Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis—A Systematic Review
Abstract
1. Introduction
2. Method
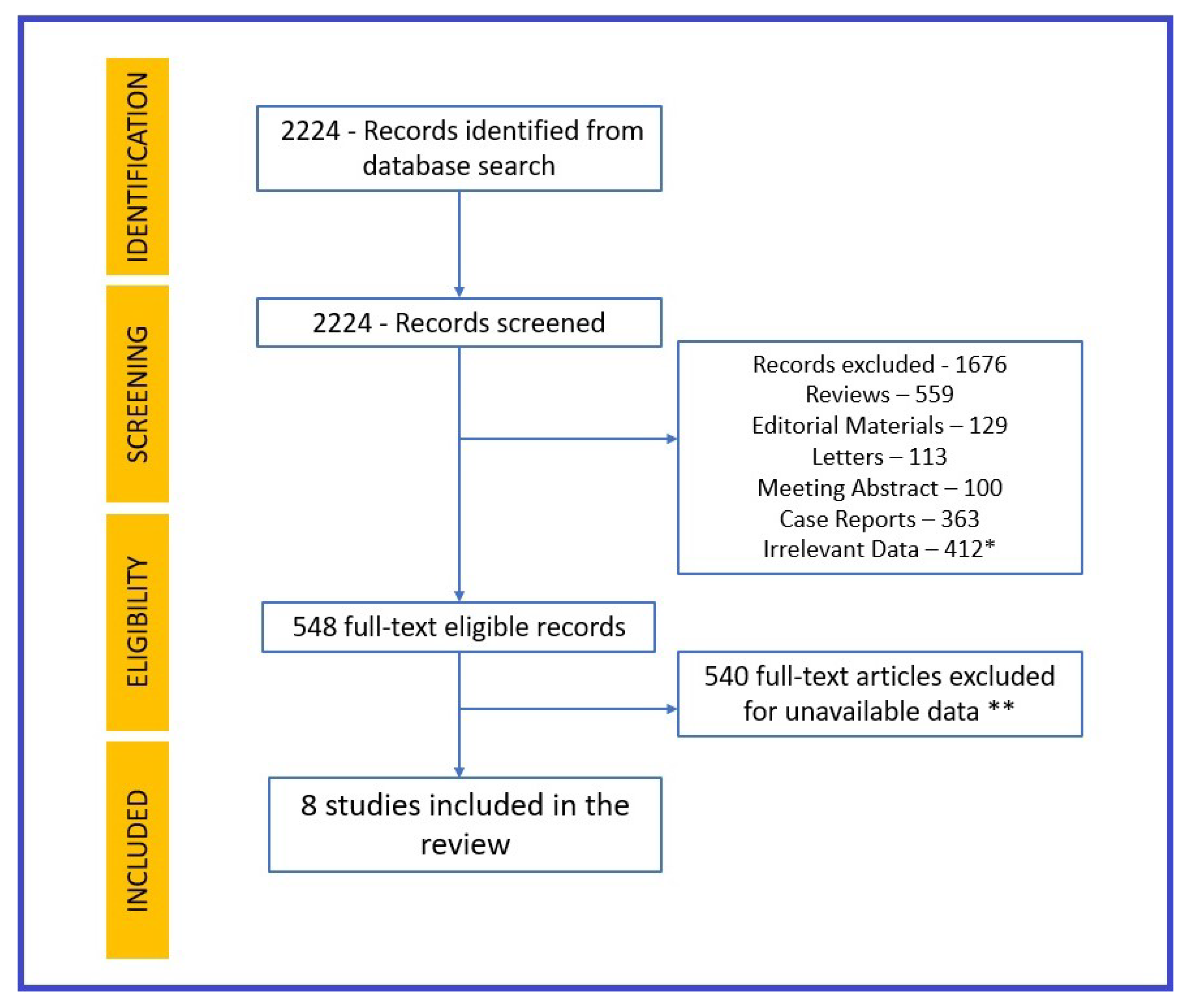
3. Results
| Authors | 1. Garcia S. et al. [8] | 2. Wei JF. et al. [10] | 3. Xie Y. et al. [9] | 4. Wang W. et al. [11] | 5. Kiris T et al. [12] | 6. Cinar T et al. [13] | 7. Choudry F.A. et al. [16] | 8. Puha K. et al. [17] |
|---|---|---|---|---|---|---|---|---|
| Country | USA | China | USA | USA | Turkey | Turkey | UK | Singapore |
| Type of study | Prospective, multicenter, observational | Prospective, observational | Retrospective, observational | Retrospective, observational | Retrospective, multicenter, observational | Prospective, observational | Retrospective, observational | Retrospective, observational |
| Patients (n) | 1191 | 101 | 5,791,407 | 2,940,988 | 1,748 | 721 | 466 | 321 |
| Type of AMI | STEMI | NA | NA | NA | STEMI | STEMI/NSTEMI | STEMI | STEMI |
| Age | 18–85 | 49 | 62.5 | 43 | 18–90 | av. 64.7 | av. 60.2 | av. 59 |
| Male | 842 (70.69%) | 54 (53.5%) | 5,228,431 (90%) | 1,241,483 (42.2%) | 1325 (75.80%) | 397 (55.06%) | 381 (81.75%) | 266 (82.86%) |
| COVID-19 confirmed | 230 | 101 | 153,760 | 690,892 | 62 | 112 | 101 | NR |
| Diabetes mellitus | 386 (32%) | 14 (13.9%) | 1,321,907 (22.82%) | 188,488 (6.4%) | 509 (29%) | 210 (29%) | 158 (33.9%) | 135 (42%) |
| Cardiac arrest | 134 (11.25%) | NR | NR | NR | 114 (6.5%) | NR | 29 (6.2%) | 19 (5.9%) |
| Cardiogenic shock | 147 (12.3%) | NR | NR | NR | 153 (8.7%) | NR | 64 (13.7%) | 30 (9.3%) |
| Smoking | 384 (32.25%) | 8 (7.9%) | 2,560,147 (44.20%) | 230,499 (7%) | 554 (31.6%) | 307 (42.5%) | 248 (53.2%) | 113 (41.4%) |
| History of CAD | 322 (27%) | 5 (5%) | NR | NR | 218 (12.5%) | 150 (21.4%) | 131 (28.1%) | 101 (31.4%) |
| Revascularization treatment | 1101 (92.44%) | NR | NA | NA | 1743 (99%) | 100% | 100% | 100% |
| Hypertension | 832 (69.85%) | 21 (21%) | 1,525,944 (26.34%) | 440,998 (14.9%) | 692 (39.5%) | 356 (49%) | 217 (46.5%) | 189 (58.9%) |
| Primary end-point | In-hospital death, stroke, recurrent myocardial infarction, unplanned revascularization | Admission to an intensive care unit, need for mechanical ventilation, vasoactive treatment or death101 | Incidence of cerebrovascular disease, dysrhythmias, ischemic heart disease, heart failure, pericarditis, myocarditis, cardiogenic shock, thrombotic disorders, MACE | Incidence of stroke, arrhythmia, pericarditis, myocarditis, ischemic coronary disease, heart failure, thrombotic disease, MACE, cardiac arrest, cardiogenic shock | MACE (all-cause mortality, heart failure, miocardialreinfarctization, cerebrovascular disease) | One-year mortality | One-year mortality | One-year cardiac-related mortality |
| Follow-up period | 5 years | 30 days | 1 year | 1 year | 542 days | 1 year | 1 year | 1 year |
4. Long-Term Risk of Myocardial Infarction
5. Long-Term Outcomes of Post-COVID-19-Positive Patients
6. Discussion
7. Limitation
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 10 June 2023).
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. Ace2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef]
