Comparative Effectiveness of Complex Telemedicine Support in Prevention of Hospitalizations and Mortality in Patients with Heart Failure: A Systematic Review and Meta-Analysis
Abstract
1. Background
2. Methods
3. Results
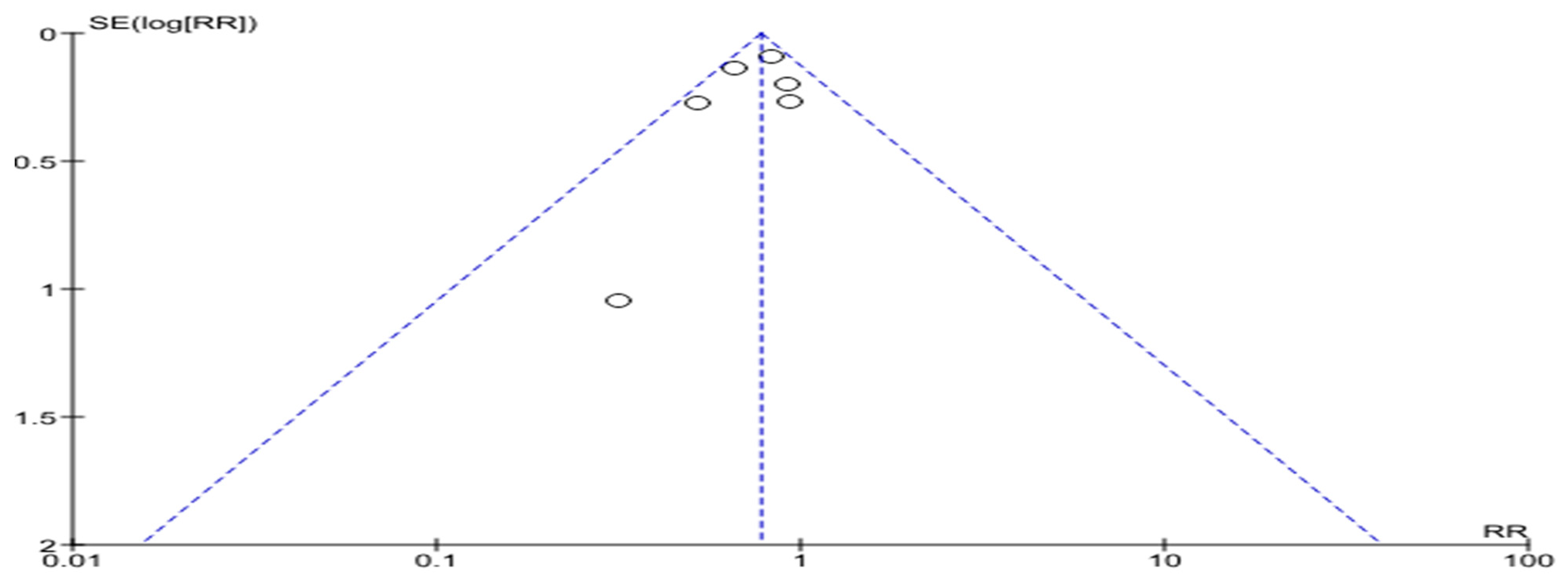
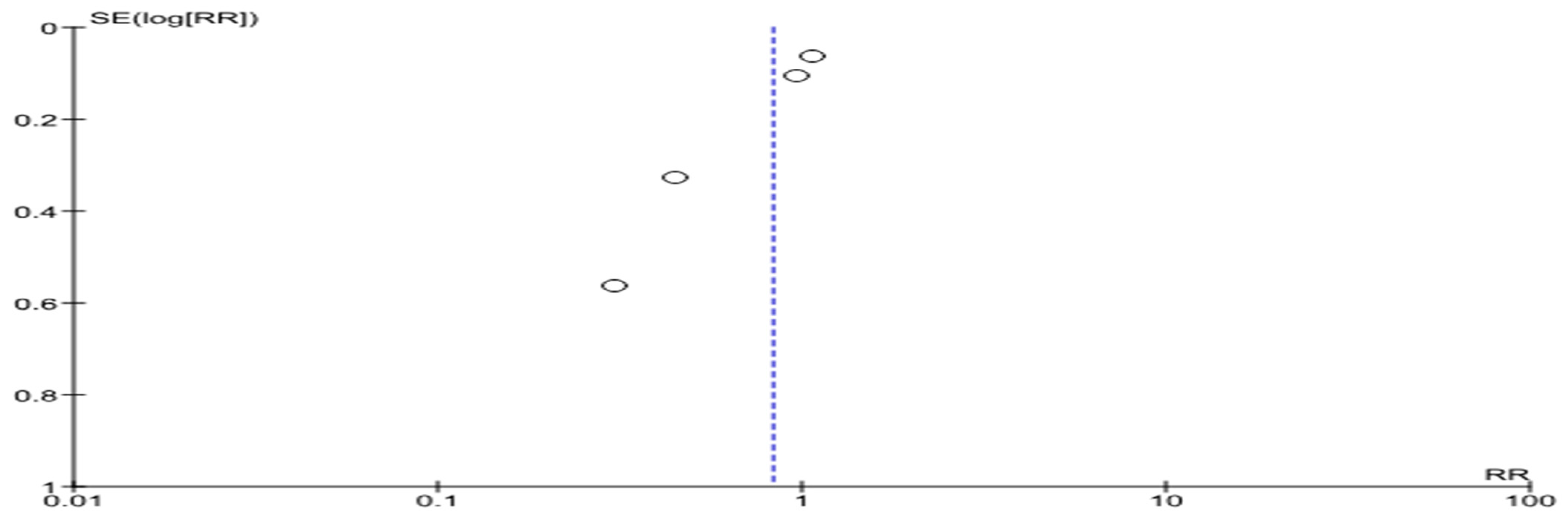
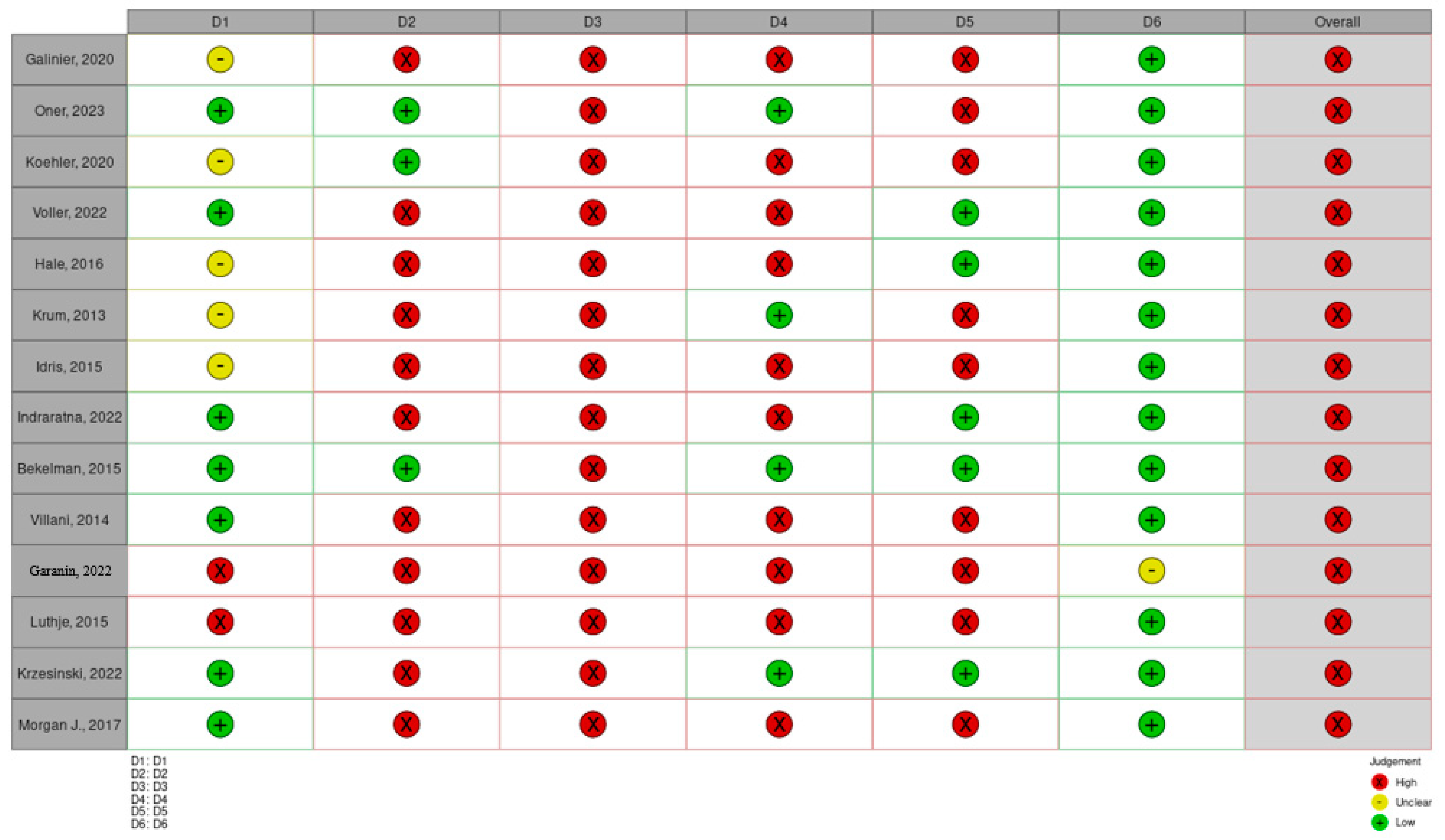
4. Assessment of the Risks of Bias
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242252. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 9961004. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure with Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018, 6, 678685. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, midrange and reduced ejection fraction: An analysis of the ESC Heart Failure LongTerm Registry. Eur. J. Heart Fail. 2017, 19, 15741585. [Google Scholar] [CrossRef] [PubMed]
- Eurlings, C.G.M.J.; Boyne, J.J.; de Boer, R.A.; Brunner-La Rocca, H.P. Telemedicine in heart failure-more than nice to have? Neth. Heart J. 2019, 27, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Pierucci, N.; Forleo, G.B.; Schiavone, M.; Bernardini, A.; Gasperetti, A.; Mitacchione, G.; Mei, M.; Giunta, G.; Piro, A.; et al. The Feasibility, Effectiveness and Acceptance of Virtual Visits as Compared to In-Person Visits among Clinical Electrophysiology Patients during the COVID-19 Pandemic. J. Clin. Med. 2023, 12, 620. [Google Scholar] [CrossRef]
- Gatto, M.C.; Frisicale, E.M.; Palopoli, P.; Sapienza, M.; Caroppo, E.; Patrizi, C.; Migliano, G.; Damiani, G. The Role of Health Institutions in Training Healthcare Personnel for the Digital Transition: The International Training Program of the Order of Physicians and Dentists of Rome. Int. Med. Educ. 2024, 3, 92–99. [Google Scholar] [CrossRef]
- Lin, M.H.; Yuan, W.L.; Huang, T.C.; Zhang, H.F.; Mai, J.T.; Wang, J.F. Clinical effectiveness of telemedicine for chronic heart failure: A systematic review and meta-analysis. J. Investig. Med. 2017, 65, 899–911. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, X.; Xu, C. Effectiveness of telemedicine systems for adults with heart failure: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2020, 25, 231–243. [Google Scholar] [CrossRef]
