Identification of Fusarium oxysporum Causing Leaf Blight on Dendrobium chrysotoxum in Yunnan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of the Pathogen
2.3. Morphological Identification
2.4. DNA Extraction
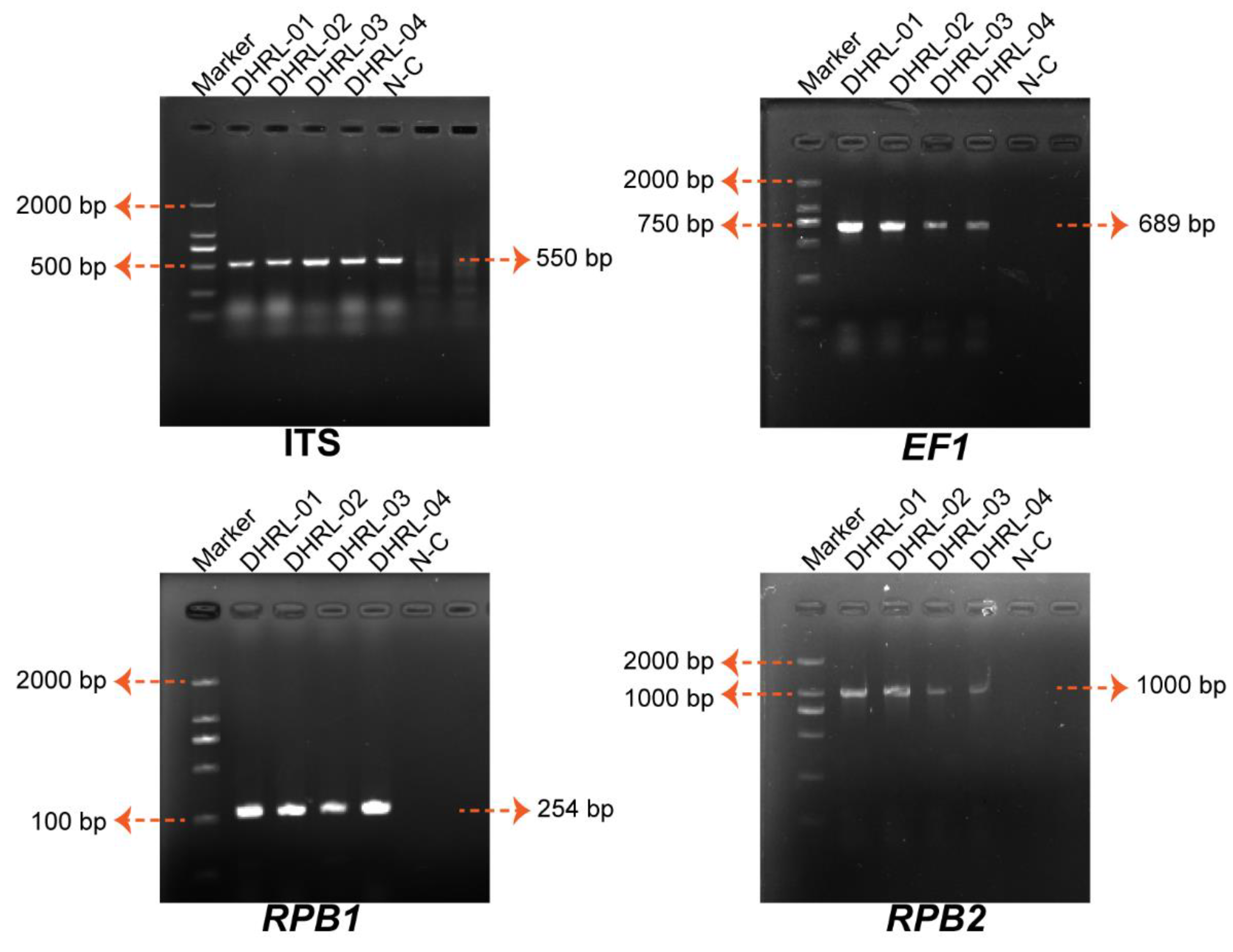
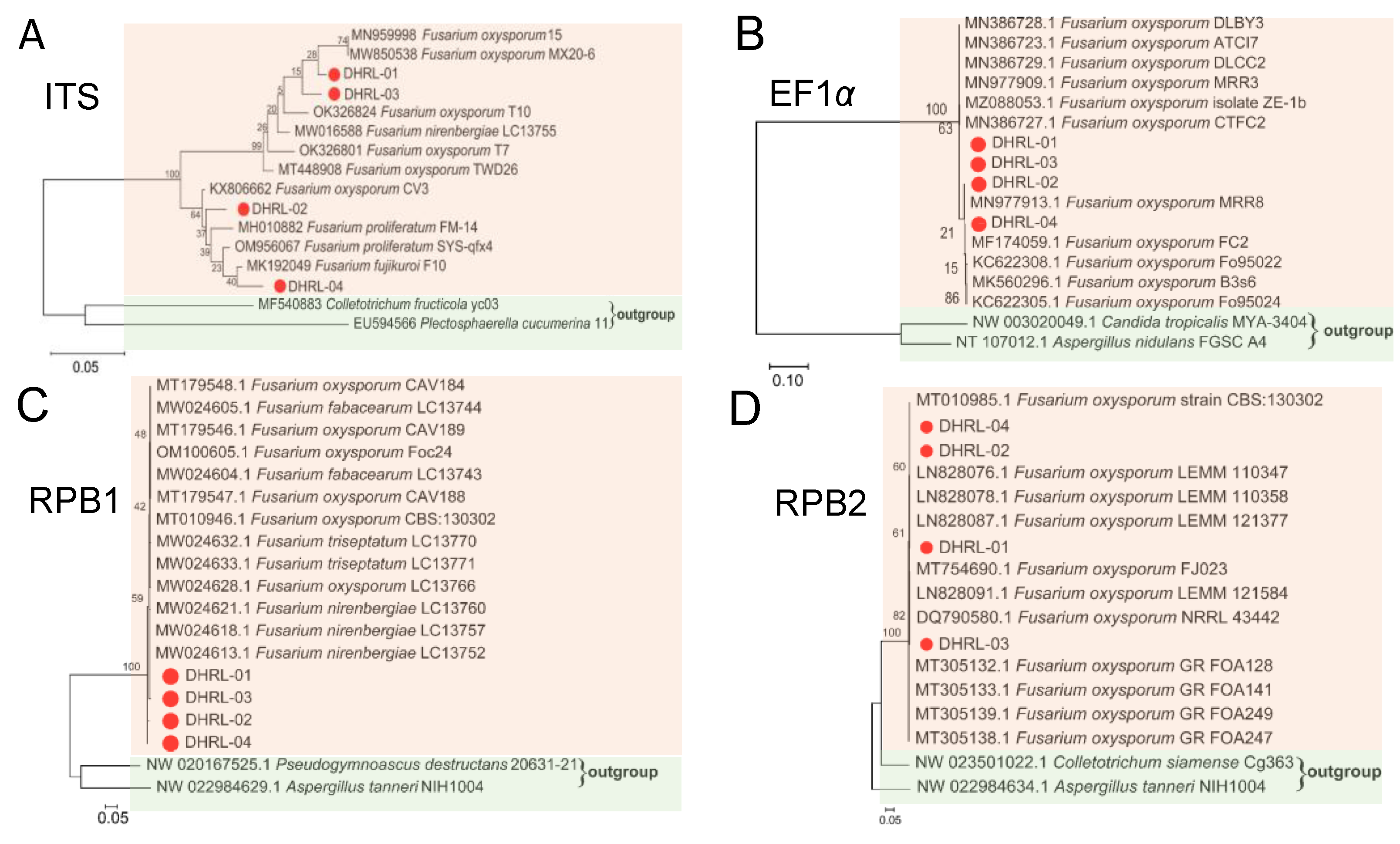
2.5. PCR Amplification, Molecular Identification, and Construction of Phylogenetic Tree
2.6. Pathogenicity Test
2.7. Pathogen Reisolation
3. Results
3.1. Nursery Observations and Isolations
3.2. Morphological Identification
3.3. Molecular Identification
3.4. Pathogenicity Test
3.5. Pathogen Reisolation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarsaiya, S.; Jain, A.; Jia, Q.; Fan, X.; Shu, F.; Chen, Z.; Zhou, Q.; Shi, J.; Chen, J. Molecular Identification of Endophytic Fungi and Their Pathogenicity Evaluation against Dendrobium nobile and Dendrobium officinale. Int. J. Mol. Sci. 2020, 21, 316. [Google Scholar] [CrossRef]
- Huang, H.; Zi, X.-M.; Lin, H.; Gao, J.-Y. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J. Microbiol. 2018, 56, 42–48. [Google Scholar] [CrossRef]
- Hou, B.; Tian, M.; Luo, J.; Ji, Y.; Xue, Q.; Ding, X. Genetic diversity assessment and ex situ conservation strategy of the endangered Dendrobium officinale (Orchidaceae) using new trinucleotide microsatellite markers. Plant Syst. Evol. 2012, 298, 1483–1491. [Google Scholar] [CrossRef]
- Hou, B.; Luo, J.; Zhang, Y.; Niu, Z.; Xue, Q.; Ding, X. Iteration expansion and regional evolution: Phylogeography of Dendrobium officinale and four related taxa in southern China. Sci. Rep. 2017, 7, 43525. [Google Scholar] [CrossRef] [PubMed]
- Tikendra, L.; Potshangbam, A.M.; Amom, T.; Dey, A.; Nongdam, P. Understanding the genetic diversity and population structure of Dendrobium chrysotoxum Lindl.—An endangered medicinal orchid and implication for its conservation. S. Afr. J. Bot. 2021, 138, 364–376. [Google Scholar] [CrossRef]
- Xu, F.-Q.; Fan, W.-W.; Zi, C.-T.; Dong, F.-W.; Yang, D.; Zhou, J.; Hu, J.-M. Four new glycosides from the stems of Dendrobium fimbriatum Hook. Nat. Prod. Res. 2016, 31, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Guo, Q.; Wang, J.; Cui, S.W. Structural characterization and conformational properties of a polysaccharide isolated from Dendrobium nobile Lindl. Food Hydrocoll. 2020, 98, 104904. [Google Scholar] [CrossRef]
