Preliminary Proteomic Study of the Porcine Pituitary Gland under Heat Stress
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Housing
2.2. Pituitary Gland Sample Collection
2.3. Protein Extraction and Quantification
2.4. iTRAQ Protein Digestion, Labeling, and High-Performance Liquid Chromatography Sample Preparation
2.5. Peptide Fractionation with Strong Cation Exchange Chromatography
2.6. LC–MS/MS Analysis
2.7. Sequence Database Searching and Data Analysis
3. Results
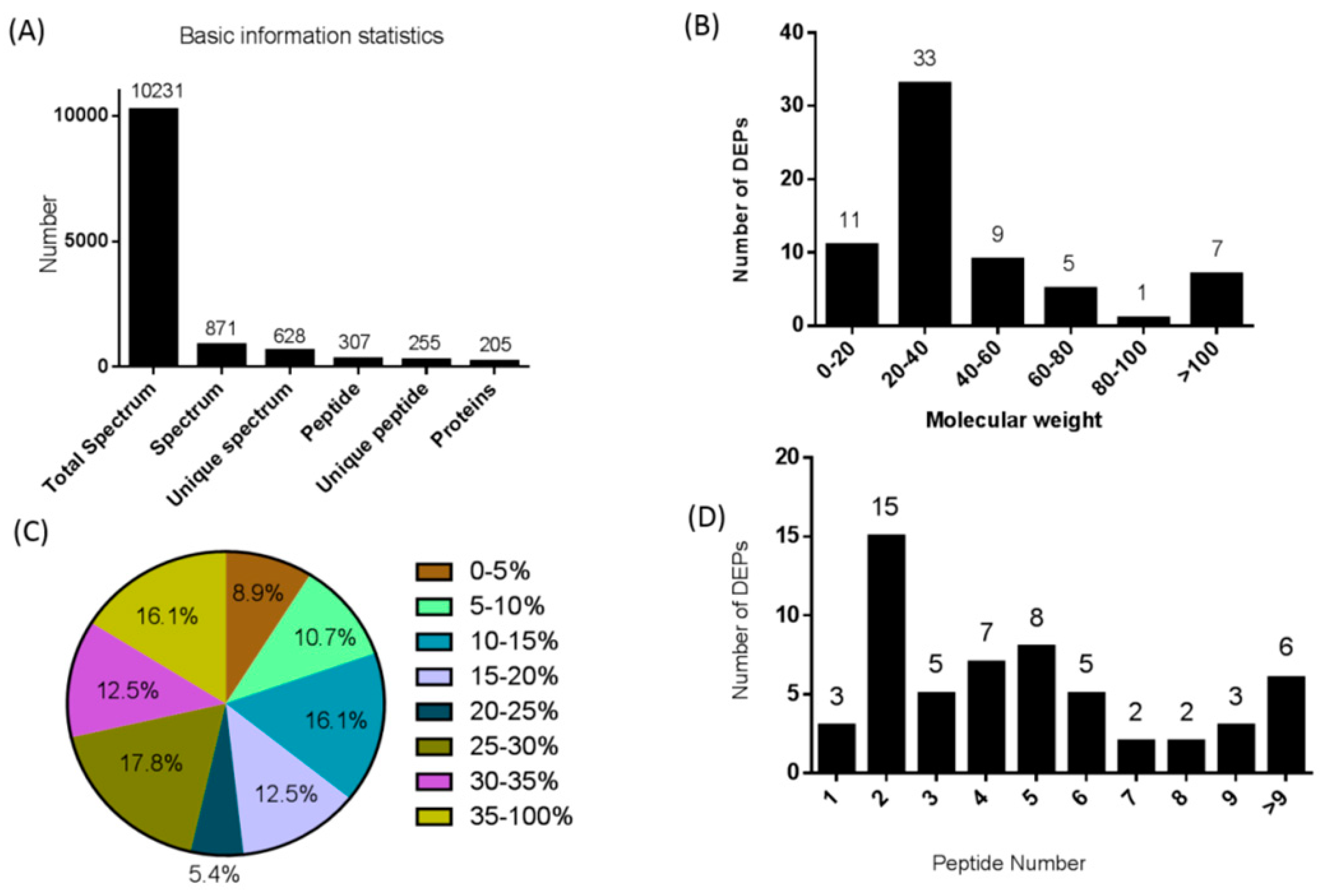
3.1. iTRAQ-Based Identification and Quantitative Proteomic Analysis of the Pituitary Gland
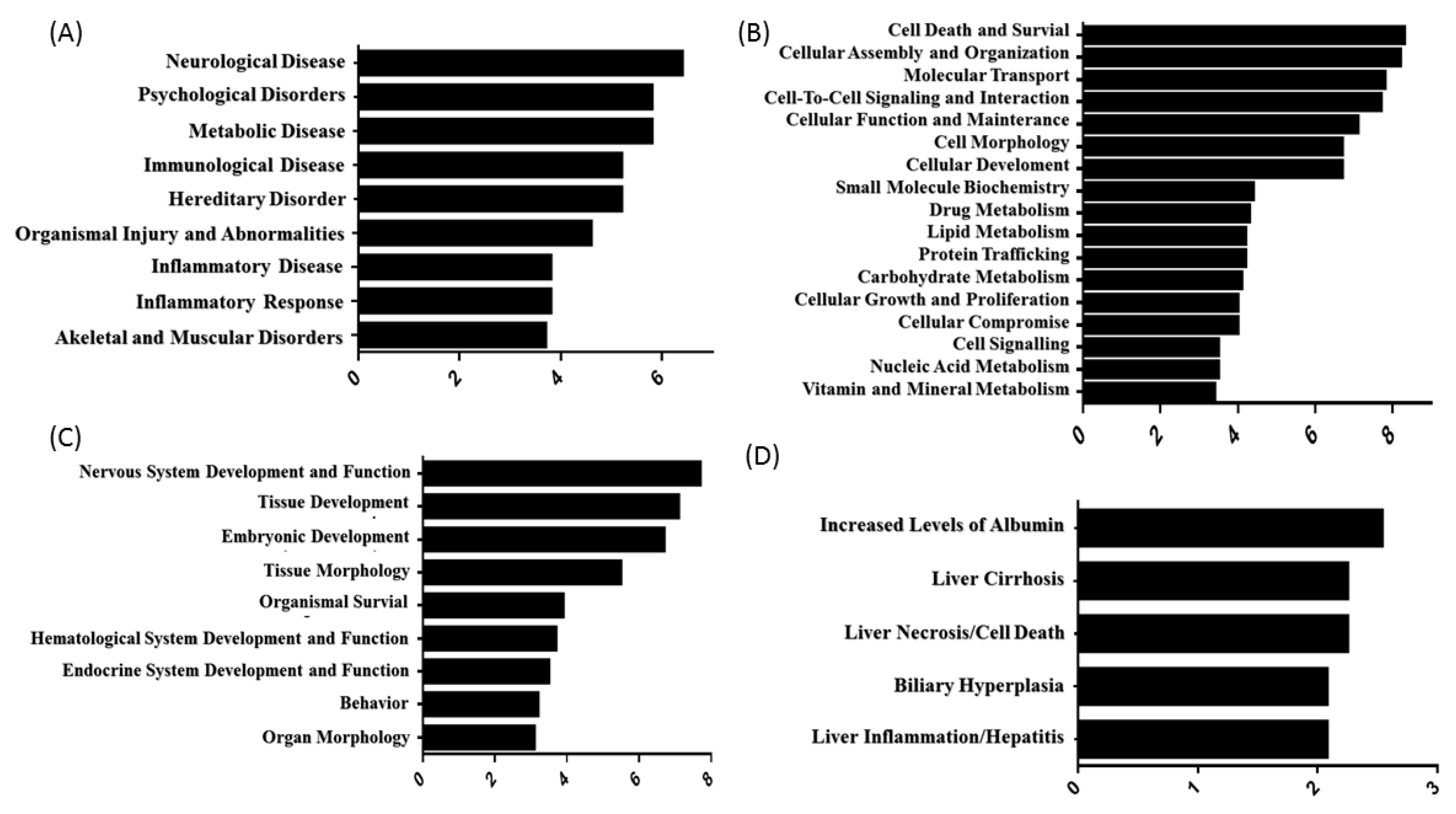
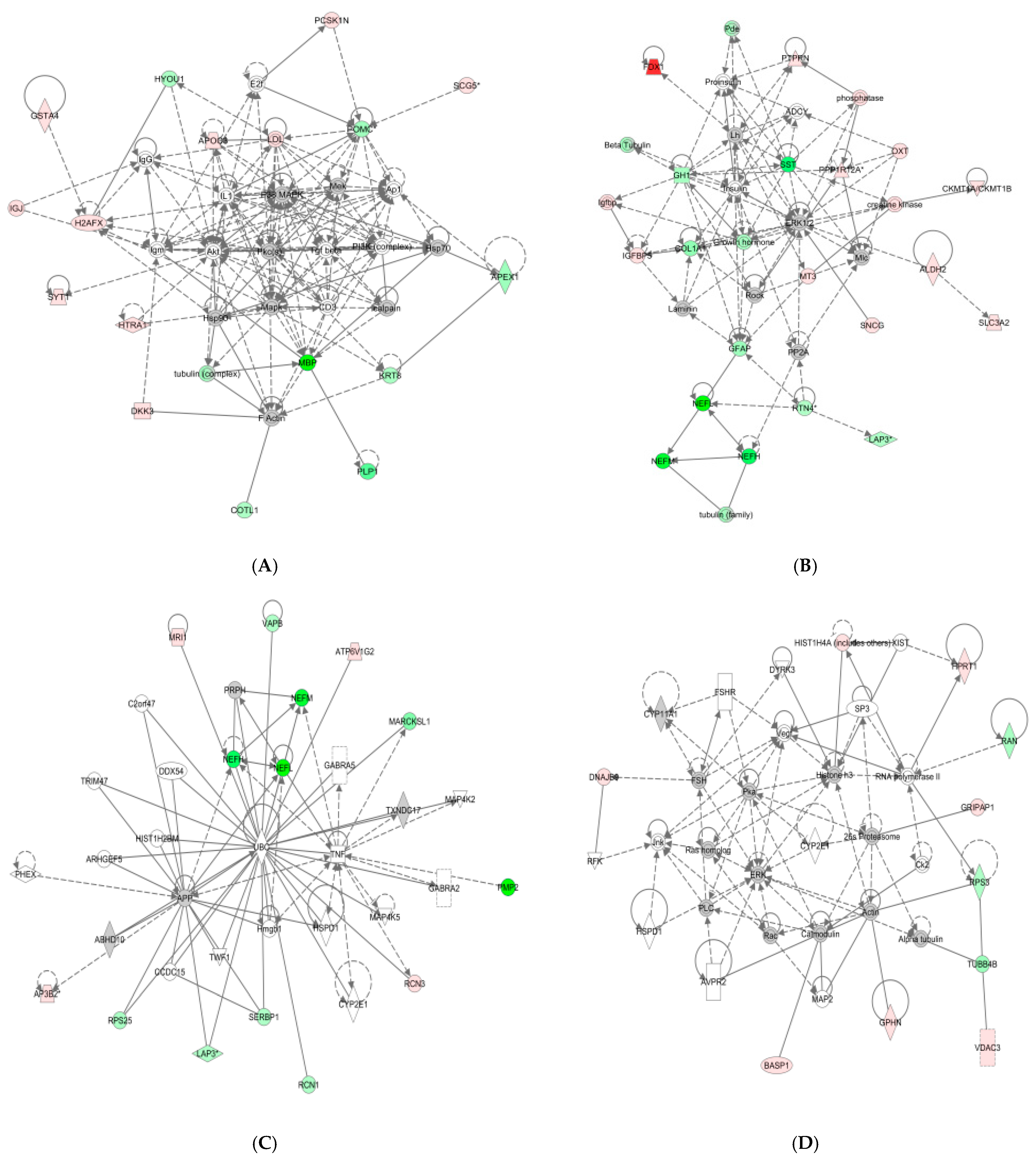
3.2. Subcellular and Functional Characterization of DEPs and Bioinformatics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, Y.; Xianyong, M.A.; Zheng, C.; Tian, Z.; Zhang, J.; Chen, W.; Youjun, H.U.; Wang, L. Effects of Heat Stress on Intestinal Health, Immune System and Meat Quality in Pigs and Its Mechanisms. Chin. J. Anim. Nutr. 2017, 29, 374–381. [Google Scholar]
- Collin, A.; Van Milgen, J.; Dubois, S.; Noblet, J. Effect of high temperature on feeding behaviour and heat production in group-housed young pigs. Br. J. Nutr. 2001, 86, 63–70. [Google Scholar] [CrossRef]
- Kerr, C.A.; Mathews, K.O.; Giles, L.R.; Jones, M.R. Effects of combined Actinobacillus pleuropneumoniae challenge and change in environmental temperature on calcitonin receptor expression levels in growing pigs. Aust. J. Agric. Res. 2004, 55, 727–732. [Google Scholar] [CrossRef]
- Lee, I.K.; Kye, Y.C.; Kim, G.; Han, W.K.; Min, J.G.; Umboh, J.; Maaruf, K.; Kim, S.W.; Yun, C.H. Stress, Nutrition, and Intestinal Immune Responses in Pigs—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1075. [Google Scholar] [CrossRef]
- Lyte, M.; Nelson, S.G.; Thompson, M.L. Innate and adaptive immune responses in a social conflict paradigm. Clin. Immunol. Immunopathol. 1990, 57, 137–147. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; Mcewen, B.S. Stress-induced enhancement of antigen-specific cell-mediated immunity. J. Immunol. 1996, 156, 2608–2615. [Google Scholar] [CrossRef]