- Rebolledo Del Toro, M.; Herrera Leaño, N.M.; Barahona-Correa, J.E.; Muñoz Velandia, O.M.; Fernández Ávila, D.G.; García Peña, Á.A. Effectiveness of mobile telemonitoring applications in heart failure patients: Systematic review of literature and meta-analysis. Heart Fail. Rev. 2023, 28, 431–452. [Google Scholar] [CrossRef] [PubMed]
- Barasa, A.; Schaufelberger, M.; Lappas, G.; Swedberg, K.; Dellborg, M.; Rosengren, A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 2532. [Google Scholar] [CrossRef]
- Kotb, A.; Cameron, C.; Hsieh, S.; Wells, G. Comparative effectiveness of different forms of telemedicine for individuals with heart failure (HF): A systematic review and network meta-analysis. PLoS ONE 2015, 10, e0118681. [Google Scholar] [CrossRef]
- Bekelman, D.B.; Plomondon, M.E.; Carey, E.P.; Sullivan, M.D.; Nelson, K.M.; Hattler, B.; McBryde, C.F.; Lehmann, K.G.; Gianola, K.; Heidenreich, P.A.; et al. Primary Results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 725–732. [Google Scholar] [CrossRef]
- Krzesiński, P.; Jankowska, E.A.; Siebert, J.; Galas, A.; Piotrowicz, K.; Stańczyk, A.; Siwołowski, P.; Gutknecht, P.; Chrom, P.; Murawski, P.; et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): A randomised controlled trial. Eur. J. Heart Fail. 2022, 24, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Garanin, A.A.; Mullova, I.S.; Shkaeva, O.V.; Duplyakova, P.D.; Duplyakov, D.V. Remote monitoring of outpatients discharged from the emergency cardiac care department. Russ. J. Cardiol. 2022, 27, 5072. [Google Scholar] [CrossRef]
- Villani, A.; Malfatto, G.; Compare, A.; Della Rosa, F.; Bellardita, L.; Branzi, G.; Molinari, E.; Parati, G. Clinical and psychological telemonitoring and telecare of high risk heart failure patients. J. Telemed. Telecare 2014, 20, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lüthje, L.; Vollmann, D.; Seegers, J.; Sohns, C.; Hasenfuß, G.; Zabel, M. A randomized study of remote monitoring and fluid monitoring for the management of patients with implanted cardiac arrhythmia devices. Europace 2015, 17, 1276–1281. [Google Scholar] [CrossRef]