- Cao, H.; Ji, Y.; Li, S.; Lu, L.; Tian, M.; Yang, W.; Li, H. Extensive Metabolic Profiles of Leaves and Stems from the Medicinal Plant Dendrobium officinale Kimura et Migo. Metabolites 2019, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fan, W.; Dong, F.; Miao, Z.; Zhou, J. Chemical Components of Dendrobium chrysotoxum. Chin. J. Chem. 2012, 30, 1327–1330. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Ng, T.B. The medicinal and pharmaceutical importance of Dendrobium species. Appl. Microbiol. Biotechnol. 2017, 101, 2227–2239. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, J.; Wen, C.; Dzah, C.S.; Juliet, I.C.; Duan, Y.; Zhang, H. Recent advances in Agaricus bisporus polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Process. Biochem. 2020, 94, 39–50. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Biological activity and phytochemical profiles of Dendrobium: A new source for specialty cosmetic materials. Ind. Crop. Prod. 2018, 120, 61–70. [Google Scholar] [CrossRef]
- Zha, X.-Q.; Luo, J.-P.; Wei, P. Identification and classification of Dendrobium candidum species by fingerprint technology with capillary electrophoresis. S. Afr. J. Bot. 2009, 75, 276–282. [Google Scholar] [CrossRef]
- Gong, C.-Y.; Yu, Z.-Y.; Lu, B.; Yang, L.; Sheng, Y.-C.; Fan, Y.-M.; Ji, L.-L.; Wang, Z.-T. Ethanol extract of Dendrobium chrysotoxum Lindl ameliorates diabetic retinopathy and its mechanism. Vasc. Pharmacol. 2014, 62, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.-Z.; Xu, T.-T.; Wang, C.-Q.; Li, Q.-M.; Zha, X.-Q.; Pan, L.-H.; Luo, J.-P. Bioactivity-guided investigation for isolation and immunoregulatory potential of polysaccharides from Dendrobium chrysotoxum stems. Process. Biochem. 2021, 104, 124–131. [Google Scholar] [CrossRef]
- Pan, L.-H.; Wang, J.; Ye, X.-Q.; Zha, X.-Q.; Luo, J.-P. Enzyme-assisted extraction of polysaccharides from Dendrobium chrysotoxum and its functional properties and immunomodulatory activity. LWT Food Sci. Technol. 2015, 60, 1149–1154. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, F.; Lin, J.; Li, Y.; Johnson, N.; Zhang, J.; Gai, C.; Su, Z.; Cheng, H.; Wang, L.; et al. Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. J. Ethnopharmacol. 2021, 279, 114399. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Jin, Y.; Wang, W.; Xia, K.; Chen, Z. Molecular and metabolic insights into floral scent biosynthesis during flowering in Dendrobium chrysotoxum. Front. Plant Sci. 2022, 13, 1030492. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-Z.; Lv, G.-P.; Hu, D.-J.; Cheong, K.L.; Xie, J.; Zhao, J.; Li, S.-P. Effects of Polysaccharides from Different Species of Dendrobium (Shihu) on Macrophage Function. Molecules 2013, 18, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Naha, S.; Majumdar, M.; Banerjee, N. Direct and callus-mediated protocorm-like body induction from shoot-tips of Dendrobium chrysotoxum Lindl. (Orchidaceae). Plant Cell Tissue Organ Cult. 2007, 90, 31–39. [Google Scholar] [CrossRef]