- Majure, D.T.; Gruberg, L.; Saba, S.G.; Kvasnovsky, C.; Hirsch, J.S.; Jauhar, R. Northwell Health COVID-19 Research Consortium. Usefulness of elevated troponin to predict death in patients with COVID-19 and myocardial injury. Am. J. Cardiol. 2021, 138, 100–106. [Google Scholar] [CrossRef]
- Bonow, R.O.; O’Gara, P.T.; Yancy, C.W. Cardiology and COVID-19. JAMA 2020, 324, 1131–1132. [Google Scholar] [CrossRef]
- Schiavone, M.; Gobbi, C.; Biondi-Zoccai, G.; D’ascenzo, F.; Palazzuoli, A.; Gasperetti, A.; Mitacchione, G.; Viecca, M.; Galli, M.; Fedele, F.; et al. Acute coronary syndromes and COVID-19: Exploring the uncertainties. J. Clin. Med. 2020, 9, 1683. [Google Scholar] [CrossRef]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef]
- Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar]
- Garcia, S.; Dehghani, P.; Grines, C.; Davidson, L.; Nayak, K.R.; Saw, J.; Waksman, R.; Blair, J.; Akshay, B.; Garberich, R.; et al. Initial Findings from the North American COVID-19 Myocardial Infarction Registry. J. Am. Coll. Cardiol. 2021, 77, 1994–2003. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Wei, J.-F.; Huang, F.-Y.; Xiong, T.-Y.; Liu, Q.; Chen, H.; Wang, H.; Huang, H.; Luo, Y.-C.; Zhou, X.; Liu, Z.-Y.; et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart 2020, 106, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.Y. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. eClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef] [PubMed]
- Kiris, T.; Avci, E.; Ekin, T.; Akgün, D.E.; Tiryaki, M.; Yidirim, A.; Hazir, K.; Murat, B.; Yeni, M.; Altindag, R.; et al. Comparison of long-term outcome of patients with ST-segment elevation myocardial infarction between pre-COVID-19 and COVID-19 era. Eur. J. Clin. Investig. 2022, 52, e13834. [Google Scholar] [CrossRef]
- Çinar, T.; Şaylik, F.; Akbulut, T.; Asal, S.; Selçuk, M.; Çiçek, V.; Kılıç, S.; Orhan, A.L. One-year outcomes of invasively managed acute coronary syndrome patients with COVID-19. Heart Lung 2022, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Lupi, L.; Zaccone, G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Choudry, F.A.; Rathod, K.S.; Baumbach, A.; Mathur, A.; Jones, D.A. Long-term outcomes of COVID-19-associated ST-elevation myocardial infarction treated with primary PCI. Cardiovasc. Revascularization Med. 2022, 43, 133–135. [Google Scholar] [CrossRef]
- Phua, K.; Chew, N.W.S.; Sim, V.; Zhang, A.A.; Rastogi, S.; Kojodjojo, P.; Chor, W.-P.D.; Koh, B.C.-P.; Leong, B.S.-H.; Ng, Z.-Y.; et al. One-year outcomes of patients with ST-segment elevation myocardial infarction during the COVID-19 pandemic. J. Thromb. Thrombolysis 2022, 53, 335–345. [Google Scholar] [CrossRef]
- Kontos, M.C.; Rennyson, S.L.; Chen, A.Y.; Alexander, K.P.; Peterson, E.D.; Roe, M.T. The Association of Myocardial Infarction Process of Care Measures and In-Hospital Mortality: A Report from the NCDR®. Am. Heart J. 2014, 168, 766–775. [Google Scholar] [CrossRef]
- Marik, P.E.; Iglesias, J.; Varon, J.; Kory, P. COVID-19 in-Hospital Mortality: A Concise Worldwide Review. J. Comm. Med. Public Health Rep. 2021, 2, 73. [Google Scholar]