- Morgan, J.M.; Kitt, S.; Gill, J.; McComb, J.M.; Ng, G.A.; Raftery, J.; Roderick, P.; Seed, A.; Williams, S.G.; Witte, K.K.; et al. Remote management of heart failure using implantable electronic devices. Eur. Heart J. 2017, 38, 2352–2360. [Google Scholar] [CrossRef]
- Galinier, M.; Roubille, F.; Berdague, P.; Brierre, G.; Cantie, P.; Dary, P.; Ferradou, J.-M.; Fondard, O.; Labarre, J.P.; Mansourati, J.; et al. Telemonitoring versus standard care in heart failure: A randomised multicentre trial. Eur. J. Heart Fail. 2020, 22, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Prescher, S.; Kirwan, B.-A.; Wegscheider, K.; Vettorazzi, E.; Lezius, S.; Winkler, S.; Moeller, V.; Fiss, G.; et al. Mortality and morbidity 1 year after stopping a remote patient management intervention: Extended follow-up results from the telemedical interventional management in patients with heart failure II (TIM-HF2) randomised trial. Lancet Digit. Health 2020, 2, e16–e24. [Google Scholar] [CrossRef]
- Indraratna, P.; Biswas, U.; McVeigh, J.; Mamo, A.; Magdy, J.; Vickers, D.; Watkins, E.; Ziegl, A.; Liu, H.; Cholerton, N.; et al. A Smartphone-Based Model of Care to Support Patients with Cardiac Disease Transitioning from Hospital to the Community (TeleClinical Care): Pilot Randomized Controlled Trial. JMIR mHealth uHealth 2022, 10, e32554. [Google Scholar] [CrossRef]
- Öner, A.; Dittrich, H.; Arslan, F.; Hintz, S.; Ortak, J.; Brandewiede, B.; Mann, M.; Krockenberger, K.; Thiéry, A.; Ziegler, A.; et al. Comparison of telemonitoring combined with intensive patient support with standard care in patients with chronic cardiovascular disease—A randomized clinical trial. Eur. J. Med. Res. 2023, 28, 22. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.M.; Jethwani, K.; Kandola, M.S.; Saldana, F.; Kvedar, J.C. A Remote Medication Monitoring System for Chronic Heart Failure Patients to Reduce Readmissions: A Two-Arm Randomized Pilot Study. J. Med. Internet Res. 2016, 18, e91, Correction in J. Med. Internet Res. 2019, 21, e13125. [Google Scholar] [CrossRef]
