Endometriosis in Menopausal Women—A New Age Is Coming? Literature Review
Abstract
1. Introduction
2. Risk Factors
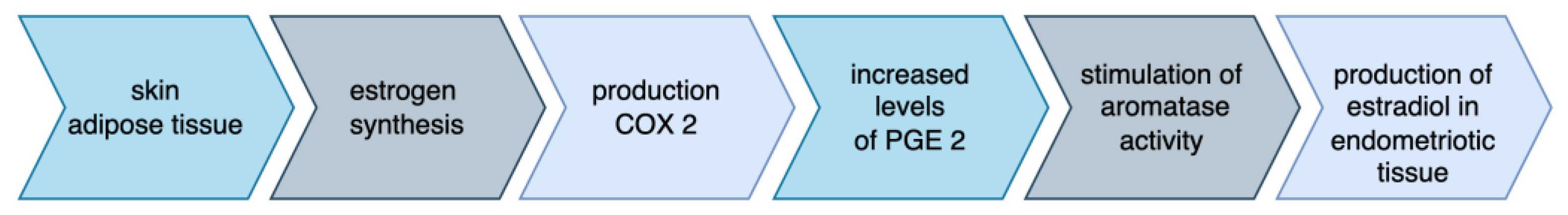
3. Pathophysiology
4. Symptomatology
5. Localization
6. Clinical Examination and Diagnosis
7. Imaging and Findings
8. Management
9. Malignant Transformation of Endometriosis Lesions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morotti, M.; Remorgida, V.; Venturini, P.L.; Ferrero, S. Endometriosis in menopause: A single institution experience. Arch. Gynecol. Obstet. 2012, 286, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Rosa-E-Silva, J.C.; Carvalho, B.R.; Barbosa, H.d.F.; Poli-Neto, O.B.; Rosa-E-Silva, A.C.J.S.; Candido-Dos-Reis, F.J.; Nogueira, A.A. Endometriosis in postmenopausal women without previous hormonal therapy: Report of three cases. Climacteric 2008, 11, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.G.; VanBuren, W.M.; Sheedy, S.P. Endometriosis in the postmenopausal female: Clinical presentation, imaging features, and management. Abdom. Radiol. 2020, 45, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, L.C.; Webster, K.E.; Kirtley, S.; Vincent, K.; Zondervan, K.T.; Becker, C.M. The management of menopause in women with a history of endometriosis: A systematic review. Hum. Reprod. Update 2017, 23, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Bendon, C.L.; Becker, C.M. Potential mechanisms of postmenopausal endometriosis. Maturitas 2012, 72, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.F.; Hillard, T.C. Hormone replacement therapy in women with past history of endometriosis. Climacteric 2006, 9, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Cumiskey, J.; Whyte, P.; Kelehan, P.; Gibbons, D. A detailed morphologic and immunohistochemical comparison of pre- and postmenopausal endometriosis. J. Clin. Pathol. 2008, 61, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, S.; Archer, D.F.; Taylor, H.S.; Pickar, J.H.; Komm, B.S. Differential effects of menopausal therapies on the endometrium. Menopause 2014, 21, 899–908. [Google Scholar] [CrossRef]
- Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol. Oncol. 2004, 94, 256–266. [Google Scholar] [CrossRef]
- de Almeida Asencio, F.; Ribeiro, H.A.; Ayrosa Ribeiro, P.; Malzoni, M.; Adamyan, L.; Ussia, A.; Gomel, V.; Martin, D.C.; Koninckx, P.R. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: A systematic review and seven case reports. Gynecol. Surg. 2019, 16, 3. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yang, S.; Fang, Z.; Gurates, B.; Tamura, M.; Sebastian, S. Estrogen production and metabolism in endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 75–85; discussion 86–88. [Google Scholar] [CrossRef] [PubMed]
- Palep-Singh, M.; Gupta, S. Endometriosis: Associations with menopause, hormone replacement therapy and cancer. Menopause Int. 2009, 15, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, G.E.; Smith, M.E.; Mendelson, C.R.; Macdonald, P.C.; Simpson, E.R. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J. Clin. Endocrinol. Metab. 1981, 53, 412–417. [Google Scholar] [CrossRef]
- Velasco, I.; Rueda, J.; Acién, P. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol. Hum. Reprod. 2006, 12, 377–381. [Google Scholar] [CrossRef]
- Zeitoun, K.M.; Bulun, S.E. Aromatase: A key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil. Steril. 1999, 72, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, G.M.; Webb, M.J.; Li, H.; Keeney, G.L. Hyperestrogenism: A relevant risk factor for the development of cancer from endometriosis. Gynecol. Oncol. 2000, 79, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Manero, M.G.; Royo, P.; Olartecoechea, B.; Alcázar, J.L. Endometriosis in a postmenopausal woman without previous hormonal therapy: A case report. J. Med Case Rep. 2009, 3, 135. [Google Scholar] [CrossRef]
