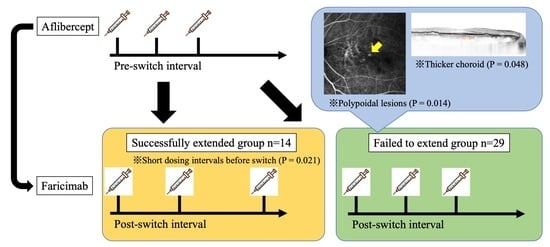
Factors Associated with Success of Switching to Faricimab for Neovascular Age-Related Macular Degeneration Refractory to Intravitreal Aflibercept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Intervention and Observation Procedure
2.3. Main Outcome Measure
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Factors Affecting Successful Switching
4.2. Switching Success Rate
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bressler, N.M. Age-related macular degeneration is the leading cause of blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; Group, A.S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Investigators, I.S.; Chakravarthy, U.; Harding, S.P.; Rogers, C.A.; Downes, S.M.; Lotery, A.J.; Wordsworth, S.; Reeves, B.C. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology 2012, 119, 1399–1411. [Google Scholar] [CrossRef]
- Rosenberg, D.; Deonarain, D.M.; Gould, J.; Sothivannan, A.; Phillips, M.R.; Sarohia, G.S.; Sivaprasad, S.; Wykoff, C.C.; Cheung, C.M.G.; Sarraf, D.; et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 2023, 37, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Ou, W.C.; Brown, D.M.; Croft, D.E.; Wang, R.; Payne, J.F.; Clark, W.L.; Abdelfattah, N.S.; Sadda, S.R. Randomized Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: 2-Year Results of the TREX-AMD Study. Ophthalmol. Retin. 2017, 1, 314–321. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Maruko, I.; Ogasawara, M.; Yamamoto, A.; Itagaki, K.; Hasegawa, T.; Arakawa, H.; Nakayama, M.; Koizumi, H.; Okada, A.A.; Sekiryu, T.; et al. Two-Year Outcomes of Treat-and-Extend Intravitreal Aflibercept for Exudative Age-Related Macular Degeneration: A Prospective Study. Ophthalmol. Retin. 2020, 4, 767–776. [Google Scholar] [CrossRef]
- Ohji, M.; Takahashi, K.; Okada, A.A.; Kobayashi, M.; Matsuda, Y.; Terano, Y. Efficacy and Safety of Intravitreal Aflibercept Treat-and-Extend Regimens in Exudative Age-Related Macular Degeneration: 52- and 96-Week Findings from ALTAIR: A Randomized Controlled Trial. Adv. Ther. 2020, 37, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Singh, R.P.; Wykoff, C.C.; Csaky, K.G.; Lai, T.Y.Y.; Loewenstein, A.; Schlottmann, P.G.; Paris, L.P.; Westenskow, P.D.; Quezada-Ruiz, C. THE ANGIOPOIETIN/TIE PATHWAY IN RETINAL VASCULAR DISEASES: A Review. Retina 2021, 41, 1–19. [Google Scholar] [CrossRef]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab. Cells 2020, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Liberski, S.; Wichrowska, M.; Kocięcki, J. Aflibercept versus Faricimab in the Treatment of Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Review. Int. J. Mol. Sci. 2022, 23, 9424. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Itagaki, K.; Hashiya, N.; Wakugawa, S.; Tanaka, K.; Nakayama, M.; Yamamoto, A.; Mukai, R.; Honjyo, J.; Maruko, I.; et al. Six-month outcomes of switching from aflibercept to faricimab in refractory cases of neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 262, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Miki, A.; Kamimura, A.; Okuda, M.; Matsumiya, W.; Imai, H.; Kusuhara, S.; Nakamura, M. Short-Term Outcomes of Faricimab Treatment in Aflibercept-Refractory Eyes with Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2023, 12, 5145. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Rush, R.B.; Rush, S.W. Intravitreal Faricimab for Aflibercept-Resistant Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2022, 16, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, R.; Falfeli, T.; Bogdanova-Bennet, A.; Varma, D.; Habib, M.; Kotagiri, A.; Steel, D.H.; Grinton, M. Outcomes of Treatment-Resistant Neovascular Age-Related Macular Degeneration Switched from Aflibercept to Faricimab. Ophthalmol. Retin. