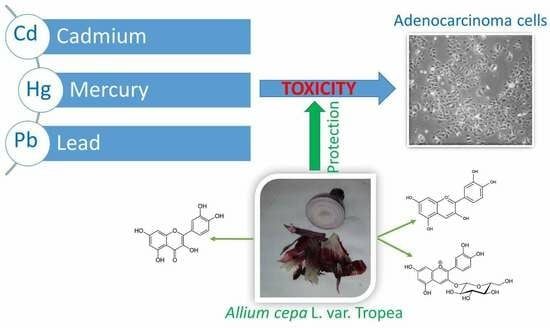
Phenolic Compounds from Tropea Red Onion as Dietary Agents for Protection against Heavy Metals Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material, Preparation of the Outer Layer Extract and Chemical Analysis
2.3. Preparation of the Sample Solutions for the Bioassays
2.4. Determination of FRAP Activity
2.5. Bovine Brain Peroxidation Assay
2.6. Preparation of “Non-Essential” Heavy Metals Solutions
2.7. Culture Cells
2.8. Determination of Cell Viability
2.9. Lactate Dehydrogenase (LDH) Assay
2.10. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.2. Cytotoxic Activity
3.3. Induction of Necrosis by “Non-Essential” Heavy Metals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffus, J.H. Heavy metals a meaningless term? Pure Appl. Chem. 2002, 74, 793G–807G. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Howard, H.; Michael, M.C. (Eds.) Human Health and Heavy Metals Exposure. In Life Support: The Environment and Human Health; MIT Press: Massachusetts, MA, USA, 2002. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects . Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Ortega, D.R.; Esquivel, D.F.G.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M.; de la Cruz, V.P. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Ghosh, D.; Chattopadhyay, A.; Firdaus, S.B.; Ghosh, A.K.; Paul, S.; Dalui, K. Lead induced oxidative stress: A health issue of global concern. J. Pharm. Res. 2014, 88, 1198–1207. [Google Scholar]
- Meleleo, D.; Sblano, C.; Storelli, M.M.; Mallamaci, R. Evidence of cadmium and mercury involvement in the Aβ42 aggregation process. Biophys. Chem. 2020, 266, 106453. [Google Scholar] [CrossRef]
- Zhukalin, M.; Blanksma, M.K.; Silva, T.D.; Suyehira, S.W.; Harvey, W.A.; Heggland, S.J.; Craig, P.R. Characterization and in vitro Cytotoxicity Testing of Ethanolamine-derived Cadmium Chelating Agents. Biometals 2007, 20, 61–72. [Google Scholar] [CrossRef]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals, and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals, and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Rajak, C.; Singh, N.; Parashar, P. Metal toxicity and natural antidotes: Prevention is better than cure. Environ. Sci. Pollut. Res. 2020, 27, 43582–43598. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Wawer, I. Anthocyanidins, structure and antioxidant properties. Farm. Pol. 2001, 15, 728–731. [Google Scholar]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; DeFreitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Rui, H.L. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Tedesco, I.; Carbone, V.; Spagnuolo, C.; Minasi, P.; Russo, G.L. Identification, and quantification of flavonoids from two southern Italian cultivars of Allium cepa L., Tropea (Red Onion) and Montoro (Copper Onion), and their capacity to protect human erythrocytes from oxidative stress. J. Agric. Food Chem. 2015, 63, 5229–5238. [Google Scholar] [CrossRef] [PubMed]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.)—A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 59, S39–S70. [Google Scholar] [CrossRef] [PubMed]
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Capasso, R.; Izzom, A.A. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. Tropea. J. Agric. Food Chem. 2005, 53, 935–940. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity-a review. Pol. J. Food Nutr. Sci. 2008, 58, 4. [Google Scholar]
- Marrelli, M.; Russo, C.; Statti, G.; Argentieri, M.P.; Meleleo, D.; Mallamaci, R.; Avato, P.; Conforti, F. Phytochemical and biological characterization of dry outer scales extract from Tropea red onion (Allium cepa L. var. Tropea)—A promising inhibitor of pancreatic lipase. Phytomedicine 2022, 2, 100235. [Google Scholar] [CrossRef]