- Völler, H.; Bindl, D.; Nagels, K.; Hofmann, R.; Vettorazzi, E.; Wegscheider, K.; Fleck, E.; Störk, S.; Nagel, E. The First Year of Noninvasive Remote Telemonitoring in Chronic Heart Failure Is not Cost Saving but Improves Quality of Life: The Randomized Controlled CardioBBEAT Trial. Telemed. J. E Health 2022, 28, 1613–1622. [Google Scholar] [CrossRef]
- Krum, H.; Forbes, A.; Yallop, J.; Driscoll, A.; Croucher, J.; Chan, B.; Clark, R.; Davidson, P.; Huynh, L.; Kasper, E.K.; et al. Telephone support to rural and remote patients with heart failure: The Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc. Ther. 2013, 31, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Degheim, G.; Ghalayini, W.; Larsen, T.R.; Nejad, D.; David, S. Home Telemedicine in Heart Failure: A Pilot Study of Integrated Telemonitoring and Virtual Provider Appointments. Rev. Cardiovasc. Med. 2015, 16, 156–162. [Google Scholar] [CrossRef]
- Lee, K.C.; Breznen, B.; Ukhova, A.; Martin, S.S.; Koehler, F. Virtual healthcare solutions in heart failure: A literature review. Front. Cardiovasc. Med. 2023, 10, 1231000. [Google Scholar] [CrossRef]
- Rosario, M.B.D.; Lovell, N.H.; Fildes, J.; Holgate, K.; Yu, J.; Ferry, C.; Schreier, G.; Ooi, S.-Y.; Redmond, S.J. Evaluation of an mHealth-Based Adjunct to Outpatient Cardiac Rehabilitation. IEEE J. Biomed. Health Inform. 2018, 22, 1938–1948. [Google Scholar] [CrossRef]
- Dendale, P.; De Keulenaer, G.; Troisfontaines, P.; Weytjens, C.; Mullens, W.; Elegeert, I.; Ector, B.; Houbrechts, M.; Willekens, K.; Hansen, D. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: The TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur. J. Heart Fail. 2012, 14, 333–340. [Google Scholar] [CrossRef] [PubMed]




| Study | Number of Patients (n) | Study Groups (n) | Study Endpoints | Observational Period | Treatment Group, Mortality | Control Group, Mortality | Treatment Group, Hospitalizations | Control Group, Hospitalizations |
