Effects of Seed Size and Cache Density on the Seed Fate of Quercus wutaishanica Mediated by Rodents
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Seed Collection and Marking
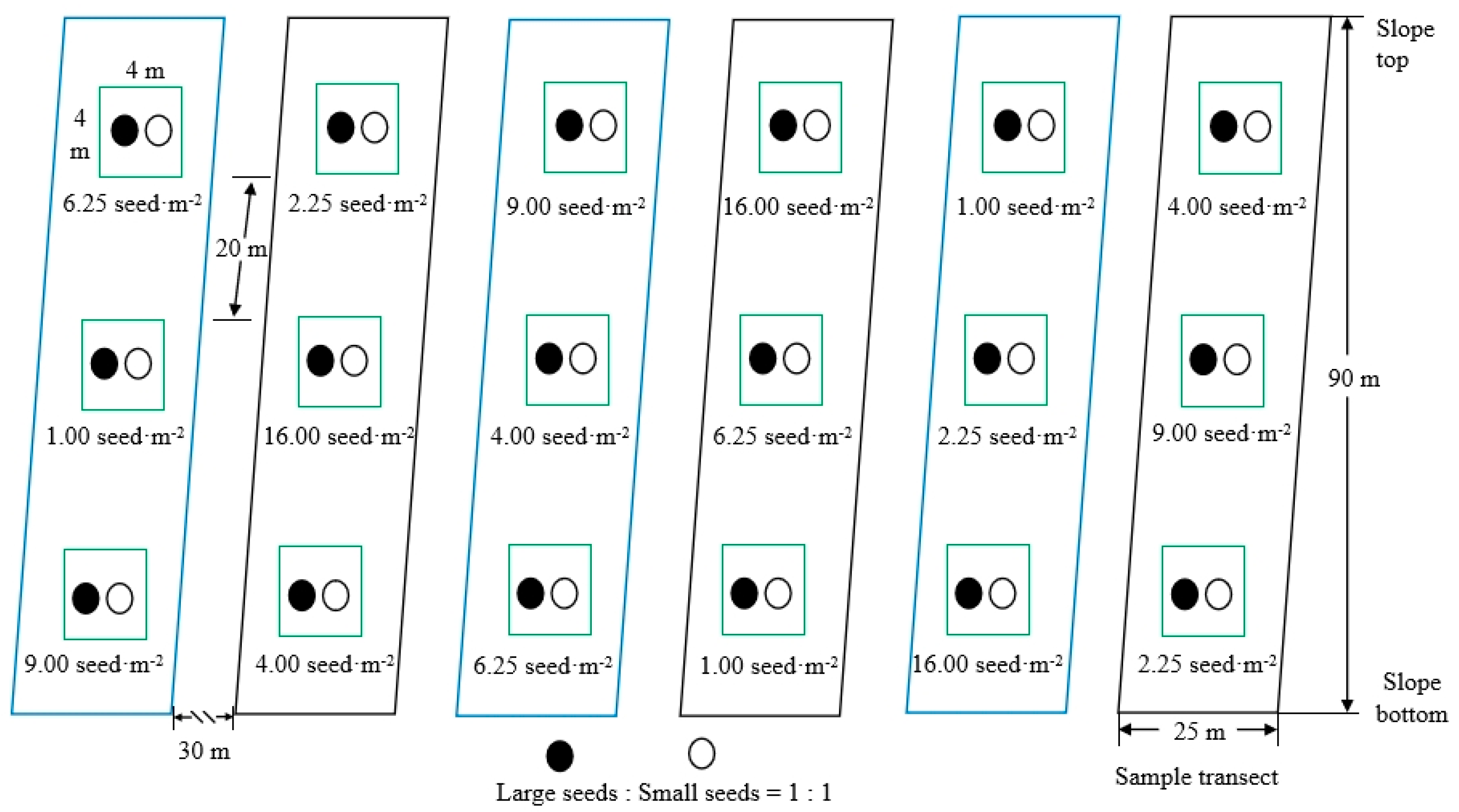
2.3. Experimental Design
2.4. Field Investigation
2.5. Seed Fates
2.6. Data Analysis
3. Results
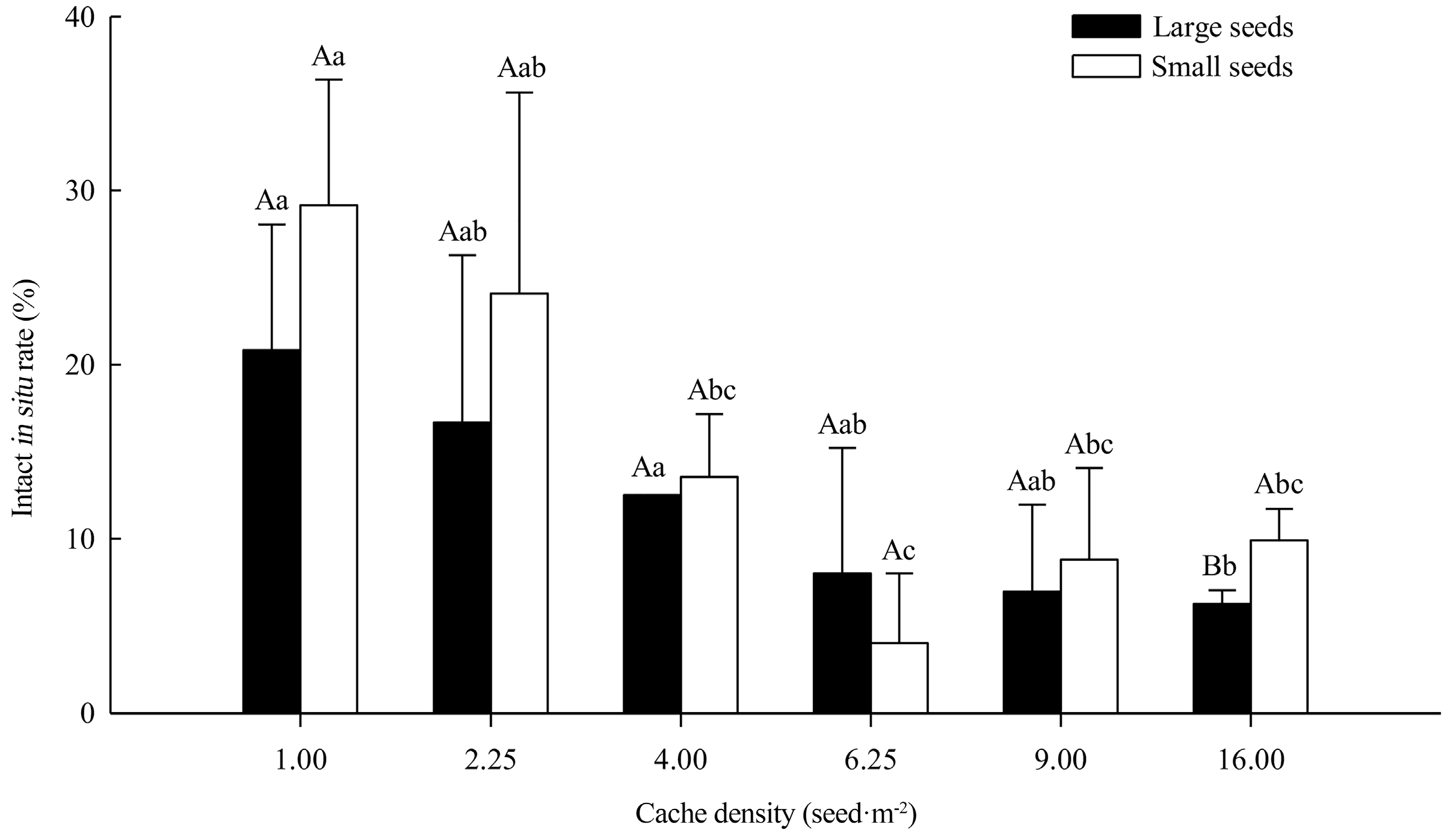
3.1. Seed Intact In Situ Rate and Dynamics
3.2. Seed Fates
3.3. Seed Dispersal Distance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vander Wall, S.B. Food Hoarding in Animals; The University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- Jansen, P.A.; Bongers, F.; Hemerik, L. Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecol. Monogr. 2004, 74, 569–589. [Google Scholar] [CrossRef]
- Zhang, Z.B. Studies on the Rodent-Seed Interaction of Forest Ecosystems—Exploring the Secrets of Cooperation Between Antagonists; Science Press: Beijing, China, 2019. [Google Scholar]
- Wang, B.; Chen, J.; Corlett, R.T. Factors influencing repeated seed movements by scatter-hoarding rodents in an alpine forest. Sci. Rep. 2014, 4, 4786. [Google Scholar] [CrossRef]
- Stapanian, M.A.; Smith, C.C. Model for seed scatter hoarding—Coevolution of fox squirrels and black walnuts. Ecology 1978, 59, 884–896. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Z.Y.; Yan, C.; Chen, J.; Guo, C.; Zhang, Z.B. Differential foraging preferences on seed size by rodents result in higher dispersal success of medium-sized seeds. Ecology 2016, 97, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.L.; Ma, J.Z.; Rong, K. Animal scatter-hoarding behavior and its impact on the regeneration of plant populations. Acta Ecol. Sin. 2016, 36, 1162–1169. [Google Scholar]
- Zhang, B.; Shi, Z.J.; Chen, X.N.; Hou, X.; Wang, J.; Li, J.G.; Chang, G. Seed dispersal of three sympatric oak species by forest rodents in the south slope of Qinling Mountains, China. Acta Ecol. Sin. 2016, 36, 6750–6757. [Google Scholar]
- Lewis, A.R. Selection of nuts by gray squirrels and optimal foraging theory. Am. Nat. 1982, 107, 250–257. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Z.B. Key factors affecting the capacity of David’s rock squirrels (Sciurotamias davidianus) to discover scatter-hoarded seeds in enclosures. Biodivers. Sci. 2007, 15, 329–336. [Google Scholar]