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, R.; Hata, M.; Tanaka, A.; Tsuchikawa, M.; Ueda-Arakawa, N.; Tamura, H.; Miyata, M.; Takahashi, A.; Kido, A.; Muraoka, Y.; et al. Therapeutic effects of faricimab on aflibercept-refractory age-related macular degeneration. Sci. Rep. 2023, 13, 21128. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Dev. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Oshima, Y.; Deering, T.; Oshima, S.; Nambu, H.; Reddy, P.S.; Kaleko, M.; Connelly, S.; Hackett, S.F.; Campochiaro, P.A. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J. Cell Physiol. 2004, 199, 412–417. [Google Scholar] [CrossRef]
- Peters, S.; Cree, I.A.; Alexander, R.; Turowski, P.; Ockrim, Z.; Patel, J.; Boyd, S.R.; Joussen, A.M.; Ziemssen, F.; Hykin, P.G.; et al. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine 2007, 40, 144–150. [Google Scholar] [CrossRef]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Inoda, S.; Takahashi, H.; Inoue, Y.; Tan, X.; Tampo, H.; Arai, Y.; Yanagi, Y.; Kawashima, H. Cytokine profiles of macular neovascularization in the elderly based on a classification from a pachychoroid/drusen perspective. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 747–758. [Google Scholar] [CrossRef]
- Chung, S.E.; Kang, S.W.; Lee, J.H.; Kim, Y.T. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 2011, 118, 840–845. [Google Scholar] [CrossRef]
- Custis, P.H.; Bressler, S.B.; Bressler, N.M. Laser management of subfoveal choroidal neovascularization in age-related macular degeneration. Curr. Opin. Ophthalmol. 1993, 4, 7–18. [Google Scholar] [CrossRef]
- Friberg, T.R.; Pandya, A.; Nazari, K. Transpupillary thermotherapy (TTT) for age-related macular degeneration. Semin. Ophthalmol. 2001, 16, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.M.; Glaser, B.M. Micropulse laser treatment of retinal-choroidal anastomoses in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Gilson, M.M.; Bressler, N.M.; Jabs, D.A.; Solomon, S.D.; Thorne, J.E.; Wilson, D.J. Periocular triamcinolone and photodynamic therapy for subfoveal choroidal neovascularization in age-related macular degeneration. Ophthalmology 2007, 114, 1713–1721. [Google Scholar] [CrossRef] [PubMed]


| All | Without Polyp | With Polyp (PCV) | p-Value | |
|---|---|---|---|---|
| (n = 43) | (n = 14) | (n = 29) | ||
| Age, years | 80.0 (73.0–84.5) | 83.5 (79.5–86.8) | 75.0 (72.0–81.0) | 0.014 |
| Sex (female) | 16 (37.2%) | 9 (64.3%) | 7 (24.1%) | 0.018 |
| Lens (IOL) | 22 (51.2%) | 9 (64.3%) | 13 (44.8%) | 0.332 |
| History of PDT | 16 (37.2%) | 1 (7.1%) | 15 (51.7%) | 0.006 |
| Total no. of previous injections | 34.0 (20.5–52.5) | 44.0 (34.0–61.8) | 27.0 (13.0–52.0) | 0.036 |
| Interval before switch, weeks | 5.0 (4.0–6.0) | 5.0 (4.3–6.8) | 5.0 (4.0–6.0) | 0.904 |
| BCVA, logMAR | 0.16 (0.05–0.30) | 0.10 (0.06–0.52) | 0.15 (0.00–0.22) | 0.412 |
| Central retinal thickness, μm | 289.0 (255.0–354.5) | 299.5 (246.3–406.5) | 289.0 (265.0–333.0) | 0.959 |
| Choroidal thickness, μm | 161.0 (109.5–237.0) | 159.0 (109.5–222.8) | 161.0 (114.0–266.0) | 0.907 |
| Maximum PED, μm | 193.0 (105.0–290.5) | 176.0 (98.5–238.5) | 201.0 (151.0–318.0) | 0.294 |
| Presence of SRH | 3 (7.0%) | 1 (7.1%) | 2 (6.9%) | 1.000 |
| Disrupted foveal ellipsoid zone | 21 (48.8%) | 7 (50.0%) | 14 (48.3%) | 1.000 |
| Successfully Extended | Failed to Extend | p-Value | |
|---|---|---|---|
| (n = 14) | (n = 29) | ||
| Age, years | 82.5 (77.8–86.8) | 77.0 (72.0–82.0) | 0.094 |
| Sex (female) | 7 (50.0%) | 9 (31.0%) | 0.316 |
| Subtype (PCV) | 6 (42.9%) | 23 (79.3%) | 0.035 |
| Lens (IOL) | 11 (78.6%) | 11 (37.9%) | 0.022 |
