Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review
Abstract
1. Introduction
2. Neuropsychological Assessments
2.1. Understanding Neurocognitive Impairment in People Living with HIV
2.2. The Role of Neuropsychological Assessments
2.3. Challenges in Neuropsychological Assessments
2.4. New Approaches to HIV-Associated NCI Definitions
3. Neuroimaging in HIV-Associated NCI
3.1. Proton Magnetic Resonance Spectroscopy
3.2. MRI Volumetrics
3.3. Diffusion Tensor Imaging
3.4. Functional Magnetic Resonance Imaging
3.5. PET
3.6. Role of Neuroimaging in Differentiating HIV-Associated NCI from Other Causes of Cognitive Decline
4. Diagnostic and Prognostic Blood and CSF Biomarkers
4.1. Monocyte/Macrophage Activation Markers
4.1.1. Neopterin
4.1.2. sCD14, sCD163, and LPS
4.1.3. MCP-1/CCL2
4.1.4. CD16+ Monocytes
4.2. Biomarkers of Neuronal Damage
4.2.1. Neurofilaments
4.2.2. S100 Calcium-Binding Protein B (S100B)
4.3. Biomarkers of Inflammation
4.4. Extracellular Vesicles
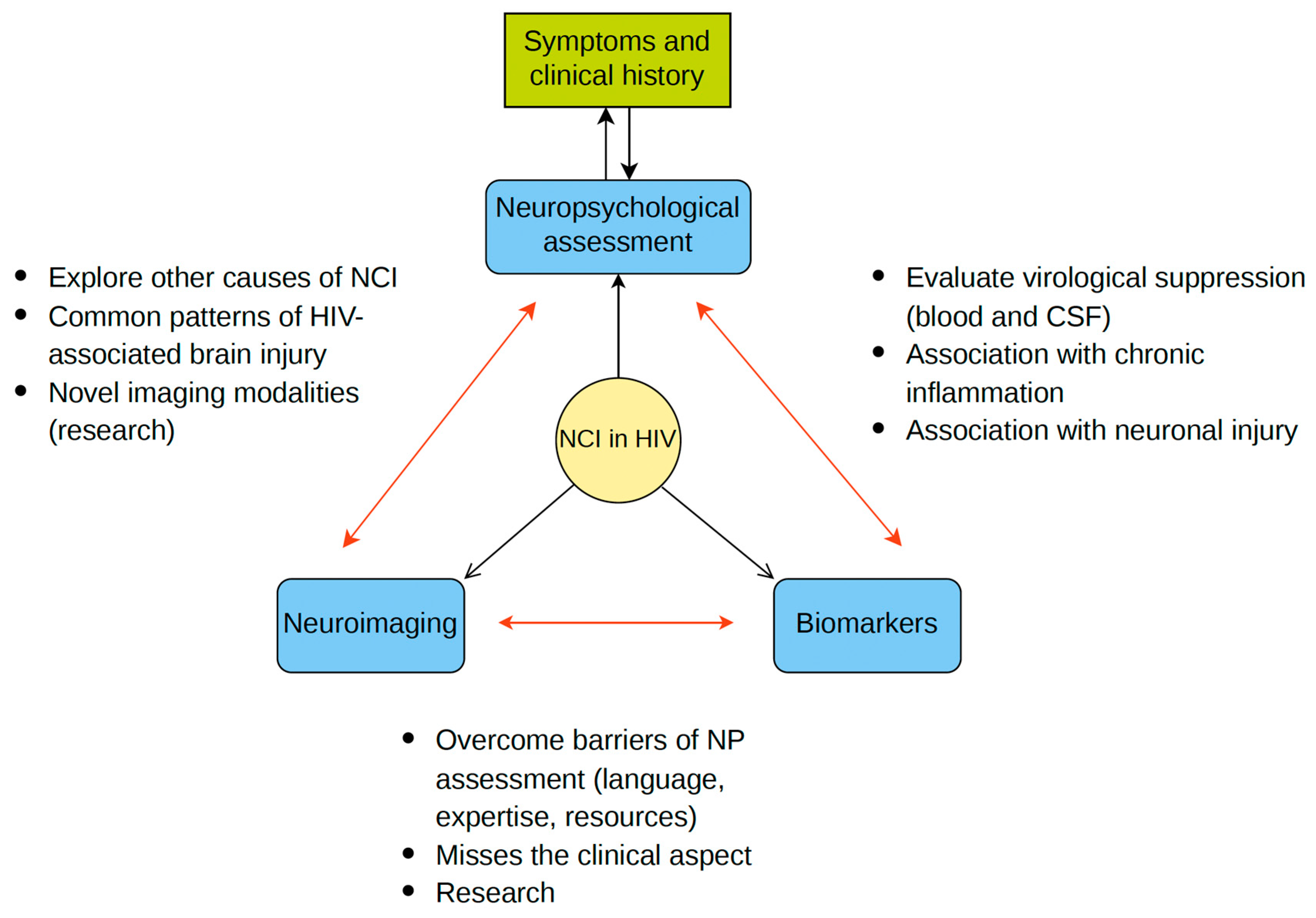
5. Combining Neuropsychology, Imaging, and Biomarkers in HIV-NCI Diagnosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Zenebe, Y.; Necho, M.; Yimam, W.; Akele, B. Worldwide Occurrence of HIV-Associated Neurocognitive Disorders and Its Associated Factors: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 814362. [Google Scholar] [CrossRef]
- Wei, J.; Hou, J.; Su, B.; Jiang, T.; Guo, C.; Wang, W.; Zhang, Y.; Chang, B.; Wu, H.; Zhang, T. The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 581346. [Google Scholar] [CrossRef]
- Keng, L.D.; Winston, A.; Sabin, C.A. The global burden of cognitive impairment in people with HIV. AIDS 2023, 37, 61–70. [Google Scholar] [CrossRef]
- Navia, B.A.; Jordan, B.D.; Price, R.W. The AIDS dementia complex: I. Clinical features. Ann. Neurol. 1986, 19, 517–524. [Google Scholar] [CrossRef]
- Hoover, D.R.; Bacellar, H.; Miller, E.N.; Cohen, B.A.; Becker, J.T.; Graham, N.; McArthur, J.H.; Selnes, O.A.; Jacobson, L.P.; Visscher, B.R.; et al. Dementia in AIDS patients: Incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993, 43, 2245–2252. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- McArthur, J.C.; Steiner, J.; Sacktor, N.; Nath, A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann. Neurol. 2010, 67, 699–714. [Google Scholar] [CrossRef]
