Investigation of the Allelopathic Effect of Two Invasive Plant Species in Rhizotron System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Application of Plant Material and Model Plant
2.2. Soil Properties, Growth Conditions, and Rhizotron System
2.3. Morphological Measurements
2.4. Statistical Analysis
3. Results
3.1. Results of Shoot Morphological Measurements
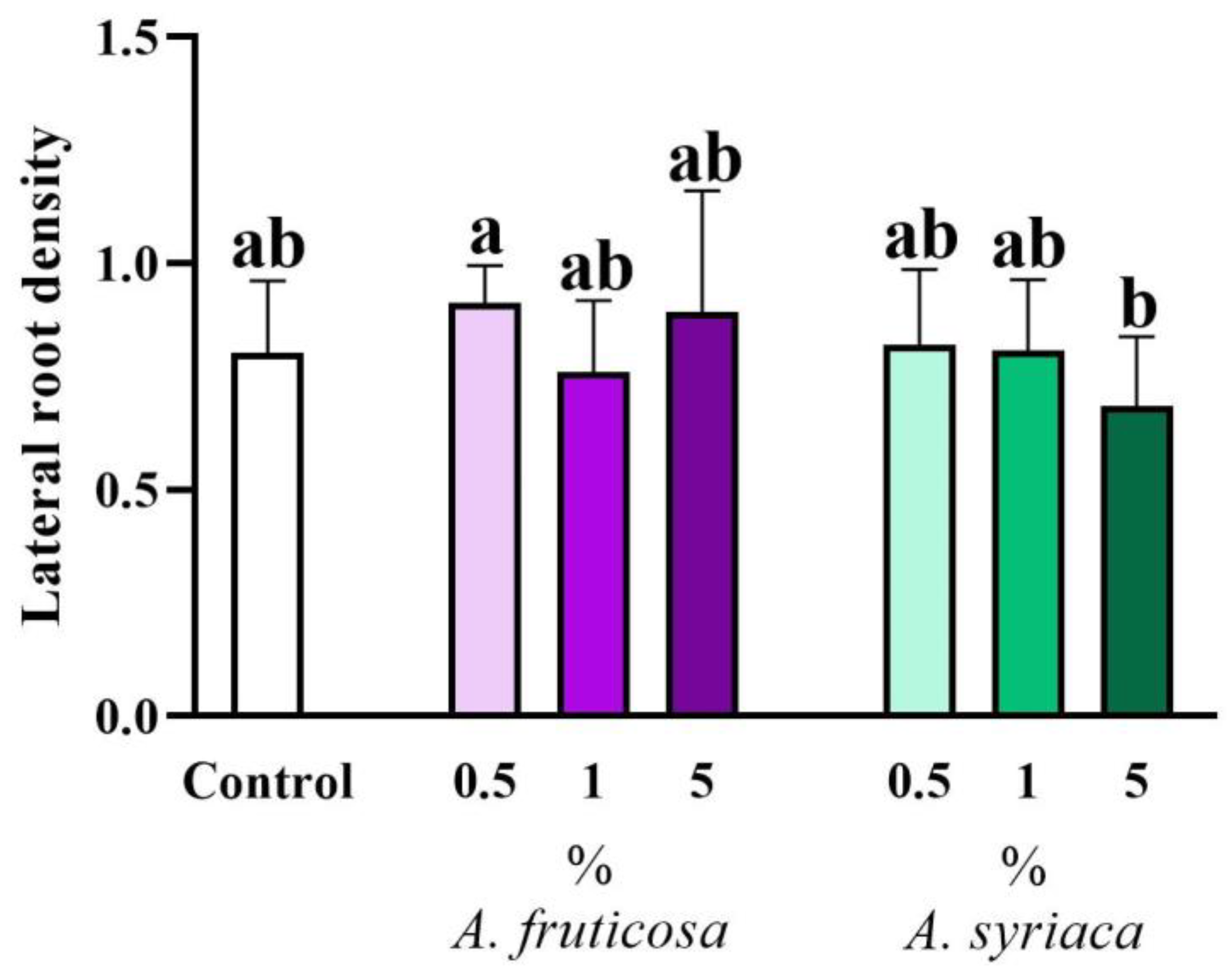
3.2. Results of Root Morphological Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M.; D’antonio, C.M.; Loope, L.L.; Rejmanek, M.; Westbrooks, R. Introduced species: A significant component of human-caused global change. N. Zldn. J. Ecol. 1997, 21, 1–16. [Google Scholar]
- Convention on Biological Diversity. Global Biodiversity Outlook 4. A Mid-Term Assessment of Progress towards the Implementation of the Strategic Plan for Biodiversity 2011–2020. 2014. Available online: https://www.cbd.int/gbo/gbo4/publication/gbo4-en-hr.pdf (accessed on 5 May 2023).