- Kohut, M.L.; Martin, A.E.; Senchina, D.S.; Lee, W. Glucocorticoids produced during exercise may be necessary for optimal virus-induced IL-2 and cell proliferation whereas both catecholamines and glucocorticoids may be required for adequate immune defense to viral infection. Brain Behav. Immun. 2005, 19, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, M.C.; Pearce, B.D.; Miller, A.H.; Biron, C.A. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J. Immunol. 1999, 162, 3527–3533. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries1. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Fabris, N.; Garaci, E. Immunoregulation; Plenum Press: New York, NY, USA, 1983; p. 141. [Google Scholar]
- Lotze, M.T. Interleukin 12: Cellular and molecular immunology of an important regulatory cytokine. Introduction. Ann. N. Y. Acad. Sci. 1996, 795, xiii–xix. [Google Scholar] [PubMed]
- Chouaib, S.; Escudier, B.; Chehimi, J. Cellular and molecular immunology of an important regulatory cytokine. Eur. Cytokine Netw. 1996, 7, 79–81. [Google Scholar]
- Xiong, Z. Effect of Chaihu Shugan Powder on Rats with Liver-depression Syndrome Induced by Chronic Restrained Stress. Chin. J. Integr. Tradit. West. Med. Gastro-Spleen 2004, 12, 220–221. [Google Scholar]
- Yu, Z.; Chen, J.; Mai, Y.; Wu, L.; Guo, Q. Effect of Hormonal Drugs on Normal SD Rat Hypothalamus-Pituitary-Adrenal Axis Based on H-E Staining and Immunohistochemical Technique. Pharm. Today 2015, 5, 496–503. [Google Scholar]
- Claudia, G.; Rossella, L.; Sabrina, C.; Emanuele, F.; Andrea, L.; Maura, A.; Anna, S.; Paolo, B.P. Effect of recombinant human growth hormone (GH) replacement on the hypothalamic-pituitary-adrenal axis in adult GH-deficient patients. J. Clin. Endocrinol. Metab. 2004, 89, 5397–5401. [Google Scholar]
- Ferrari, E.; Cravello, L.; Muzzoni, B.; Casarotti, D.; Paltro, M.; Solerte, S.; Fioravanti, M.; Cuzzoni, G.; Pontiggia, B.; Magri, F. Age-related changes of the hypothalamic-pituitary-adrenal axis: Pathophysiological correlates. Eur. J. Endocrinol. 2001, 144, 319–329. [Google Scholar] [CrossRef]
- Gual, C. Progress in Endocrinology: Proceedings of the Third International Congress of Endocrinology, Mexico, June 30–July 5, 1968; Excerpta Medica Foundation: Amsterdam, The Netherlands, 1969. [Google Scholar]