- Tao, Y.; Zeng, F.; Ho, H.; Wei, J.; Wu, Y.; Yang, L.; He, Y. Pythium vexans Causing Stem Rot of Dendrobium in Yunnan Province, China. J. Phytopathol. 2010, 159, 255–259. [Google Scholar] [CrossRef]
- Nhung, N.P.; Thu, P.Q.; Dell, B.; Chi, N.M. First report of canker disease in Dalbergia tonkinensis caused by Fusarium lateritium and Fusarium decemcellulare. Australas. Plant Pathol. 2018, 47, 317–323. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Hanisia, R.H.; Signorelli, S.; Fauziah, T.; Iriawati; Esyanti, R.R. Extracellular DNA of Fusarium oxysporum f. sp. cubense as a Priming Agent for Inducing the Resistance of Banana Plantlets. Agronomy 2023, 13, 441. [Google Scholar] [CrossRef]
- Matengu, T.T.; Bullock, P.R.; Mkhabela, M.S.; Zvomuya, F.; Henriquez, M.A.; Ojo, E.R.; Fernando, W.G.D. Weather-based models for forecasting Fusarium head blight risks in wheat and barley: A review. Plant Pathol. 2023. [Google Scholar] [CrossRef]
- Zhu, Y.; Abdelraheem, A.; Cooke, P.; Wheeler, T.; Dever, J.K.; Hake, K.; Bissonnette, K.; Zhang, J. Comparative analysis of infection process in Upland cottons differing in resistance to Fusarium wilt caused by Fusarium oxysporum f. sp. vasinfectum race. Crop Sci. 2023, 63, 1330–1343. [Google Scholar] [CrossRef]
- Srivastava, S.; Kadooka, C.; Uchida, J.Y. Fusarium species as pathogen on orchids. Microbiol. Res. 2018, 207, 188–195. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2017, 217, 1203–1212. [Google Scholar] [CrossRef]
- Trabelsi, R.; Sellami, H.; Gharbi, Y.; Krid, S.; Cheffi, M.; Kammoun, S.; Dammak, M.; Mseddi, A.; Gdoura, R.; Triki, M.A. Morphological and molecular characterization of Fusarium spp. associated with olive trees dieback in Tunisia. 3 Biotech 2017, 7, 28. [Google Scholar] [CrossRef]
- Brandfass, C.; Karlovsky, P. Upscaled CTAB-Based DNA Extraction and Real-Time PCR Assays for Fusarium culmorum and F. graminearum DNA in Plant Material with Reduced Sampling Error. Int. J. Mol. Sci. 2008, 9, 2306–2321. [Google Scholar] [CrossRef]
- Shanuja, S.; Iswarya, S.; Gnanamani, A. Marine fungal DHICA as a UVB protectant: Assessment under in vitro and in vivo conditions. J. Photochem. Photobiol. B Biol. 2018, 179, 139–148. [Google Scholar] [CrossRef]