- Nightingale, S.; Winston, A.; Letendre, S.; Michael, B.D.; McArthur, J.C.; Khoo, S.; Solomon, T. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014, 13, 1139–1151. [Google Scholar] [CrossRef]
- Underwood, J.; Robertson, K.R.; Winston, A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 2015, 29, 253–261. [Google Scholar] [CrossRef]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.-C.; Mpongo, N.; et al. Cognitive impairment in people living with HIV: Consensus recommendations for a new approach. Nat. Rev. Neurol. 2023, 19, 424–433. [Google Scholar] [CrossRef]
- Robertson, K.; Liner, J.; Heaton, R. Neuropsychological assessment of HIV-infected populations in international settings. Neuropsychol. Rev. 2009, 19, 232–249. [Google Scholar] [CrossRef]
- McLaurin, K.A.; Booze, R.M.; Mactutus, C.F. Diagnostic and prognostic biomarkers for HAND. J. Neurovirol. 2019, 25, 686–701. [Google Scholar] [CrossRef]
- Pulliam, L. Evolving biomarkers for HIV-associated neurocognitive disorders (HAND). In HIV-Associated Neurocognitive Disorders; Elsevier: Amsterdam, The Netherlands, 2024; pp. 295–306. [Google Scholar] [CrossRef]
- Anderson, A.M.; Ma, Q.; Letendre, S.L.; Iudicello, J. Soluble Biomarkers of Cognition and Depression in Adults with HIV Infection in the Combination Therapy Era. Curr. HIV/AIDS Rep 2021, 18, 558–568. [Google Scholar] [CrossRef]
- Alagaratnam, J.; Winston, A. Molecular neuroimaging of inflammation in HIV. Clin. Exp. Immunol. 2022, 210, 14–23. [Google Scholar] [CrossRef]
- Woods, S.P.; Moore, D.J.; Weber, E.; Grant, I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol. Rev. 2009, 19, 152–168. [Google Scholar] [CrossRef]
- HNRC Group; Dawes, S.; Suarez, P.; Casey, C.Y.; Cherner, M.; Marcotte, T.D.; Letendre, S.; Grant, I.; Heaton, R.K. Variable patterns of neuropsychological performance in HIV-1 infection. J. Clin. Exp. Neuropsychol. 2008, 30, 613–626. [Google Scholar] [CrossRef]
- Molsberry, S.A.; Cheng, Y.; Kingsley, L.; Jacobson, L.; Levine, A.J.; Martin, E.; Miller, E.N.; Munro, C.A.; Ragin, A.; Sacktor, N.; et al. Neuropsychological phenotypes among men with and without HIV disease in the multicenter AIDS cohort study. AIDS 2018, 32, 1679–1688. [Google Scholar] [CrossRef]