- Zuin, M.; Rigatelli, G.; Battisti, V.; Costola, G.; Roncon, L.; Bilato, C. Increased risk of acute myocardial infarction after COVID-19 recovery: A systematic review and meta-analysis. Int. J. Cardiol. 2023, 372, 138–143. [Google Scholar] [CrossRef]
- Zając, P.; Kaziród-Wolski, K.; Sielski, J.; Wolska, M.; Malinowski, K.P.; Siudak, Z. COVID-19 as an Independent Predictor of Aspiration Thrombectomy in STEMI. National Data from the ORPKI Register in the Years 2020–2022. Postep. Kardiol. Interv. 2023, 19, 119–126. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Primessnig, U.; Pieske, B.M.; Sherif, M. Increased mortality and worse cardiac outcome of acute myocardial infarction during the early COVID-19 pandemic. ESC Heart Fail. 2021, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Song, W.C.; FitzGerald, G.A. COVID-19, microangiopathy, hemostatic activation, and complement. J. Clin. Investig. 2020, 130, 3950–3953. [Google Scholar] [CrossRef] [PubMed]
- Lasica, R.; Djukanovic, L.; Mrdovic, I.; Savic, L.; Ristic, A.; Zdravkovic, M.; Simic, D.; Krljanac, G.; Popovic, D.; Simeunovic, D.; et al. Acute Coronary Syndrome in the COVID-19 Era—Differences and Dilemmas Compared to the Pre-COVID-19 Era. Clin. Med. 2022, 11, 3024. [Google Scholar] [CrossRef]
- Shibata, T.; Kawakami, S.; Noguchi, T.; Tanaka, T.; Asaumi, Y.; Kanaya, T.; Nagai, T.; Nakao, K.; Fujino, M.; Nagatsuka, K.; et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation 2015, 132, 241–250. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Nicin, L.; Abplanalp, W.T.; Mellentin, H.; Kattih, B.; Tombor, L.; John, D.; Schmitto, J.D.; Heineke, J.; Emrich, F.; Arsalan, M.; et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020, 41, 1804–1806. [Google Scholar] [CrossRef]
- Choudry, F.A.; Hamshere, S.M.; Rathod, K.S.; Akhtar, M.M.; Archbold, R.A.; Guttmann, O.P.; Woldman, S.; Jain, A.K.; Knight, C.J.; Baumbach, A.; et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2020, 76, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; AlOtaiby, S.; Badarin, F.A.; Khraibi, A.; Nader, M. Interaction of SARS-CoV-2 with cardiomyocytes: Insight into the underlying molecular mechanisms of cardiac injury and pharmacotherapy. Biomed. Pharmacother. 2022, 146, 112518. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Carrabba, M.; Milligan, R.; Williamson, M.K.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K.; et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667–2689. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. SARS-CoV-2 spike protein causes cardiovascular disease independent of viral infection. Clin. Sci. 2022, 136, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target Ther. 2021, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Sasidhar, M.V.; Chevooru, S.K.; Eickelberg, O.; Hartung, H.P.; Neuhaus, O. Downregulation of monocytic differentiation via modulation of CD147 by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. PLoS ONE 2017, 12, e0189701. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol.-Cell Physiol. 2021, 322, C1–C11. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098. [Google Scholar] [CrossRef]