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, M.; Genovesi, P.; Gollasch, S.; Pagad, S.; Starfinger, U.; Ten Brink, P.; Shine, C. Technical Support to EU Strategy on Invasive Species (IAS)–Assessment of the Impacts of IAS in Europe and the EU; Institute for European Environmental Policy (IEEP): Brussels, Belgium, 2008. [Google Scholar]
- Mazza, G.; Tricarico, E.; Genovesi, P.; Gherardi, F. Biological invaders are threats to human health: An overview. Ethol. Ecol. Evol. 2014, 26, 112–129. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Cuthbert, R.N.; Essl, F.; Haubrock, P.J.; Ricciardi, A.; Courchamp, F. Biological invasions are as costly as natural hazards. Perspect. Ecol. Conserv. 2023, 21, 143–150. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Novoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits associated with invasiveness in alien plants: Where do we stand? In Biological Invasions, 1st ed.; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 193, pp. 97–125. [Google Scholar]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press Inc.: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Pinto, M.; Soares, C.; Martins, M.; Sousa, B.; Valente, I.; Pereira, R.; Fidalgo, F. Herbicidal effects and cellular targets of aqueous extracts from young Eucalyptus globulus Labill. leaves. Plants 2021, 10, 1159. [Google Scholar] [CrossRef]
- Bakacsy, L.; Sípos, L.; Barta, A.; Stefkó, D.; Vasas, A.; Szepesi, Á. Concentration dependent effects of effusol and juncusol from Juncus compressus on seedling development of Arabidopsis thaliana. Sci. Rep. 2022, 12, 13870. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Gallet, C.; Zackrisson, O. An ecosystem-level perspective of allelopathy. Biol. Rev. 1998, 73, 305–319. [Google Scholar] [CrossRef]
- Macías, F.A.; Durán, A.G.; Molinillo, J.M. Allelopathy: The chemical language of plants. In Progress in the Chemistry of Organic Natural Products 112; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–84. [Google Scholar]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Stinson, K.A.; Campbell, S.; Powell, J.; Wolfe, B.; Callaway, R.; Thelen, G.; Hallett, S.; Prati, D.; Klironomos, J. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 2006, 4, e140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jin, Y.; Tang, J.; Chen, X. The invasive plant Solidago canadensis L. suppresses local soil pathogens through allelopathy. Appl. Soil. Ecol. 2009, 41, 215–222. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2021, 11, 3. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef]
- Glinwood, R.; Ninkovic, V.; Pettersson, J. Chemical interaction between undamaged plants–effects on herbivores and natural enemies. Phytochemistry 2011, 72, 1683–1689. [Google Scholar] [CrossRef]
- Dias, M.C.; Conceição, I.L.; Abrantes, I.; Cunha, M.J. Solanum sisymbriifolium—A new approach for the management of plant-parasitic nematodes. Eur. J. Plant Pathol. 2012, 133, 171–179. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Homolová, Z. Nematode communities indicate the negative impact of Reynoutria japonica invasion on soil fauna in ruderal habitats of tatra national park in Slovakia. Glob. Ecol. Conserv. 2021, 26, e01470. [Google Scholar] [CrossRef]
- Vivanco, J.M.; Bais, H.P.; Stermitz, F.R.; Thelen, G.C.; Callaway, R.M. Biogeographical variation in community response to root allelochemistry: Novel weapons and exotic invasion. Ecol. Lett. 2004, 7, 285–292. [Google Scholar] [CrossRef]