- Cabral, C.S.; Fonseca, M.E.d.N.; Brunelli, K.R.; Rossato, M.; Costa, H.; Boiteux, L.S.; Reis, A. Relationships among Brazilian and worldwide isolates of Fusarium oxysporum f. sp. lactucae race 1 inferred from ribosomal intergenic spacer (IGS-rDNA) region and EF-1α gene sequences. Eur. J. Plant Pathol. 2018, 152, 81–94. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-Accessible DNA Sequence Database for Identifying Fusaria from Human and Animal Infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef]
- O’donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Han, K.-S.; Park, J.-H.; Back, C.-G.; Park, M.-J. First Report of Fusarium subglutinans Causing Leaf Spot Disease on Cymbidium orchids in Korea. Mycobiology 2015, 43, 343–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.-J.; Chen, Y.-J.; Jain, Y.-C.; Chung, W.-C.; Wang, C.-L.; Chung, W.-H. Identification of Fusarium proliferatum causing leaf spots on Cymbidium orchids in Taiwan. J. Phytopathol. 2018, 166, 675–685. [Google Scholar] [CrossRef]
- Baek, J.M.; Kim, J.-Y.; Ahn, S.-J.; Cheon, Y.-H.; Yang, M.; Oh, J.; Choi, M.K. Dendrobium moniliforme Exerts Inhibitory Effects on Both Receptor Activator of Nuclear Factor Kappa-B Ligand-Mediated Osteoclast Differentiation in vitro and Lipopolysaccharide-Induced Bone Erosion in vivo. Molecules 2016, 21, 295. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, B.; Peng, A.; Song, X.; Chen, X. The genome of banana leaf blight pathogen Fusarium sacchari str. FS66 harbors widespread gene transfer from Fusarium oxysporum. Front. Plant Sci. 2021, 12, 629859. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Li, Q.; Huang, S.; Tang, L.; Chen, X.; Guo, T.; Mo, J.; Ma, L. First Report of Leaf Blight Caused by Fusarium pernambucanum and Fusarium sulawesiense on Plum in Sichuan, China. Plant Dis. 2022, 106, 2759. [Google Scholar] [CrossRef]
- Thirumalaisamy, P.P.; Dutta, R.; Jadon, K.S.; Nataraja, M.V.; Padvi, R.D.; Rajyaguru, R.; Yusufzai, S. Association and characterization of the Fusarium incarnatum—F. equiseti species complex with leaf blight and wilt of peanut in India. J. Gen. Plant Pathol. 2018, 85, 83–89. [Google Scholar] [CrossRef]
- Guo, M.; Li, B.; Wang, R.; Liu, P.; Chen, Q. Occurrence of dieback disease caused by Fusarium equiseti on Dendrobium officinale in China. Crop. Prot. 2020, 137, 105209. [Google Scholar] [CrossRef]