- Yi, X.F.; Rachel, C.; Bartlow, A.W.; Agosta, S.J.; Steele, M.A. Ability of chestnut oak to tolerate acorn pruning by rodents: The role of the cotyledonary petiole. Naturwissenschaften 2013, 100, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.F.; Steele, M.A.; Stratford, J.A.; Wang, Z.Y.; Yang, Y.Q. The use of spatial memory for cache management by a scatter-hoarding rodent. Behav. Ecol. Sociobiol. 2016, 70, 1527–1534. [Google Scholar] [CrossRef]
- Moore, J.E.; McEuen, A.B.; Swihart, R.K.; Contreras, T.A.; Steele, M.A. Determinants of seed removal distance by scatter-hoarding rodents in deciduous forests. Ecology 2007, 88, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, D.; Kranstauber, B.; Kays, R.W.; Jansen, P.A. Scatter hoarding by the Central American agouti: A test of optimal cache spacing theory. Anim. Behav. 2009, 78, 1327–1333. [Google Scholar] [CrossRef]
- Male, L.H.; Smulders, T.V. Hyperdispersed cache distributions reduce pilferage: A field study. Anim. Behav. 2007, 73, 717–726. [Google Scholar] [CrossRef]
- Luo, Y.H.; Cheng, J.M.; Yan, X.F.; Yang, H.; Shen, Y.; Ge, J.R.; Zhang, M.; Zhang, J.F.; Xu, Z.W. Density-dependent seed predation of Quercus wutaishanica by rodents in response to different seed states. Animals 2023, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.B.; Yan, X.F.; Wang, J.L.; Zhou, Y.F. Predation and removal of rodents on the seeds with different size and pericarp traits. Chin. J. Appl. Ecol. 2013, 24, 2325–2332. [Google Scholar]
- Yan, X.F.; Zhou, L.B.; Zhang, K.W.; Zhou, Y.F. Cotyledon loss and its effects on survival and growth of Quercus wutaishanica seedlings under different densities. Chin. J. Plant. Ecol. 2012, 36, 831–840. [Google Scholar] [CrossRef]
- Yan, X.F.; Zhou, L.B.; Liu, J.L. Effects of different habitats and coverage treatments on the fates of Quercus wutaishanica seeds under the predation pressure of rodents. Acta Ecol. Sin. 2012, 32, 2778–2787. [Google Scholar]