- Suchońska, B.; Gajewska, M.; Zyguła, A.; Wielgoś, M. Endometriosis resembling endometrial cancer in a postmenopausal patient. Climacteric 2018, 21, 88–91. [Google Scholar] [CrossRef]
- Gompel, A.; Stuenkel, C.A. Neurokinin 3 receptor antagonists for menopausal vasomotor symptoms, an appraisal. Cell Rep. Med. 2023, 4, 101076. [Google Scholar] [CrossRef]
- Shaukat, A.; Mujeeb, A.; Shahnoor, S.; Nasser, N.; Khan, A.M. “Veozah (Fezolinetant): A Promising Non-Hormonal Treatment for Vasomotor Symptoms in Menopause”. Health Sci. Rep. 2023, 6, e1610. [Google Scholar] [CrossRef]
- Fraser, G.L.; Hoveyda, H.R.; Clarke, I.J.; Ramaswamy, S.; Plant, T.M.; Rose, C.; Millar, R.P. The NK3 Receptor Antagonist ESN364 Interrupts Pulsatile LH Secretion and Moderates Levels of Ovarian Hormones Throughout the Menstrual Cycle. Endocrinology 2015, 156, 4214–4225. [Google Scholar] [CrossRef] [PubMed]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.-R.; Leng, J.-H.; Jia, S.-Z.; Lang, J.-H. Postmenopausal endometriosis: A retrospective analysis of 69 patients during a 20-year period. Chin. Med. J. 2013, 126, 4588–4589. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Uchida, Y.; Nakajima, F. Vesical endometriosis following the menopause. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 2009, 20, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.A.; Selim, Y.A.R.M.; Arif, M.A.; Albroumi, S.A. Gastric wall endometriosis in a postmenopausal woman. Egypt. J. Radiol. Nucl. Med. 2016, 47, 1783–1786. [Google Scholar] [CrossRef]
- Izuishi, K.; Sano, T.; Shiota, A.; Mori, H.; Ebara, K. Small bowel obstruction caused by endometriosis in a postmenopausal woman. Asian J. Endosc. Surg. 2015, 8, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Andriola, V.; Battaglia, M.; Ditonno, P.; Fiore, M.G.; De Fazio, M.; Memeo, R.; Altomare, D.F. The unexpected conundrum of endometrioid carcinoma in deep rectal endometriosis arising 11 years after total hysterectomy bilateral salpingo-oophorectomy. Int. J. Color. Dis. 2016, 31, 475–477. [Google Scholar] [CrossRef]
- Threadcraft, M.; Fouad, L.; Bruce, A.; Nakhleh, R.; Dinh, T. Endometriosis in a Postmenopausal Patient Presenting as an Erythematous Vaginal Plaque. J. Minim. Invasive Gynecol. 2017, 24, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Thylan, S. Re: Renal and diaphragmatic endometriosis de novo associated with hormone replacement therapy. J. Urol. 1995, 154, 1143, Erratum in J. Urol. 1995, 154, 1872. [Google Scholar] [CrossRef]
- Flyckt, R.; Lyden, S.; Roma, A.; Falcone, T. Post-menopausal endometriosis with inferior vena cava invasion requiring surgical management. Hum. Reprod. 2011, 26, 2709–2712. [Google Scholar] [CrossRef][Green Version]
- Cameron, M.; Westwell, S.; Subramanian, A.; Ramesar, K.; Howlett, D. Postmenopausal Cutaneous Endometriosis: Mimicking Breast Metastasis. Breast J. 2017, 23, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, A.; Achilarre, M.T.; Scardapane, A.; Lorusso, F.; Ceci, O.; Mangiatordi, G.; Angelelli, G.; Van Herendael, B.; Selvaggi, L.; Bettocchi, S. Accuracy of transvaginal sonography and contrast-enhanced magnetic resonance-colonography for the presurgical staging of deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2012, 40, 592–603. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research 2019, 8, 529. [Google Scholar] [CrossRef]
- Bäumler, M.; Heiss, N.; Druckmann, R. Endometriosis at all ages: Diagnostic ultrasound. Horm. Mol. Biol. Clin. Investig. 2022, 43, 151–157. [Google Scholar] [CrossRef]