- Marrelli, M.; Argentieri, M.P.; Alexa, E.; Meleleo, D.; Statti, G.; Avato, P.; Conforti, F.; Mallamaci, R. Antioxidant activity and protective effect of the outer scales hydroalcoholic extract of Allium cepa L. var. Tropea on toxicity damage induced by Cadmium in Caco-2 cells. Food Chem. Toxicol. 2022, 170, 113495. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Marek Kuś, P.; Jerković, I. Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Houghton, P.J. Antioxidant, and cytotoxic activities of Retama raetam subsp. Gussonei. Phytother. Res. 2004, 18, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Storelli, M.M.; Barbarossa, A.; Messina, G.; Valenzano, A.; Meleleo, D. Potential Protective Effects of Spirulina (Spirulina platensis) against In Vitro Toxicity Induced by Heavy Metals (Cadmium, Mercury, and Lead) on SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 17076. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, S.; Gioielli, A.; Cataldi, M.; Di Renzo, G.; Annunziato, L. In the neuronal cell line SH-SY5Y, oxidative stress-induced free radical overproduction causes cell death without any participation of intracellular Ca2+ increase Biochim. Biophys. Acta Mol. Cell Res. 1999, 1452, 151–160. [Google Scholar] [CrossRef]
- Kaja, S.; Payne, A.J.; Naumchuk, Y.; Koulen, P. Quantification of lactate dehydrogenase for cell viability testing using cell lines and primary cultured astrocytes. Curr. Protoc. Toxicol. 2017, 72, 2–26. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity, and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar]
- El Ati-Hellal, M.; Fayçal, H. Heavy metals in the environment and health impact. In Environmental Health; IntechOpen: London, UK, 2021; Volume 51. [Google Scholar]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Hemmaphan, S.; Bordeerat, N.K. Bordeerat. Genotoxic effects of lead and their impact on the expression of DNA repair genes. Int. J. Environ. Res. Public Health 2022, 19, 4307. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury Toxicity and Neurodegenerative Effects. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: Cham, Switzerland, 2014; Volume 229. [Google Scholar]
- Oyugi, A.M.; Kibet, J.K.; Adongo, J.O. A review of the health implications of heavy metals and pesticide residues on khat users. Bull. Natl. Res. Cent. 2021, 45, 158. [Google Scholar] [CrossRef]
- Gade, M.; Comfort, N.; Re, D.B. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ. Res. 2021, 201, 111558. [Google Scholar] [CrossRef]
- Crespo, I.; García-Mediavilla, M.V.; Almar, M.; González, P.; Tuñón, M.J.; Sánchez-Campos, S.; González-Gallego, J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. 2008, 46, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [PubMed]
- Yan, Y.; Guo, F.; Liu, K.; Ding, R.; Wang, Y. The effect of endocrine-disrupting chemicals on placental development. Front. Endocrinol. 2023, 14, 1059854. [Google Scholar] [CrossRef] [PubMed]
- Kianoush, S.; Balali-Mood, M.; Mousavi, S.R.; Moradi, V.; Sadeghi, M.; Dadpour, B.; Shakeri, M.T. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic Clin. Pharmacol. Toxicol. 2012, 110, 476–481. [Google Scholar] [CrossRef]
- Nwokocha, C.R.; Nwokocha, M.I.; Aneto, I.; Obi, J.; Udekweleze, D.C.; Olatunde, B.; Iwuala, M.O. Comparative analysis on the effect of Lycopersicon esculentum (tomato) in reducing cadmium, mercury, and lead accumulation in liver. Food Chem. Toxicol. 2012, 50, 2070–2073. [Google Scholar] [CrossRef]








| Concentration | % Cell Viability | |
|---|---|---|
| 24 h | 72 h | |
| CdCl2 | ||