- McMahan, C.; Dietrich, D.K.; Horne, E.F.; Kelly, E.; Geannopoulos, K.; Julnes, P.S.S.; Ham, L.; Santamaria, U.; Lau, C.-Y.; Wu, T.; et al. Neurocognitive Dysfunction With Neuronal Injury in People With HIV on Long-Duration Antiretroviral Therapy. Neurology 2023, 100, e2466–e2476. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R.; Deutsch, R.; Letendre, S.; Ellis, R.J.; Casaletto, K.; Marquine, M.J.; Woods, S.P.; Vaida, F.; Atkinson, J.H.; et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clin. Infect. Dis. 2015, 60, 473–480. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Johnson, T.P.; Nath, A. Biotypes of HIV-associated neurocognitive disorders based on viral and immune pathogenesis. Curr. Opin. Infect. Dis. 2022, 35, 223–230. [Google Scholar] [CrossRef]
- Mukerji, S.S.; Petersen, K.J.; Pohl, K.M.; Dastgheyb, R.M.; Fox, H.S.; Bilder, R.M.; Brouillette, M.-J.; Gross, A.L.; Scott-Sheldon, L.A.J.; Paul, R.H.; et al. Machine Learning Approaches to Understand Cognitive Phenotypes in People With HIV. J. Infect. Dis. 2023, 227, S48–S57. [Google Scholar] [CrossRef]
- Sacktor, N.; Skolasky, R.L.; Seaberg, E.; Munro, C.; Becker, J.T.; Martin, E.; Ragin, A.; Levine, A.; Miller, E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016, 86, 334–340. [Google Scholar] [CrossRef]
- American Psychiatric Association. Neurocognitive Disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; pp. 632–634. [Google Scholar]
- American Psychiatric Association. Other Conditions That May Be a Focus of Clinical Attention. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2022; pp. 717–721. [Google Scholar]
- Tierney, S.M.; Sheppard, D.P.; Kordovski, V.M.; Faytell, M.P.; Avci, G.; Woods, S.P. A comparison of the sensitivity, stability, and reliability of three diagnostic schemes for HIV-associated neurocognitive disorders. J. Neurovirol. 2017, 23, 404–421. [Google Scholar] [CrossRef]
- Woods, S.P.; Rippeth, J.D.; Frol, A.B.; Levy, J.K.; Ryan, E.; Soukup, V.M.; Hinkin, C.H.; Lazzaretto, D.; Cherner, M.; Marcotte, T.D.; et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J. Clin. Exp. Neuropsychol. 2004, 26, 759–778. [Google Scholar] [CrossRef]
- Matchanova, A.; Woods, S.P.; Kordovski, V.M. Operationalizing and evaluating the Frascati criteria for functional decline in diagnosing HIV-associated neurocognitive disorders in adults. J. Neurovirol. 2020, 26, 155–167. [Google Scholar] [CrossRef]
- Rubin, L.H.; Maki, P.M. HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Curr. HIV/AIDS Rep. 2019, 16, 82–95. [Google Scholar] [CrossRef]