- Pal, R.W.; Chen, S.; Nagy, D.U.; Callaway, R.M. Impacts of Solidago gigantea on other species at home and away. Biol. Invasions 2015, 17, 3317–3325. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Alford, É.R.; Perry, L.G.; Qin, B.; Vivanco, J.M.; Paschke, M.W. A putative allelopathic agent of Russian knapweed occurs in invaded soils. Soil. Biol. Biochem. 2007, 39, 1812–1815. [Google Scholar] [CrossRef]
- Bossdorf, O.; Auge, H.; Lafuma, L.; Rogers, W.E.; Siemann, E.; Prati, D. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 2005, 144, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ridenour, W.M.; Vivanco, J.M.; Feng, Y.; Horiuchi, J.I.; Callaway, R.M. No evidence for trade-offs: Centaurea plants from America are better competitors and defenders. Ecol. Monogr. 2008, 78, 369–386. [Google Scholar] [CrossRef]
- Inderjit; Callaway, R.M. Experimental designs for the study of allelopathy. Plant Soil 2003, 256, 1–11. [Google Scholar] [CrossRef]
- Csiszár, Á. Allelopathic effects of invasive woody plant species in Hungary. Acta Silv. Lign. Hun. 2009, 5, 9–17. [Google Scholar] [CrossRef]
- Kruse, M.; Strandberg, M.; Strandberg, B. Ecological Effects of Allelopathic Plants—A Review; National Environmental Research Institute: Silkeborg, Denmark, 2000. [Google Scholar]
- Brückner, D.J.; Szabó, L.G. Az allelopátia modern értelmezése. Kitaibelia 2001, 4, 93–106. [Google Scholar]
- Fekete, G. Populációk közötti interakciók. In Növényföldrajzi Társulástan és Ökológia; Hortobágyi, T., Simon, T., Eds.; Tankönyvkiadó: Budapest, Hungary, 1981; pp. 180–191. [Google Scholar]
- Blum, U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Blum, U.; Austin, M.F.; Shafer, S.R. The fates and effects of phenolic acids in a plant-microbial-soil model system. In Recent Advances on Allelopathy. A Science for the Future; Macías, F.A., Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; Cadiz University Press: Cadiz, Spain, 1999; pp. 159–166. [Google Scholar]
- Blum, U.; Shafer, S.R.; Lehman, M.E. Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: Concepts vs. an experimental model. Crit. Rev. Plant Sci. 1999, 18, 673–693. [Google Scholar] [CrossRef]
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemicals interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principles and Practices in Plant Ecology: Allelochemicals Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74. [Google Scholar]
- Novak, J.M.; Jayachandran, K.; Moorman, T.B.; Weber, J.B. Sorption and binding of organic compounds in soils and their relation to bioavailability. In Bioremediation: Science and Applications; Skipper, H.D., Turco, R.F., Eds.; Soil Science Society of America Publication: Madison, WI, USA, 1995; pp. 13–31. [Google Scholar]
- Prati, D.; Bossdorf, O. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am. J. Bot. 2004, 91, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.O.; Ning, L.; Tang, M.; Du, D.L.; van Kleunen, M.; Yu, F.H. Diversity-and density-mediated allelopathic effects of resident plant communities on invasion by an exotic plant. Plant Soil 2019, 440, 581–592. [Google Scholar] [CrossRef]
- Power, G.; Sánchez Vilas, J. Competition between the invasive Impatiens glandulifera and UK native species: The role of soil conditioning and pre-existing resident communities. Biol. Invasions 2020, 22, 1527–1537. [Google Scholar] [CrossRef]