- Quinteiro-Filho, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Sa, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: Role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012, 90, 1986–1994. [Google Scholar] [CrossRef]
- Fontana, J.M.; Bankamp, B.; Rota, P.A. Inhibition of interferon induction and signaling by paramyxoviruses. Immunol. Rev. 2010, 225, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Schaulies, J.; Schneider-Schaulies, S. Chapter 4 Receptor Interactions, Tropism, and Mechanisms Involved in Morbillivirus-Induced Immunomodulation. Adv. Virus Res. 2008, 71, 173–205. [Google Scholar] [PubMed]
- Smyth, G.P.; Stapleton, P.P.; Freeman, T.A.; Concannon, E.M.; Mestre, J.R.; Duff, M.; Maddali, S.; Daly, J.M. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-κB activation in macrophages. J. Surg. Res. 2004, 116, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, J.D.; Peterson-Burch, B.D.; Reinhardt, T.A. Differential expression analysis of proteins from neutrophils in the periparturient period and neutrophils from dexamethasone-treated dairy cows. Vet. Immunol. Immunopathol. 2006, 111, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, J.D.; Reinhardt, T.A.; Goff, J.P.; Horst, R.L. Neutrophil extracellular trap formation by bovine neutrophils is not inhibited by milk. Vet. Immunol. Immunopathol. 2006, 113, 248–255. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D. Developmental Changes in the Milk Fat Globule Membrane Proteome During the Transition from Colostrum to Milk. J. Dairy Sci. 2008, 91, 2307–2318. [Google Scholar] [CrossRef]
- Cho, D.; Sung, N.; Collins, M.T. Identification of proteins of potential diagnostic value for bovine paratuberculosis. Proteomics 2006, 6, 5785–5794. [Google Scholar] [CrossRef]
- Connolly, J.P.; Comerci, D.; Alefantis, T.G.; Walz, A.; Delvecchio, V.G. Proteomic analysis of Brucella abortus cell envelope and identification of immunogenic candidate proteins for vaccine development. Proteomics 2010, 6, 3767–3780. [Google Scholar] [CrossRef]
- Boyce, J.D.; Cullen, P.A.; Nguyen, V.; Wilkie, I.; Adler, B. Analysis of the Pasteurella multocida outer membrane sub-proteome and its response to the in vivo environment of the natural host. Proteomics 2010, 6, 870–880. [Google Scholar] [CrossRef]