- Xiao, Z.S.; Jansen, P.A.; Zhang, Z.B. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. For. Ecol. Manag. 2006, 223, 18–23. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, Y.; Zhang, Z.B. Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. For. Ecol. Manag. 2008, 255, 124–1250. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Henry, H.M. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2019, 24, 127–135. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Vander Wall, S.B.; Jenkins, S.H. Reciprocal pilferage and the evolution of food-hoarding behavior. Behav. Ecol. 2003, 14, 656–667. [Google Scholar] [CrossRef]
- Shen, Z.; Dong, Z.; Cao, L.L.; Zhang, M.M.; Liu, G.Q.; Yi, X.F. Effects of conspecific and interspecific interference competitions on cache site selection of Siberian chipmunks (Tamias sibiricus). Acta Ecol. Sin. 2012, 32, 7264–7269. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Zhang, Z.B.; Lu, J.Q.; Cheng, J.R. Repeated caching of plant seeds by small rodents. Chin. J. Zool. 2004, 39, 94–99. [Google Scholar]
- Huang, Z.Y.; Wang, Y.; Zhang, H.M.; Wu, F.Q.; Zhang, Z.B. Behavioural responses of sympatric rodents to complete pilferage. Anim. Behav. 2011, 81, 831–836. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, H.; Wang, Y.; Zhang, Z.B. Response of seed-hoarding behaviour to consepecific audiences in scatter-and/or lader-hoarding ordents. Behaviour 2011, 148, 825–842. [Google Scholar]
- Teng, W.; Liu, B.B.; Rong, K. Predation pattern of seed predators on coniferous seeds and their defense adaptation. Chin. J. Ecol. 2018, 37, 2180–2188. [Google Scholar]
- Wang, B.; Wang, G.; Chen, J. Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol. 2012, 213, 1329–1336. [Google Scholar] [CrossRef]
- Tamura, N.; Hayashi, F. Geographic variation in walnut seed size correlates with hoarding behaviour of two rodent species. Ecol. Res. 2008, 23, 607–614. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Z.B. Effects of soil depth, cache spacing and cache size of sunflower (Helianthus annuus) seeds on seed discovery by Siberian chipmunk (Tamias sibiricus senescens). Acta Theriol. Sin. 2006, 26, 398–402. [Google Scholar]