- Xiao, C.; Li, R. Detection and Control of Fusarium oxysporum from Soft Rot in Dendrobium officinale by Loop-Mediated Isothermal Amplification Assays. Biology 2021, 10, 1136. [Google Scholar] [CrossRef]
- Huda-Shakirah, A.R.; Mohd, M.H. First report of Fusarium sacchari causing leaf blotch of orchid (Dendrobium antennatum) in Malaysia. Crop. Prot. 2021, 143, 105559. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Mohd, M.H.; Nor, N.M.I.M.; Zakaria, L. First Report of Fusarium oxysporum and F. solani Associated With Pineapple Rot in Peninsular Malaysia. Plant Dis. 2015, 99, 1650. [Google Scholar] [CrossRef]
- Meena, R.P.; Roy, S. Morphological and molecular characterization of Fusarium sp. causing wilt disease of isabgol (Plantago ovata Forsk.) and its management strategies. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100244. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia 2018, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kauserud, H. ITS alchemy: On the use of ITS as a DNA marker in fungal ecology. Fungal Ecol. 2023, 65, 101274. [Google Scholar] [CrossRef]
- Bickerstaff, J.R.M.; Jordal, B.H.; Riegler, M. Two sympatric lineages of Australian Cnestus solidus share Ambrosiella symbionts but not Wolbachia. Heredity 2024, 132, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Hiremani, N.S.; Dubey, S.C. Phylogenetic relationship among Indian population of Fusarium oxysporum f. sp. lentis infecting lentil and development of specific SCAR markers for detection. 3 Biotech 2019, 9, 196. [Google Scholar] [CrossRef]
- Abd Murad, N.B.; Mohamed Nor, N.M.I.; Shohaimi, S.; Mohd Zainudin, N.A.I. Genetic diversity and pathogenicity of Fusarium species associated with fruit rot disease in banana across Peninsular Malaysia. J. Appl. Microbiol. 2017, 123, 1533–1546. [Google Scholar] [CrossRef]
- Patel, R.; Mehta, K.; Prajapati, J.; Shukla, A.; Parmar, P.; Goswami, D.; Saraf, M. An anecdote of mechanics for Fusarium biocontrol by plant growth promoting microbes. Biol. Control. 2022, 174, 105012. [Google Scholar] [CrossRef]
- Dangi, S.R.; Tirado-Corbalá, R.; Gerik, J.; Hanson, B.D. Effect of Long-Term Continuous Fumigation on Soil Microbial Communities. Agronomy 2017, 7, 37. [Google Scholar] [CrossRef]
- Van Esse, H.P.; Reuber, T.L.; van der Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2019, 225, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, K.; Wang, H.; Liu, Z.; Shi, Y.; Gao, Z.; Wang, Z. Evaluation of the biocontrol potential of Bacillus sp. WB against Fusarium oxysporum f. sp. niveum. Biol. Control. 2020, 147, 104288. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Shahzad, R.; Tayade, R.; Shahid, M.; Hussain, A.; Ali, M.W.; Yun, B.-W. Evaluation potential of PGPR to protect tomato against Fusarium wilt and promote plant growth. PeerJ 2021, 9, e11194. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control. 2020, 144, 104240. [Google Scholar] [CrossRef]



