hydroxydesmethylimipramine | Hydoxy derivative | 0.1 ± 0.02 |
| 74 | 27.24 b | 2331 | 2-ethylacridine | Heterocyclic compound | 0.1 ± 0.08 |
| 75 | 30.25 b | 2707 | Octadecanamide | Amide | 0.6 ± 0.38 |
| 76 | 30.49 a | 2734 | Squalene | Triterpene | 2.7 ± 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niassy, S.; Omuse, E.R.; Khang’ati, J.E.; Bächinger, I.; Kupesa, D.M.; Cheseto, X.; Mbatha, B.W.; Copeland, R.S.; Mohamed, S.A.; Gama, M.; et al. Validating Indigenous Farmers’ Practice in the Management of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) in Maize Cropping Systems in Africa. Life 2024, 14, 180. https://doi.org/10.3390/life14020180
Niassy S, Omuse ER, Khang’ati JE, Bächinger I, Kupesa DM, Cheseto X, Mbatha BW, Copeland RS, Mohamed SA, Gama M, et al. Validating Indigenous Farmers’ Practice in the Management of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) in Maize Cropping Systems in Africa. Life. 2024; 14(2):180. https://doi.org/10.3390/life14020180
Chicago/Turabian StyleNiassy, Saliou, Evanson Rigan Omuse, John Emanuel Khang’ati, Ines Bächinger, David Mfuti Kupesa, Xavier Cheseto, Benjamin W. Mbatha, Robert S. Copeland, Samira Abuelgasim Mohamed, Mphatso Gama, and et al. 2024. "Validating Indigenous Farmers’ Practice in the Management of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) in Maize Cropping Systems in Africa" Life 14, no. 2: 180. https://doi.org/10.3390/life14020180