- Cohen, K.; Ren, S.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Guo, Y.; Lipsitch, M.; Daugherty, S.E. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2022, 376, e068414. [Google Scholar] [CrossRef]
- Sutherland, N.; Dayawansa, N.H.; Filipopoulos, B.; Vasanthakumar, S.; Narayan, O.; Ponnuthurai, F.A.; van Gaal, W. Acute coronary syndrome in the COVID-19 pandemic: Reduced cases and increased ischaemic time. Heart Lung Circ. 2022, 31, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Nanavaty, D.; Sinha, R.; Kaul, D.; Sanghvi, A.; Kumar, V.; Vachhani, B.; Singh, S.; Devarakonda, P.; Reddy, S.; Verghese, D. Impact of COVID-19 on Acute Myocardial Infarction: A National Inpatient Sample Analysis. Curr. Probl. Cardiol. 2024, 49, 102030. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Claggett, B.; Sindet-Pedersen, C.; Lassen, M.C.H.; Skaarup, K.G.; Jensen, J.U.S.; Fralick, M.; Schou, M.; Lamberts, M.; Gerds, T.; et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 2020, 142, 2080–2082. [Google Scholar] [CrossRef] [PubMed]
- Toscano, O.; Cosentino, N.; Campodonico, J.; Bartorelli, A.L.; Marenzi, G. Acute Myocardial Infarction during the COVID-19 Pandemic: An Update on Clinical Characteristics and Outcomes. Front. Cardiovasc. Med. 2021, 8, 648290. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.N.; Şahin, M. Comparison of the Efficacy and Safety Profiles of Different P2Y12 Inhibitors in Patients with ST-Segment Elevation Myocardial Infarction in the COVID-19 Era. Cureus 2023, 15, e43829. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Majmundar, M.; Khawaja, T.; Doshi, R.; Kaur, A.; Mehta, H.; Gupta, K. Causes and Predictors of 30-Day Readmission in Patients with COVID-19 and ST-Segment–Elevation Myocardial Infarction in the United States: A Nationwide Readmission Database Analysis. J. Am. Heart Assoc. 2023, 12, e029738. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Albaghdadi, M.S.; Meraj, P.M.; Schmidt, C.; Garberich, R.; Jaffer, F.A.; Dixon, S.; Rade, J.J.; Tannenbaum, M.; Chambers, J.; et al. Reduction in ST segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J. Am. Coll. Cardiol. 2020, 75, 2871–2872. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Macedo, A.V.S.; de Barros, E.S.P.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; D’Andréa Saba Arruda, G.; de Albuquerque, D.C.; Camiletti, A.S.; et al. BRACE CORONA Investigators: Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: A randomized clinical trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef]
- Andreß, S.; Stephan, T.; Felbel, D.; Mack, A.; Baumhardt, M.; Kersten, J.; Buckert, D.; Pott, A.; Dahme, T.; Rottbauer, W.; et al. Deferral of non-emergency cardiac procedures is associated with increased early emergency cardiovascular hospitalizations. Clin. Res. Cardiol. 2022, 111, 1121–1129. [Google Scholar] [CrossRef]
- Rabail, R.; Ahmed, W.; Ilyas, M.; Rajoka, M.S.R.; Hassoun, A.; Khalid, A.R.; Khan, M.R.; Aadil, R.M. The Side Effects and Adverse Clinical Cases Reported after COVID-19 Immunization. Vaccines 2022, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Coronavirus Disease 2019 (COVID-19) Vaccines. Available online: https://www.cdc.gov/vaccines/acip/ (accessed on 6 July 2021).