|---|---|---|---|---|---|---|---|---|
| Bekelman D.B., 2015 [15] | 392 | Home telemonitoring (n = 187) Traditional monitoring (n = 197) | All-cause mortality, all-cause hospitalizations | 12 months | 8/187 | 19/197 | 55/187 | 59/197 |
| Krzesinski P., 2022 [16] | 605 | Telemonitoring (n = 300) Traditional monitoring (n = 305) | All-cause mortality, HF-related hospitalizations | 12 months | 28/298 | 29/305 | 62/298 | 97/305 |
| Garanin A.A., 2022 [17] | 392 | Telemonitoring (n = 197) Traditional monitoring (n = 195) | All-cause mortality, cardiovascular-related hospitalizations | 3 months | 6/197 | 11/195 | 4/197 | 13/195 |
| Villani A., 2014 [18] | 80 | Telemonitoring (n = 40) Traditional monitoring (n = 40) | Mortality, HF-related hospitalizations | 12 months | 5/40 | 9/40 | 12/40 | 23/40 |
| Lars Luthje, 2015 [19] | 176 | Remote monitoring (n = 87) Traditional monitoring (n = 89) | All-cause mortality, HF-related hospitalizations | 15 months | 8/87 | 6/89 | 20/87 | 22/89 |
| Morgan J.M. [20] | 1650 | Remote monitoring (n = 824) Traditional monitoring (n = 826) | All-cause mortality, cardiovascular-related hospitalizations | At mean 2.8 years | 128/824 | 152/826 | 315/824 | 297/826 |
| Galinier M., 2020 [21] | 937 | Telemonitoring (n = 482) Traditional monitoring (n = 455) | All-cause mortality, HF-related hospitalizations, all-cause hospitalizations | 18 months | 91/482 | 89/455 | 141/482 242/482 | 160/455 241/455 |
| Koehler F., 2020 [22] | 1538 | Telemonitoring (n = 765) Traditional monitoring (n = 773) | All-cause mortality | 24 months | 129/765 | 152/773 | - | - |
| Indraratna P., 2022 [23] | 164 | Telemonitoring (n = 81) Traditional monitoring (n = 83) | All-cause mortality, all-cause hospitalizations | 193 days | 1/81 | 4/83 | 21/81 | 41/83 |
| cardiovascular-related hospitalizations | - | - | 11/81 | 25/83 | ||||
| Oner A., 2023 [24] | 960 | Telemonitoring (n = 478) Traditional monitoring (n = 482) | All-cause mortality | 12 months | 4/477 | 23/480 | - | - |
| Hale T.M., 2016 [25] | 29 | Telemonitoring (n = 13) Traditional monitoring (n = 16) | All-cause hospitalizations, | 3 months | - | - | 1/11 | 7/14 |