- Liu, C.Q.; Wang, Z.Y.; Yi, X.F.; Yang, Y.Q. Effects of cache depth, cache size and soil moisture on cache discovery of Pinus koraiensis seeds by Tamias sibiricus. Acta Theriol. Sin. 2016, 36, 72–76. [Google Scholar]
- Zhang, H.M.; Cheng, J.R.; Xiao, Z.S.; Zhang, Z.B. Effects of seed abundance on seed scatter-hoarding of Edward’s rat (Leopoldamys edwardsi Muridae.) at the individual level. Oecologia 2008, 158, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.S.; Zhang, Z.B.; Krebs, C.J. Long-term seed survival and dispersal dynamics in a rodent-dispersed tree: Testing the predator satiation hypothesis and the predator dispersal hypothesis. J. Ecol. 2013, 101, 1256–1264. [Google Scholar] [CrossRef]
- Niu, H.Y.; Wang, Z.Y.; Huang, G.C.; Peng, C.; Zhang, Z.B.; Zhang, H.M. Responses of a scatter-hoarding squirrel to conspecific pilfering: A test of the reciprocal pilferage hypothesis. Anim. Behav. 2020, 170, 147–155. [Google Scholar] [CrossRef]
- Vander Wall, S.B.; Enders, M.S.; Waitman, B.A. Asymmetrical cache pilfering between yellow pine chipmurks and golden-mantled ground squirrels. Anim. Behav. 2009, 78, 555–561. [Google Scholar] [CrossRef]
- Vander Wall, S.B. How plants manipulate the scatter-hoarding behaviour of seed-dispersing animals. Philos. Trans. R. Soc. B. 2010, 365, 989–997. [Google Scholar] [CrossRef]
| Density (Seed·m−2) | EIS | EAR | SH | M | ||||
|---|---|---|---|---|---|---|---|---|
| Large Seed | Small Seed | Large Seed | Small Seed | Large Seed | Small Seed | Large Seed | Small Seed | |
| 1.00 | 4.17 ± 7.22 a | 25.00 ± 12.50 a | 16.67 ± 14.43 a | 8.33 ± 14.43 c | 4.17 ± 7.22 a | 0.00 | 54.17 ± 7.22 Aa | 37.50 ± 21.65 Aac |
| 2.25 | 3.70 ± 3.21 Aa | 12.96 ± 6.42 Aab | 22.22 ± 14.70 Aa | 48.15 ± 11.56 Aabc | 11.11 ± 5.56 Aa | 3.70 ± 3.21 Aab | 46.30 ± 26.25 Aa | 11.11 ± 0.00 Ac |
| 4.00 | 2.08 ± 3.61 Aa | 5.21 ± 3.61 Ab | 29.17 ± 25.26 Aa | 60.42 ± 6.51 Aa | 11.46 ± 7.86 Aa | 1.04 ± 1.80 Ab | 44.79 ± 20.81 Aa | 19.79 ± 7.86 Abc |
| 6.25 | 0.67 ± 1.15 Aa | 4.67 ± 1.15 Ab | 22.00 ± 4.00 Aa | 42.67 ± 19.63 Aabc | 13.33 ± 4.16 Aa | 13.33 ± 4.16 Aa | 56.00 ± 12.49 Aa | 35.33 ± 12.70 Aab |