| Gene | Primers | Sequence (5′–3′) | Annealing Temperature (°C) |
|---|---|---|---|
| ITS | ITS1-F | TCCGTAGGTGAACCTGCGG | 55 |
| ITS4-R | TCCTCCGCTTATTGATATGC | ||
| EF-1α | EF1-F | ATGGGTAAGGARGACAAGAC | 55 |
| EF2-R | GGARGTACCAGTSATCATG | ||
| RPB1 | F7-F | CRACACAGAAGAGTTTGAAGG | 55 |
| R8-R | CAATGAGACCTTCTCGACCAGC | ||
| RPB2 | 5F2-F | GGGGWGAYCAGAAGAAGGC | 55 |
| 7CR-R | CCCATRGCTTGYTTRCCCAT |
| Isolate | Fusarium MLST (Number) | Fusarium MLST Similarity % | GenBank Accession Number | |||
|---|---|---|---|---|---|---|
| ITS | EF-1α | RPB1 | RPB2 | |||
| DHRL-01 | Fusarium oxysporum species complex (NRRL 22549) | 100 | MW599746 | MW703468 | MW703472 | MW703476 |
| DHRL-02 | Fusarium oxysporum species complex (NRRL 22549) | 100 | MW599747 | MW703469 | MW703473 | MW703477 |
| DHRL-03 | Fusarium oxysporum species complex (NRRL 22549) | 100 | MW599748 | MW703470 | MW703474 | MW703478 |
| DHRL-04 | Fusarium oxysporum species complex (NRRL 22549) | 100 | MW599749 | MW703471 | MW703475 | MW703479 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ahmed, W.; Zhang, J.; Gao, S.; Wang, Z.; Yang, H.; Bai, X.; Luo, K.; Xu, C.; Ji, G. Identification of Fusarium oxysporum Causing Leaf Blight on Dendrobium chrysotoxum in Yunnan Province, China. Life 2024, 14, 285. https://doi.org/10.3390/life14030285
Yang J, Ahmed W, Zhang J, Gao S, Wang Z, Yang H, Bai X, Luo K, Xu C, Ji G. Identification of Fusarium oxysporum Causing Leaf Blight on Dendrobium chrysotoxum in Yunnan Province, China. Life. 2024; 14(3):285. https://doi.org/10.3390/life14030285
Chicago/Turabian StyleYang, Jun, Waqar Ahmed, Jinhao Zhang, Shunyu Gao, Zhenji Wang, Haiyan Yang, Xuehui Bai, Kai Luo, Chengdong Xu, and Guanghai Ji. 2024. "Identification of Fusarium oxysporum Causing Leaf Blight on Dendrobium chrysotoxum in Yunnan Province, China" Life 14, no. 3: 285. https://doi.org/10.3390/life14030285
APA StyleYang, J., Ahmed, W., Zhang, J., Gao, S., Wang, Z., Yang, H., Bai, X., Luo, K., Xu, C., & Ji, G. (2024). Identification of Fusarium oxysporum Causing Leaf Blight on Dendrobium chrysotoxum in Yunnan Province, China. Life, 14(3), 285. https://doi.org/10.3390/life14030285