- Nafisi Moghadam, R.; Tamizi, F.; Kazem Razavi Ratki, S.; Nafisi Moghadam, A.; Javaheri, A.; Namiranian, N. Evaluation of diagnostic value of pelvic MRI in endometriosis in comparison with surgical findings: A cross-sectional study. Int. J. Reprod. Biomed. (IJRM) 2024, 22, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef]
- Brulport, A.; Bourdon, M.; Vaiman, D.; Drouet, C.; Pocate-Cheriet, K.; Bouzid, K.; Marcellin, L.; Santulli, P.; Abo, C.; Jeljeli, M.; et al. An integrated multi-tissue approach for endometriosis candidate biomarkers: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 21. [Google Scholar] [CrossRef]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935, Erratum in BMJ 2017, 358, j4227. [Google Scholar] [CrossRef] [PubMed]
- Schipper, E.; Nezhat, C. Video-assisted laparoscopy for the detection and diagnosis of endometriosis: Safety, reliability, and invasiveness. Int. J. Women’s Health 2012, 4, 383–393. [Google Scholar] [CrossRef]
- Alborzi, S.; Rasekhi, A.; Shomali, Z.; Madadi, G.; Alborzi, M.; Kazemi, M.; Nohandani, A.H. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine 2018, 97, e9536. [Google Scholar] [CrossRef]
- Oxholm, D.; Knudsen, U.B.; Kryger-Baggesen, N.; Ravn, P. Postmenopausal endometriosis. Acta Obstet. Gynecol. Scand. 2007, 86, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ozyurek, E.S.; Yoldemir, T.; Kalkan, U. Surgical challenges in the treatment of perimenopausal and postmenopausal endometriosis. Climacteric 2018, 21, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.A.; Almaria, M.J.G. Postmenopausal endometriosis: Drawing a clearer clinical picture. Climacteric 2018, 21, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mathey, M.P.; Bouquet de Jolinière, J.; Major, A.; Pugin, F.; Monnard, E.; Fiche, M.; Sandmeier, D.; Khomsi, F.; Feki, A. Endometriotic Mass After Hysterectomy in a 61 Year Old Post-menopausal Woman: A Case Report and Update. Front. Surg. 2019, 6, 14. [Google Scholar] [CrossRef]
- Zanello, M.; Borghese, G.; Manzara, F.; Degli Esposti, E.; Moro, E.; Raimondo, D.; Abdullahi, L.O.; Arena, A.; Terzano, P.; Meriggiola, M.C.; et al. Hormonal Replacement Therapy in Menopausal Women with History of Endometriosis: A Review of Literature. Medicina 2019, 55, 477. [Google Scholar] [CrossRef] [PubMed]
- Słopień, R.; Męczekalski, B. Aromatase inhibitors in the treatment of endometriosis. Menopausal Rev./Prz. Menopauzalny 2016, 1, 43–47. [Google Scholar] [CrossRef]
- Hajjar, L.R.; Kim, W.; Nolan, G.H.; Turner, S.; Raju, U.R. Intestinal and pelvic endometriosis presenting as a tumor and associated with tamoxifen therapy: Report of a case. Obstet. Gynecol. 1993, 82, 642. [Google Scholar]
- Bese, T.; Simsek, Y.; Bese, N.; Ilvan, S.; Arvas, M. Extensive pelvic endometriosis with malignant change in tamoxifen-treated postmenopausal women. Int. J. Gynecol. Cancer 2003, 13, 376–380. [Google Scholar] [CrossRef]
- Okugawa, K.; Hirakawa, T.; Ogawa, S.; Kaku, T.; Nakano, H. Ovarian endometrioid adenocarcinoma arising from an endometriotic cyst in a postmenopausal woman under tamoxifen therapy for breast cancer: A case report. Gynecol. Oncol. 2002, 87, 231–234. [Google Scholar] [CrossRef]
- Schlesinger, C.; Silverberg, S.G. Tamoxifen-associated polyps (basalomas) arising in multiple endometriotic foci: A case report and review of the literature. Gynecol. Oncol. 1999, 73, 305–311. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Tang, S.; Feng, F.; Liu, C.; Wang, L.; Zhao, W.; Zhang, T.; Yao, Y.; Wang, X.; et al. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Streuli, I.; Gaitzsch, H.; Wenger, J.-M.; Petignat, P. Endometriosis after menopause: Physiopathology and management of an uncommon condition. Climacteric 2017, 20, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Marie-Scemama, L.; Even, M.; De La Joliniere, J.B.; Ayoubi, J.-M. Endometriosis and the menopause: Why the question merits our full attention. Horm. Mol. Biol. Clin. Investig. 2019, 37, 20180071. [Google Scholar] [CrossRef] [PubMed]