- Mollenkopf, H.J.; Grode, L.; Mattow, J.; Stein, M.; Mann, P.; Knapp, B.; Ulmer, J.; Kaufmann, S.H.E. Application of Mycobacterial Proteomics to Vaccine Design: Improved Protection by Mycobacterium bovis BCG Prime-Rv3407 DNA Boost Vaccination against Tuberculosis. Infect. Immun. 2004, 72, 6471–6479. [Google Scholar] [CrossRef]
- WiśNiewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Krninger, L.; Steiniger, F.; Berger, S.; Kraus, S.; Welte, C.U.; Deppenmeier, U. Energy conservation in the gut microbe Methanomassiliicoccus luminyensis is based on membrane-bound ferredoxin oxidation coupled to heterodisulfide reduction. FEBS J. 2019, 286, 3831–3843. [Google Scholar] [CrossRef] [PubMed]
- Kamalaskar, L.; Kapse, N.; Pore, S.; Dhakephalkar, A.P.; Ranade, D.R.; Dhakephalkar, P.K. Genome sequence and gene expression studies reveal novel hydrogenases mediated hydrogen production by Clostridium biohydrogenum sp. nov., MCM B-509T. Int. J. Hydrogen Energy 2016, 41, 11990–11999. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Tao, C.; Guo, H.; Yao, W. Heat stress during late gestation disrupts maternal microbial transmission with altered offspring’s gut microbial colonization and serum metabolites in a pig model. Environ. Pollut. 2020, 266, 115111. [Google Scholar] [CrossRef] [PubMed]
- Choii, G.; Ko, J. Gephyrin: A central GABAergic synapse organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.; Rostaing, P.; Guigonis, J.M.; Renner, M.; Dumoulin, A.; Samson, M.; Vannier, C.; Triller, A. Heat Shock Cognate Protein 70 Regulates Gephyrin Clustering. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Shabb, J.B.; Muhonen, W.W.; Mehus, A.A. Quantitation of Human Metallothionein Isoforms in Cells, Tissues, and Cerebrospinal Fluid by Mass Spectrometry. Methods Enzymol. 2016, 586, 413. [Google Scholar] [PubMed]
- Luo, J.; Fang, J.; Lili, L.I.; Zhang, B.; Lizhuan, W.U.; Zijun, L.I.; Peng, Y.; Fan, J.X.; Lan, X.Y.; Zhan, J.S. Effect of Metallothionein on Cell Cycle, Apoptosis Rate and Subsets Distribution of Lymphocytes in Peripheral Blood of Dairy Cattle under Heat Stress. Agric. Ence Technol. 2013, 14, 1457–1461. [Google Scholar]
- Zheng, J.; Zhang, Y.; Xu, W.; Luo, Y.B.; Hao, J.; Shen, X.L.; Yang, X.; Li, X.; Huang, K. Zinc protects HepG2 cells against the oxidative damage and DNA damage induced by ochratoxin A. Toxicol. Appl. Pharmacol. 2013, 268, 123–131. [Google Scholar] [CrossRef]