- Ito, I.; Kobayashi, K.; Yoneyama, T. Fate of dehydromatricaria ester added to soil and its implications for the allelopathic effect of Solidago altissima L. Ann. Bot. 1998, 82, 625–630. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Ley, R.E. Microbial competition and soil structure limit the expression of allelochemicals in nature. In Allelopathy: Procedures and Processes; Inderjit, Dakshini, D.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 339–351. [Google Scholar]
- Csiszár, Á.; Korda, M.; Schmidt, D.; Šporčić, D.; Teleki, B.; Tiborcz, V.; Zagyvai, G.; Bartha, D. Néhány inváziós és potenciálisan inváziós neofiton allelopátiás hatásának vizsgálata. Bot. Közlem. 2012, 99, 159–171. [Google Scholar]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Rewald, B.; Ephrath, J.E. Minirhizotron techniques. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 728–743. [Google Scholar]
- Feigl, G.; Molnár, Á.; Szőllősi, R.; Ördög, A.; Törőcsik, K.; Oláh, D.; Bodor, A.; Perei, K.; Kolbert, Z. Zinc-induced root architectural changes of rhizotron grown B. napus correlate with a differential nitro-oxidative response. Nitric Oxide 2019, 90, 55–65. [Google Scholar] [CrossRef]
- Feigl, G.; Varga, V.; Molnár, Á.; Dimitrakopoulos, P.G.; Kolbert, Z. Different nitro-oxidative response of Odontarrhena lesbiaca plants from geographically separated habitats to excess nickel. Antioxidants 2020, 9, 837. [Google Scholar] [CrossRef]
- Feigl, G.; Czifra, Á.; Molnár, Á.; Bodor, A.; Kovács, E.; Perei, K.; Jebet, V.; Kolbert, Z. Reorganization of protein tyrosine nitration pattern indicates the relative tolerance of Brassica napus (L.) over Helianthus annuus (L.) to combined heavy metal treatment. Plants 2020, 9, 902. [Google Scholar] [CrossRef]
- Mészáros, E.; Bodor, A.; Szierer, Á.; Kovács, E.; Perei, K.; Tölgyesi, C.; Bátori, Z.; Feigl, G. Indirect effects of COVID-19 on the environment: How plastic contamination from disposable surgical masks affect early development of plants. J. Hazard. Mater. 2022, 436, 129255. [Google Scholar] [CrossRef]
- Bodor, A.; Bellahsen, N.; Perei, K.; Hodúr, C.; Feigl, G. Phytotoxicity evaluation of nutrient-fortified pomegranate peel powders prepared from food waste and their feasibility as biofertilizers. Environ. Dev. Sustain. [CrossRef]
- Csiszár, Á.; Korda, M.; Schmidt, D.; Sporcic, D.; Süle, P.; Teleki, B.; Tiborcz, V.; Zagyvai, G.; Bartha, D. Allelopathic potential of some invasive plant species occurring in Hungary. Allelopath. J. 2013, 31, 309–318. [Google Scholar]
- Pellegrini, E.; Boscutti, F.; Alberti, G.; Casolo, V.; Contin, M.; De Nobili, M. Stand age, degree of encroachment and soil characteristics modulate changes of C and N cycles in dry grassland soils invaded by the N2-fixing shrub Amorpha fruticosa. Sci. Total Environ. 2021, 792, 148295. [Google Scholar] [CrossRef]
- Krstin, L.; Katanić, Z.; Žuna Pfeiffer, T.; Špoljarić Maronić, D.; Marinčić, D.; Martinović, A.; Štolfa Čamagajevac, I. Phytotoxic effect of invasive species Amorpha fruticosa L. on germination and the early growth of forage and agricultural crop plants. Ecol. Res. 2021, 36, 97–106. [Google Scholar] [CrossRef]
- Szigetvári, C.; Tóth, T. Cserjés gyalogakác (Amorpha fruticosa). In Inváziós Növényfajok Magyarországon; Csiszár, Á., Ed.; Nyugat-magyarországi Egyetem Kiadó: Sopron, Hungary, 2012; pp. 121–126. [Google Scholar]
- Bakacsy, L. Invasion impact is conditioned by initial vegetation states. Community Ecol. 2019, 20, 11–19. [Google Scholar] [CrossRef]