| 9.00 | 0.46 ± 0.80 Aa | 6.48 ± 2.12 Ab | 12.95 ± 9.76 Aa | 38.43 ± 10.42 Abc | 14.81 ± 11.31 Aa | 8.80 ± 5.78 Aab | 6.48 ± 2.12 Ba | 37.50 ± 2.78 Aa |
| 16.00 | 0.78 ± 0.35 Aa | 7.55 ± 1.63 Ab | 18.75 ± 7.69 Ba | 53.65 ± 2.26 Aab | 15.36 ± 5.86 Aa | 8.85 ± 5.86 Bab | 58.85 ± 11.38 Aa | 20.05 ± 1.90 Bbc |
| Cache Density (Seed·m−2) | SEED Size | EDAR | SHD |
|---|---|---|---|
| Mean ± Std (Number) | Mean ± Std (Number) | ||
| 1.00 | Large seed | 2.03 ± 1.96 c (n = 4) | 1.10 ± 0 b (n = 1) |
| Small seed | 0.15 ± 0.09 b (n = 2) | — | |
| 2.25 | Large seed | 2.00 ± 1.55 Ac(n = 14) | 4.54 ± 1.56 Ab (n = 6) |
| Small seed | 0.88 ± 0.49 Ab (n = 27) | 0.65 ± 0.38 Bb (n = 2) | |
| 4.00 | Large seed | 5.43 ± 1.25 Abc (n = 29) | 5.75 ± 1.78 Ab (n = 11) |
| Small seed | 1.39 ± 0.71 Bb (n = 59) | 0.40 ± 0 Bb (n = 1) | |
| 6.25 | Large seed | 9.76 ± 1.70 Aa (n = 36) | 10.31 ± 3.39 Aa (n = 20) |
| Small seed | 4.39 ± 0.18 Ba (n = 66) | 4.19 ± 0.88 Ba (n = 20) | |
| 9.00 | Large seed | 7.58 ± 1.49 Aabc (n = 35) | 7.35 ± 4.21 Aa (n = 32) |
| Small seed | 2.49 ± 1.75 Bab (n = 91) | 5.55 ± 0.68 Aa (n = 19) | |
| 16.00 | Large seed | 5.87 ± 1.19 Abc (n = 77) | 6.63 ± 1.00 Aab (n = 60) |
| Small seed | 2.07 ± 0.40 Aab (n = 213) | 2.55 ± 0.82 Aab (n = 34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Zhang, M.; Yan, X. Effects of Seed Size and Cache Density on the Seed Fate of Quercus wutaishanica Mediated by Rodents. Life 2024, 14, 286. https://doi.org/10.3390/life14030286
Cheng J, Zhang M, Yan X. Effects of Seed Size and Cache Density on the Seed Fate of Quercus wutaishanica Mediated by Rodents. Life. 2024; 14(3):286. https://doi.org/10.3390/life14030286
Chicago/Turabian StyleCheng, Jiming, Min Zhang, and Xingfu Yan. 2024. "Effects of Seed Size and Cache Density on the Seed Fate of Quercus wutaishanica Mediated by Rodents" Life 14, no. 3: 286. https://doi.org/10.3390/life14030286
APA StyleCheng, J., Zhang, M., & Yan, X. (2024). Effects of Seed Size and Cache Density on the Seed Fate of Quercus wutaishanica Mediated by Rodents. Life, 14(3), 286. https://doi.org/10.3390/life14030286