- Bartiromo, L.; Schimberni, M.; Villanacci, R.; Mangili, G.; Ferrari, S.; Ottolina, J.; Salmeri, N.; Dolci, C.; Tandoi, I.; Candiani, M. A Systematic Review of Atypical Endometriosis-Associated Biomarkers. Int. J. Mol. Sci. 2022, 23, 4425. [Google Scholar] [CrossRef] [PubMed]
- Ñiguez Sevilla, I.; Machado Linde, F.; Marín Sánchez, M.D.P.; Arense, J.J.; Torroba, A.; Nieto Díaz, A.; Sánchez Ferrer, M.L. Prognostic importance of atypical endometriosis with architectural hyperplasia versus cytologic atypia in endometriosis-associated ovarian cancer. J. Gynecol. Oncol. 2019, 30, e63. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vigano’, P.; Parazzini, F.; Stoppelli, S.; Giambattista, E.; Vercellini, P. Association between endometriosis and cancer: A comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol. Oncol. 2006, 101, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, L.; Mellemkjær, L.; Frederiksen, K.; Kjær, S.K.; Brinton, L.A.; Sakoda, L.C.; van Valkengoed, I.; Olsen, J.H. Risk for breast cancer among women with endometriosis. Int. J. Cancer 2006, 120, 1372–1375. [Google Scholar] [CrossRef]
- Dhanda, S.; Thakur, M.; Kerkar, R.; Jagmohan, P. Diffusion-weighted imaging of gynecologic tumors: Diagnostic pearls and potential pitfalls. RadioGraphics 2014, 34, 1393–1416. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.O.; Okada, S.; Yagi, T.; Satoh, T.; Oki, A.; Tsunoda, H.; Yoshikawa, H. MRI of endometriotic cysts in association with ovarian carcinoma. Am. J. Roentgenol. 2010, 194, 355–361. [Google Scholar] [CrossRef]
- Robinson, K.A.; Menias, C.O.; Chen, L.; Schiappacasse, G.; Shaaban, A.M.; Caserta, M.P.; Elsayes, K.M.; VanBuren, W.M.; Bolan, C.W. Understanding malignant transformation of endometriosis: Imaging features with pathologic correlation. Abdom. Radiol. 2020, 45, 1762–1775. [Google Scholar] [CrossRef]
| Risk factors | History of symptoms before menopause |
| Therapy with hormone replacement | |
| Obesity | |
| Therapy with tamoxifen | |
| Evidence of prior endometriosis | |
| Symptomatology | Non-specific clinical symptoms |
| Pelvic pain | |
| Intestinal symptoms | |
| Localization | Ovaries |
| Utero-sacral ligaments | |
| Pelvic peritoneal surfaces | |
| Other: urinary tract, stomach, large and small bowel, vagina, diaphragm, inferior vena cava and skin | |
| Imaging | Transvaginal sonography |
| Computerized tomography | |
| Magnetic resonance imaging (MRI) | |
| Management | Surgical removal of all the visible endometriotic lesions |
| Medical therapy (e.g., progesterone, aromatase inhibitors) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.-D.; Haj Hamoud, B.; Amza, M.; Gorecki, G.-P.; Sima, R.-M.; Gică, N.; Pleș, L. Endometriosis in Menopausal Women—A New Age Is Coming? Literature Review. Life 2024, 14, 485. https://doi.org/10.3390/life14040485
Dinu M-D, Haj Hamoud B, Amza M, Gorecki G-P, Sima R-M, Gică N, Pleș L. Endometriosis in Menopausal Women—A New Age Is Coming? Literature Review. Life. 2024; 14(4):485. https://doi.org/10.3390/life14040485
Chicago/Turabian StyleDinu, Mihai-Daniel, Bashar Haj Hamoud, Mihaela Amza, Gabriel-Petre Gorecki, Romina-Marina Sima, Nicolae Gică, and Liana Pleș. 2024. "Endometriosis in Menopausal Women—A New Age Is Coming? Literature Review" Life 14, no. 4: 485. https://doi.org/10.3390/life14040485
APA StyleDinu, M.-D., Haj Hamoud, B., Amza, M., Gorecki, G.-P., Sima, R.-M., Gică, N., & Pleș, L. (2024). Endometriosis in Menopausal Women—A New Age Is Coming? Literature Review. Life, 14(4), 485. https://doi.org/10.3390/life14040485