| 0.01 µM | 96.4 ± 1.5 | 52.9 ± 4.2 *** |
| 0.05 µM | 94.4 ± 5.0 | 58.7 ± 5.1 *** |
| 0.25 µM | 92.7 ± 2.5 | 44.9 ± 2.9 *** |
| 2.5 µM | 85.7 ± 3.0 * | 23.3 ± 2.3 *** |
| 25 µM | 42.7 ± 3.0 *** | 17.2 ± 1.5 *** |
| 100 µM | 23.8 ± 3.0 *** | 14.7 ± 2.2 *** |
| 250 µM | 16.4 ± 2.5 *** | 12.6 ± 2.6 *** |
| HgCl2 | ||
| 0.01 µM | 82.6 ± 2.5 * | 62.4 ± 0.6 *** |
| 0.05 µM | 82.2 ± 2.2 * | 60.0 ± 0.5 *** |
| 0.25 µM | 75.3 ± 1.7 ** | 58.9 ± 0.8 *** |
| 2.5 µM | 62.2 ± 3.8 *** | 52.1 ± 1.8 *** |
| 25 µM | 45.1 ± 1.7 *** | 42.4 ± 0.6 *** |
| 100 µM | 23.9 ± 1.8 *** | 21.0 ± 0.2 *** |
| 250 µM | 19.1 ± 1.2 *** | 18.8 ± 0.8 *** |
| PbCl2 | ||
| 0.01 µM | 81.7 ± 2.6 * | 64.1 ± 1.2 *** |
| 0.05 µM | 80.6 ± 2.5 * | 47.3 ± 1.0 *** |
| 0.25 µM | 75.0 ± 2.4 ** | 46.2 ± 0.6 *** |
| 2.5 µM | 71.8 ± 2.5 ** | 41.0 ± 0.7 *** |
| 25 µM | 64.7 ± 1.6 *** | 40.6 ± 1.0 *** |
| 100 µM | 66.1 ± 1.2 *** | 28.5 ± 1.1 *** |
| 250 µM | 25.9 ± 1.1 *** | 24.6 ± 1.0 *** |
| Ctrl | 100 ± 0.0 | 100 ± 0.0 |
| Concentration | % Cell Viability | |||
|---|---|---|---|---|
| 24 h | 72 h | |||
| Cd 25 µM | 45.8 ± 3.5 | 14.4 ± 1.5 | ||
| TRO | TRO + CdCl2 | TRO | TRO + CdCl2 | |
| 25 µg/mL | 143.9 ± 13.9 | 39.4 ± 3.8 * | 110.2 ± 8.4 | 103.3 ± 18.2 |
| 50 µg/mL | 203.9 ± 19.7 *** | 189.7 ± 18.3 ** | 17.3 ± 1.1 *** | 57.6 ± 2.9 ** |
| 100 µg/mL | 207.2 ± 22.6 *** | 142.9 ± 17.9 | 54.3 ± 1.5 ** | 16.3 ± 9.1 *** |
| Hg 25 µM | 44.5 ± 1.7 | 34.6 ± 4.3 | ||
| TRO | TRO + HgCl2 | TRO | TRO + HgCl2 | |
| 25 µg/mL | 143.9 ± 13.9 ** | 183.6 ± 5.3 ** | 110.2 ± 8.4 | 54.3 ± 2.0 |
| 50 µg/mL | 203.9 ± 19.7 *** | 192.3 ± 6.0 *** | 17.3 ± 1.1 ** | 57.6 ± 2.9 |
| 100 µg/mL | 207.2 ± 22.6 *** | 228.4 ± 9.9 *** | 54.3 ± 1.5 * | 74.3 ± 2.0 |
| Pb 25 µM | 64.3 ± 3.9 | 42.1 ± 0.7 | ||
| TRO | TRO + PbCl2 | TRO | TRO + PbCl2 | |
| 25 µg/mL | 143.9 ± 13.9 | 138.7 ± 8.9 | 110.2 ± 8.4 | 95.6 ± 6.5 |
| 50 µg/mL | 203.9 ± 19.7 ** | 166.4 ± 12.2 * | 17.3 ± 1.1 ** | 78.7 ± 17.5 |
| 100 µg/mL | 207.2 ± 22.6 ** | 203.4 ± 6.5 *** | 54.3 ± 1.5 * | 103.3 ± 18.2 |
| Ctrl | 100 | 100 | ||
| Concentration | % Cell Viability | |||
|---|---|---|---|---|
| 24 h | ||||
| 25 µM Metal | Cd 144.4 ± 14.8 | Hg 135.4 ± 14.9 | Pb 126.6 ± 2.8 | |
| TRO | TRO + CdCl2 | TRO + HgCl2 | TRO + PbCl2 | |
| 25 µg/mL | 105.5 ± 5.0 ** | 93.4 ± 5.3 | 113.0 ± 6.8 | 114.6 ± 5.5 |
| 50 µg/mL | 66.7 ± 9.9 *** | 55.5 ± 7.4 *** | 50.3 ± 7.8 *** | 65.3 ± 0.7 *** |
| 100 µg/mL | 85.0 ± 2.4 ** | 46.7 ± 4.0 *** | 30.3 ± 5.5 *** | 27.6 ± 3.0 *** |
| 72 h | ||||
| 25 µM Metal | Cd 149.3 ± 10.0 | Hg 144.4 ± 6.9 | Pb 160.9 ± 8.0 | |
| TRO | TRO + CdCl2 | TRO + HgCl2 | TRO + PbCl2 | |
| 25 µg/mL | 100.5 ± 10.0 ** | 98.7 ± 6.0 *** | 106.5 ± 5.1 | 102.5 ± 8.0 |
| 50 µg/mL | 110.1 ± 9.5 ** | 96.9 ± 6.0 *** | 149.2 ± 4.9 | 53.7 ± 4.3 *** |
| 100 µg/mL | 96.6 ± 6.0 *** | 134.7 ± 12.0 | 158.9 ± 0.5 | 70.0 ± 2.0 *** |
| Ctrl | 100 | 100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamaci, R.; Conforti, F.; Statti, G.; Avato, P.; Barbarossa, A.; Meleleo, D. Phenolic Compounds from Tropea Red Onion as Dietary Agents for Protection against Heavy Metals Toxicity. Life 2024, 14, 495. https://doi.org/10.3390/life14040495
Mallamaci R, Conforti F, Statti G, Avato P, Barbarossa A, Meleleo D. Phenolic Compounds from Tropea Red Onion as Dietary Agents for Protection against Heavy Metals Toxicity. Life. 2024; 14(4):495. https://doi.org/10.3390/life14040495
Chicago/Turabian StyleMallamaci, Rosanna, Filomena Conforti, Giancarlo Statti, Pinarosa Avato, Alexia Barbarossa, and Daniela Meleleo. 2024. "Phenolic Compounds from Tropea Red Onion as Dietary Agents for Protection against Heavy Metals Toxicity" Life 14, no. 4: 495. https://doi.org/10.3390/life14040495