- Follak, S.; Bakacsy, L.; Essl, F.; Hochfellner, L.; Lapin, K.; Schwarz, M.; Tokarska-Guzik, B.; Wołkowycki, D. Monograph of invasive plants in Europe No. 6: Asclepias syriaca L. Bot. Lett. 2021, 168, 422–451. [Google Scholar] [CrossRef]
- Varga, L.; Dancza, I. Selyemkóró (Asclepias syriaca L.). In Az Ötödik Országos Gyomfelvételezés Magyarország Szántóföldjein; Novák, R., Dancza, I., Szentey, L., Karamán, J., Eds.; Vidékfejlesztési Minisztérium Élelmiszerlánc-felügyeleti Főosztály: Budapest, Hungary, 2011; pp. 272–282. [Google Scholar]
- Yenish, J.P.; Durgan, B.R.; Miller, D.W.; Wyse, D.L. Wheat (Triticum aestivum) yield reduction from common milkweed (Asclepias syriaca) competition. Weed Sci. 1997, 45, 127–131. [Google Scholar] [CrossRef]
- Kazinczi, G.; Béres, I.; Mikulás, J.; Nádasy, E. Allelopathic effect of Cirsium arvense and Asclepias syriaca. J. Plant Dis. Prot. 2004, 19, 301–308. [Google Scholar]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Complex synergistic interactions among volatile and phenolic compounds underlie the effectiveness of allelopathic residues added to the soil for weed control. Plants 2022, 11, 1114. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Cipollini, D.; Morris, K.; Gurusinghe, S.; Weston, L.A. Ecological realism and rigor in the study of plant-plant allelopathic interactions. Plant Soil 2023, 489, 1–39. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of troble? Trends. Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kazinczi, G.; Béres, I.; Horvath, J. Weed-crop interferences in Hungary. In Allelopathy: New Concepts & Methodology; Fujii, Y., Hiradate, S., Eds.; CRC Press: Enfield, CT, USA, 2007; pp. 165–170. [Google Scholar]
- Nagy, K.N.; Kardos, L.V.; Orbán, Z.; Bakacsy, L. The allelochemical potential of an invasive ornamental plant, the Indian blanket flower (Gaillardia pulchella Foug.). Plant Species Biol. 2023, 39, 1–7. [Google Scholar] [CrossRef]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef]
- Popov, M.; Prvulović, D.; Šućur, J.; Vidović, S.; Samardžić, N.; Stojanović, T.; Konstantinović, B. Chemical characterization of common milkweed (Asclepias syriaca L.) root extracts and their influence on maize (Zea mays L.), soybean (Glycine max (L.) Merr.) and sunflower (Helianthus annuus L.) seed germination and seedling growth. Appl. Ecol. Environ. Res. 2021, 19, 4219–4230. [Google Scholar] [CrossRef]
- Nádasy, E.; Pásztor, G.; Béres, I.; Szilágyi, G. Allelopathic effects of Abutilon theophrasti, Asclepias syriaca and Panicum ruderale on maize. Julius-Kühn-Archiv 2018, 454–458. [Google Scholar] [CrossRef]
- Szilágyi, A.; Radócz, L.; Tóth, T. Allelopathic effect of invasive plants (Eriochloa villosa, Asclepias syriaca, Fallopia × bohemica, Solidago gigantea) on seed germination. Acta Agrar. Debr. 2018, 179–182. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakacsy, L.; Kardos, L.V.; Szepesi, Á.; Nagy, K.N.; Vasas, A.; Feigl, G. Investigation of the Allelopathic Effect of Two Invasive Plant Species in Rhizotron System. Life 2024, 14, 475. https://doi.org/10.3390/life14040475
Bakacsy L, Kardos LV, Szepesi Á, Nagy KN, Vasas A, Feigl G. Investigation of the Allelopathic Effect of Two Invasive Plant Species in Rhizotron System. Life. 2024; 14(4):475. https://doi.org/10.3390/life14040475
Chicago/Turabian StyleBakacsy, László, Luca Viktória Kardos, Ágnes Szepesi, Krisztina Napsugár Nagy, Andrea Vasas, and Gábor Feigl. 2024. "Investigation of the Allelopathic Effect of Two Invasive Plant Species in Rhizotron System" Life 14, no. 4: 475. https://doi.org/10.3390/life14040475