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
| Authors | 1. Garcia S. et al. [8] | 2. Xie Y. et al. [9] | 3. CinarT. et al. [13] | 3. Kiris T. et al. [12] | 4. Wang W. et al. [11] |
|---|---|---|---|---|---|
| COVID-19 (+)/COVID-19 (−) | 230/436 | 153,760/5,637,647 | 112/609 | 1686/62 | 691,455/2,249,533 |
| Male/Female | 71%/29% | 89%/11% | 55%/45% | 75%/25% | 43.2%/56.8% |
| MACE (COVID+/COVID−) | 33%/18% | Hazard Ration 1.26 (CI 95%) for non-hospitalized COVID+/2.41 for hospitalized COVID + patient | 21.3%/6.3% | 22%/22% | Hazard Ratio (CI = 95%) in COVID-19+ was 2.26 |
| 30-day Outcome | 1 out of 3 COVID-19 (+) patients deceased | Incidence of MI increases 3 times for post-COVID-19 patients | Mortality COVID-19 = 21%. Non-COVID-19 = 7.1% | NA | HR for MI at 30-days outcome = 2.32, HR for death at 30 days = 2.067 |
| Incidence of Miocardial Infarcion in COVID-19 (+) Patient Non-Hospitalized/Hospitalized | NA | 3 times higher MI incidence in hospitalized MI patients | NA | NA | Similar MI incidence for hospitalized/non-hospitalized COVID-19+ |
| Incidence of Miocardial Infarction at 12-Month Follow-up | NA | Hazard Ratio (CI 95%) 1.71, burden/1000 pers at 12 M 7.59 for COVID-19+ survivors | NR | 6.5% pre-COVID era/5.3% in COVID era | HR (95% CI) = 1.49 |
| Study | 1. Xie Y. et al. [9] | 2. Wang W. et al. [11] | 3. Garcia S et al. [8] | 4. Kiris T et al. [12] |
|---|---|---|---|---|
| Mace | HR (CI 95%) = 1.55 (COVID-19 + 67.67 vs. COVID-19 − 44.19) | 10,530 patients HR = 1.871 | 36% | 22% |
| Cerebrovascular | HR = 1.53 (COVID-19 + 15.95 vs. COVID-19 − 10.48) | 4793 patients HR = 1.68 | 5% | 1% |
| Arhythmyas | HR = 1.69 (COVID-19 + 49.37 vs. COVID-19 − 29.51) | 20,927 patients HR = 2.407 | NA | NA |
| Ischemic heart disease | HR = 1.66 (COVID-19 + 18.47 vs. COVID-19 − 11.19) | 3651 patients HR = 2.8 | 6% (Recurrent MI and unplanned revascularization) | 15% (Recurrent MI, unplanned revascularization) |
| Heart failure | HR = 1.72 (COVID-19 + 27.92 vs. COVID-19 − 16.31) | 5831 patients HR = 2.29 | 54% | 12% |
| Thrombotic disease | HR = 2.39 (COVID-19 + 17.07 vs. COVID-19 − 7.19 | 4599 patients HR = 2.64 | NA | NA |
| Cardiac arrest | HR = 2.45 (COVID-19 + 1.20 vs. COVID-19 − 0.49) | 474 patients HR = 1.75 | 11% | 8.50% |
| Cardiogenic shock | HR = 2.43 (COVID-19 + 0.87 vs. COVID-19 − 0.36) | 204 patients HR = 1.98 | 18% | 21% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, M.; Ardelean, A.I.; Andronie-Cioara, F.L.; Filimon, G.C. Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis—A Systematic Review. Life 2024, 14, 202. https://doi.org/10.3390/life14020202
Rus M, Ardelean AI, Andronie-Cioara FL, Filimon GC. Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis—A Systematic Review. Life. 2024; 14(2):202. https://doi.org/10.3390/life14020202
Chicago/Turabian StyleRus, Marius, Adriana Ioana Ardelean, Felicia Liana Andronie-Cioara, and Georgiana Carmen Filimon. 2024. "Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis—A Systematic Review" Life 14, no. 2: 202. https://doi.org/10.3390/life14020202
APA StyleRus, M., Ardelean, A. I., Andronie-Cioara, F. L., & Filimon, G. C. (2024). Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis—A Systematic Review. Life, 14(2), 202. https://doi.org/10.3390/life14020202