| HF-related hospitalizations | - | - | 1/11 | 4/14 | ||||
| Voller H., 2022 [26] | 621 | Telemonitoring (n = 302) Traditional monitoring (n = 319) | All-cause mortality, All-cause hospitalizations, | 12 months | 20/241 | 26/251 | 139/241 | 150/251 |
| HF-related hospitalizations, | - | - | 39/241 | 44/251 | ||||
| cardiovascular-related hospitalizations | - | - | 99/241 | 106/251 | ||||
| Krum H., 2013 [27] | 313 | Telemonitoring (n = 188) Traditional monitoring (n = 217) | All-cause mortality, all-cause hospitalizations | 12 months | 17/170 | 16/209 | 74/161 | 114/204 |
| Idris, 2015 [28] | 28 | Telemonitoring (n = 14) Traditional monitoring (n = 14) | All-cause mortality, all-cause hospitalizations | 6 months | 1/14 | 2/14 | 1/14 | 7/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garanin, A.; Rubanenko, A.; Trusov, Y.; Rubanenko, O.; Kolsanov, A. Comparative Effectiveness of Complex Telemedicine Support in Prevention of Hospitalizations and Mortality in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Life 2024, 14, 507. https://doi.org/10.3390/life14040507
Garanin A, Rubanenko A, Trusov Y, Rubanenko O, Kolsanov A. Comparative Effectiveness of Complex Telemedicine Support in Prevention of Hospitalizations and Mortality in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Life. 2024; 14(4):507. https://doi.org/10.3390/life14040507
Chicago/Turabian StyleGaranin, Andrey, Anatoly Rubanenko, Yuriy Trusov, Olesya Rubanenko, and Alexandr Kolsanov. 2024. "Comparative Effectiveness of Complex Telemedicine Support in Prevention of Hospitalizations and Mortality in Patients with Heart Failure: A Systematic Review and Meta-Analysis" Life 14, no. 4: 507. https://doi.org/10.3390/life14040507
APA StyleGaranin, A., Rubanenko, A., Trusov, Y., Rubanenko, O., & Kolsanov, A. (2024). Comparative Effectiveness of Complex Telemedicine Support in Prevention of Hospitalizations and Mortality in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Life, 14(4), 507. https://doi.org/10.3390/life14040507