- Li, H.; Malyar, R.M.; Zhai, N.; Wang, H.; Chen, X. Zinc supplementation alleviates OTA-induced oxidative stress and apoptosis in MDCK cells by up-regulating metallothioneins. Life Ences 2019, 234, 116735. [Google Scholar] [CrossRef]
- Sharma, H.S.; Sharma, A. Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog. Brain Res. 2007, 162, 245–273. [Google Scholar]
- Sharma, H.S.; Nyberg, F.; Cervos-Navarro, J.; Dey, P.K. Histamine modulates heat stress-induced changes in blood-brain barrier permeability, cerebral blood flow, brain oedema and serotonin levels: An experimental study in conscious young rats. Neuroence 1992, 50, 445–454. [Google Scholar] [CrossRef]
- Sharma, H.; Drieu, K.; Alm, P.; Westman, J. Role of nitric oxide in blood-brain barrier permeability, brain edema and cell damage following hyperthermic brain injury. An experimental study using EGB-761 and Gingkolide B pretreatment in the rat. Acta Neurochir. Suppl. 2000, 76, 81–86. [Google Scholar]
- Sharma, H.S.; Kretzschmar, R.; Cervós-Navarro, J.; Ermisch, A.; Dey, P.K. Chapter 27: Age-related pathophysiology of the blood-brain barrier in heat stress. Prog. Brain Res. 1992, 91, 189. [Google Scholar] [PubMed]
- Aberg, D. Role of the Growth Hormone/Insulin-Like Growth Factor 1 Axis in Neurogenesis. Endocr. Dev. 2010, 17, 63–76. [Google Scholar] [PubMed]
- Parkhie, M.R.; Johnson, H.D. Growth Hormone Releasing Activity in the Hypothalamus of Rats Subjected to Prolonged Heat Stress. Exp. Biol. Med. 1969, 130, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Roushdy, E.M.; Zaglool, A.W.; El-Tarabany, M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018, 74, 337–343. [Google Scholar] [CrossRef] [PubMed]


| Protein Name | Accession Number | Ratio (Heat Stress/ Control) | Peptides | Functions |
|---|---|---|---|---|
| Downregulated protein in pituitary gland | ||||
| RTN4-Aw | gi|38488990 | 0.666 | 4 | |
| Cytosol aminopeptidase-like | gi|350587377 | 0.66 | 2 | manganese ion binding |
| Plasminogen activator inhibitor 1 RNA-binding protein-like isoform 2 | gi|311259195 | 0.652 | 3 | |
| Reticulocalbin-1-like isoform 2 | gi|350580184 | 0.642 | 5 | protein binding and calcium ion binding |