| History of PDT | 2 (14.3%) | 14 (48.3%) | 0.045 |
| Total no. of previous injections | 36.5 (26.5–62.0) | 34.0 (17.0–52.0) | 0.300 |
| Treatment interval, weeks | 5.0 (4.0–5.0) | 6.0 (5.0–6.0) | 0.035 |
| BCVA, logMAR | 0.13 (0.10–0.28) | 0.16 (0.00–0.30) | 0.620 |
| Central retinal thickness, μm | 277.0 (246.3–310.5) | 319.0 (265.0–387.0) | 0.097 |
| Choroidal thickness, μm | 149.5 (115.3–179.0) | 190.0 (102.0–286.0) | 0.271 |
| Maximum PED, μm | 178.5 (98.5–238.3) | 198.0 (151.0–318.0) | 0.318 |
| Presence of SRH | 1 (7.1%) | 2 (6.9%) | 1.000 |
| Disrupted foveal ellipsoid zone | 7 (50.0%) | 14 (48.3%) | 1.000 |
| Covariates | β | Z-Value | Odd Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| With polypoidal lesions | −117.8 | −0.001 | 0.109 | 0.019–0.642 | 0.014 |
| Choroidal thickness, per 10 μm increase | −12.2 | −0.001 | 0.888 | 0.789–0.999 | 0.048 |
| Interval before switch, per week extension | −77.8 | −0.002 | 0.381 | 0.168–0.864 | 0.021 |
| All | Successfully Extended | Failed to Extend | p-Value | |
|---|---|---|---|---|
| (n = 43) | (n = 14) | (n = 29) | ||
| BCVA, logMAR | 0.00 (−0.08–0.00) | −0.05 (−0.38–0.15) | 0.00 (−0.10–0.30) | 0.003 |
| Central retinal thickness, μm | −2.0 (−36.0–25.0) | −29.0 (−51.5–−7.8) | 13.0 (−12.0–32.0) | 0.006 |
| Choroidal thickness, μm | −1.0 (−13.5–8.0) | −5.0 (−18.8–5.3) | 0.0 (−11.0–9.0) | 0.190 |
| Maximum PED, μm | −20.0 (−51.5–2.5) | −26.0 (−102.8–−13.3) | −16.0 (−33.0–9.0) | 0.080 |
| Exudative change | 0.002 | |||
| No fluid | 4 (9.3%) | 4 (28.6%) | 0 (0.0%) | |
| Reduced | 17 (39.5%) | 7 (50.0%) | 10 (34.5%) | |
| Worsened | 22 (51.2%) | 3 (21.4%) | 19 (65.5%) | |
| Extended intervals, weeks | 1.0 (0.0–3.0) | 3.0 (3.0–4.8) | 0.0 (0.0–1.0) | 0.001 |
| Switched Back Cases | Others | p-Value | |
|---|---|---|---|
| (n = 5) | (n = 38) | ||
| Age, years | 77.0 (73.0–82.0) | 80.0 (73.3–84.8) | 0.622 |
| Sex (female) | 2 (40.0%) | 14 (36.8%) | 1.000 |
| Subtype (PCV) | 5 (100.0%) | 24 (63.2%) | 0.156 |
| Lens (IOL) | 3 (60.0%) | 19 (50.0%) | 1.000 |
| History of PDT | 4 (80.0%) | 12 (31.6%) | 0.056 |
| Total no. of previous injections | 27.0 (23.0–38.0) | 34.5 (19.8–52.8) | 0.733 |
| Treatment interval, weeks | 5.0 (5.0–5.0) | 5.0 (4.0–6.0) | 0.583 |
| BCVA, logMAR | 0.16 (−0.08–0.22) | 0.13 (0.05–0.30) | 0.746 |
| Central retinal thickness, μm | 340.0 (320.0–416.0) | 283.5 (251.8–345.8) | 0.099 |
| Choroidal thickness, μm | 127.0 (102.0–295.0) | 161.0 (114.0–226.0) | 0.880 |
| Maximum PED, μm | 202.0 (179.0–318.0) | 190.5 (103.8–263.3) | 0.405 |
| Presence of SRH | 0 (0.0%) | 3 (7.9%) | 1.000 |
| Disrupted foveal ellipsoid zone | 2 (40.0%) | 19 (50.0%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machida, A.; Oishi, A.; Ikeda, J.; Kurihara, J.; Yoneda, A.; Tsuiki, E.; Hirata, Y.; Murakami, R.; Kitaoka, T. Factors Associated with Success of Switching to Faricimab for Neovascular Age-Related Macular Degeneration Refractory to Intravitreal Aflibercept. Life 2024, 14, 476. https://doi.org/10.3390/life14040476
Machida A, Oishi A, Ikeda J, Kurihara J, Yoneda A, Tsuiki E, Hirata Y, Murakami R, Kitaoka T. Factors Associated with Success of Switching to Faricimab for Neovascular Age-Related Macular Degeneration Refractory to Intravitreal Aflibercept. Life. 2024; 14(4):476. https://doi.org/10.3390/life14040476
Chicago/Turabian StyleMachida, Akira, Akio Oishi, Junichiro Ikeda, Junko Kurihara, Ai Yoneda, Eiko Tsuiki, Yuki Hirata, Ryuya Murakami, and Takashi Kitaoka. 2024. "Factors Associated with Success of Switching to Faricimab for Neovascular Age-Related Macular Degeneration Refractory to Intravitreal Aflibercept" Life 14, no. 4: 476. https://doi.org/10.3390/life14040476