| 40S ribosomal protein S25-like isoform 1 | gi|311264034 | 0.629 | 4 | RNA binding and structural constituent of ribosome |
| Growth hormone | gi|134715 | 0.601 | 13 | growth hormone receptor binding, ion binding and hormone activity |
| APEX nuclease 1 | gi|210062866 | 0.555 | 3 | transcription corepressor activity |
| Collagen alpha-1(I) chain | gi|350590450 | 0.493 | 2 | |
| Proteolipid protein | gi|5679718 | 0.363 | 6 | structural constituent of myelin sheath |
| Neurofilament heavy polypeptide | gi|311270880 | 0.237 | 9 | |
| Protein, myelin basic | gi|224358 | 0.175 | 4 | structural constituent of myelin sheath |
| Neurofilament light polypeptide | gi|346716234 | 0.171 | 16 | protein C-terminus binding |
| Myelin P2 protein | gi|297307127 | 0.158 | 4 | cholesterol binding |
| Upregulated protein in pituitary gland | ||||
| Ferredoxin | gi|164449 | 4.945 | 5 | metal ion binding and electron carrier activity |
| Oxytocin-neurophysin 1 | gi|585553 | 4.469 | 5 | neurohypophyseal hormone activity and oxytocin receptor binding |
| Glutathione S-transferase A4 | gi|343403759 | 3.183 | 1 | glutathione transferase activity |
| Immunoglobulin J chain-like isoform 1 | gi|335293621 | 3.029 | 1 | antigen binding |
| Gephyrin | gi|343183313 | 2.924 | 3 | cytoskeletal protein binding and protein complex scaffold |
| DnaJ homolog subfamily B member 9-like isoform 1 | gi|311275610 | 2.375 | 2 | heat shock protein binding |
| Protein tyrosine phosphatase, receptor type, N isoform 1 | gi|311273106 | 2.37 | 4 | transmembrane receptor protein tyrosine phosphatase activity |
| Metallothionein-III | gi|2073002 | 2.18 | 2 | - |
| Serine protease HTRA1-like | gi|311271945 | 2.011 | 2 | serine-type endopeptidase activity |
| Gamma-synuclein | gi|132269870 | 1.969 | 7 | - |
| Putative V-ATPase G subunit | gi|6624727 | 1.947 | 2 | hydrogen-exporting ATPase activity |
| Secreted neuronal and endocrine protein | gi|120564449 | 1.836 | 5 | enzyme inhibitor activity |
| Ig heavy chain variable VDJ region | gi|558859 | 1.832 | 1 | - |
| Methylthioribose-1-phosphate isomerase | gi|311248954 | 1.822 | 2 | identical protein binding |
| Brain acid soluble protein 1-like isoform 1 | gi|311273514 | 1.704 | 9 | transcription corepressor activity |
| Insulin-like growth factor-binding protein 5 precursor | gi|300679422 | 1.701 | 6 | insulin-like growth factor I binding |
| GRIP1-associated protein 1 | gi|350595677 | 1.696 | 8 | - |
| Dickkopf homolog 3 | gi|88606665 | 1.691 | 7 | |
| Hypoxanthine phosphoribosyltransferase 1 | gi|71842219 | 1.683 | 5 | hypoxanthine phosphoribosyltransferase activity |
| Creatine kinase U-type, mitochondrial-like isoform 1 | gi|311244870 | 1.675 | 9 | creatine kinase activity |
| AP-3 complex subunit beta-2 | gi|350596485 | 1.668 | 2 | transporter activity |
| Voltage-dependent anion channel 3 | gi|8745556 | 1.636 | 2 | porin activity and nucleotide binding |
| Reticulocalbin-3-like | gi|311257971 | 1.632 | 6 | calcium ion binding |
| Histone H2A.x-like | gi|335294990 | 1.629 | 3 | histone binding and damaged DNA binding |
| Neuroendocrine protein 7B2 | gi|112850 | 1.628 | 5 | enzyme inhibitor activity and unfolded protein binding |
| Synaptotagmin-1 | gi|350584732 | 1.6 | 4 | 1-phosphatidylinositol binding |
| Apolipoprotein C-III | gi|164361 | 1.559 | 4 | phospholipid binding and lipase inhibitor activity |
| Mitochondrial aldehyde dehydrogenase 2 | gi|81295909 | 1.536 | 6 | aldehyde dehydrogenase [NAD(P)+] activity |
| Protein phosphatase 1 regulatory subunit 12A | gi|350584736 | 1.536 | 2 | signal transducer activity |
| Solute carrier family 3 member 2 | gi|171465894 | 1.521 | 6 | calcium:sodium antiporter activity |
| Histone H4 | gi|51317314 | 1.508 | 5 | Chromatin structure and dynamics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Gao, Y.; Li, Y.; Xie, H.; Liu, X.; Yong, Y.; Li, Y.; Yu, Z.; Ma, X.; Ju, X. Preliminary Proteomic Study of the Porcine Pituitary Gland under Heat Stress. Life 2024, 14, 366. https://doi.org/10.3390/life14030366
Zhou Q, Gao Y, Li Y, Xie H, Liu X, Yong Y, Li Y, Yu Z, Ma X, Ju X. Preliminary Proteomic Study of the Porcine Pituitary Gland under Heat Stress. Life. 2024; 14(3):366. https://doi.org/10.3390/life14030366
Chicago/Turabian StyleZhou, Qiu, Yuan Gao, Yin Li, Huili Xie, Xiaoxi Liu, Yanhong Yong, Youquan Li, Zhichao Yu, Xingbin Ma, and Xianghong Ju. 2024. "Preliminary Proteomic Study of the Porcine Pituitary Gland under Heat Stress" Life 14, no. 3: 366. https://doi.org/10.3390/life14030366