- Lang, R.; Hogan, B.; Zhu, J.; McArthur, K.; Lee, J.; Zandi, P.; Nestadt, P.; Silverberg, M.J.; Parcesepe, A.M.; Cook, J.A.; et al. The prevalence of mental health disorders in people with HIV and the effects on the HIV care continuum. AIDS 2023, 37, 259–269. [Google Scholar] [CrossRef]
- Ayano, G.; Duko, B.; Bedaso, A. The Prevalence of Post-Traumatic Stress Disorder among People Living with HIV/AIDS: A Systematic Review and Meta-Analysis. Psychiatr. Q. 2020, 91, 1317–1332. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Ma, Y.; Jia, L.; Cai, M.; Li, Z.; Zhang, T.; Guo, C. People who living with HIV/AIDS also have a high prevalence of anxiety disorders: A systematic review and meta-analysis. Front. Psychiatry 2024, 15, 1259290. [Google Scholar] [CrossRef]
- Too, E.K.; Abubakar, A.; Nasambu, C.; Koot, H.M.; Cuijpers, P.; Newton, C.R.; Nyongesa, M.K. Prevalence and factors associated with common mental disorders in young people living with HIV in sub-Saharan Africa: A systematic review. J. Int. AIDS Soc. 2021, 24, e25705. [Google Scholar] [CrossRef]
- Yen, Y.-F.; Lai, H.-H.; Kuo, Y.-C.; Chan, S.-Y.; Chen, L.-Y.; Chen, C.-C.; Wang, T.-H.; Wang, C.C.; Chen, M.; Yen, T.-F.; et al. Association of depression and antidepressant therapy with antiretroviral therapy adherence and health-related quality of life in men who have sex with men. PLoS ONE 2022, 17, e0264503. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Smith, F.A.; Trivedi, M.H.; Beach, S.R. Depressive Disorders in HIV/AIDS: A Clinically Focused Narrative Review. Harv. Rev. Psychiatry 2020, 28, 146–158. [Google Scholar] [CrossRef]
- Bayes-Marin, I.; Egea-Cortés, L.; Palacio-Vieira, J.; Bruguera, A.; Mesías-Gazmuri, J.; Llibre, J.M.; Fernández, E.; Imaz, A.; Forero, C.G.; Agustí, C.; et al. Determinants of Depressive Symptoms in People Living with HIV: Findings from a Population-Based Study with a Gender Perspective. Int. J. Environ. Res. Public Health 2023, 20, 3687. [Google Scholar] [CrossRef]
- Namagga, J.K.; Rukundo, G.Z.; Niyonzima, V.; Voss, J. Depression and HIV associated neurocognitive disorders among HIV infected adults in rural southwestern Uganda: A cross-sectional quantitative study. BMC Psychiatry 2021, 21, 350. [Google Scholar] [CrossRef]
- Salis, F.; Belfiori, M.; Bellisai, A.; Bernardini, E.; Murtas, M.; Piras, R.; Serreli, S.; Ortu, F.; Piano, P.; Del Giacco, S.; et al. Cognitive Impairment in People Living with HIV and the Impact of Mood: Results from a Cross-Sectional Study. J. Clin. Med. 2024, 13, 1631. [Google Scholar] [CrossRef]
- Lukasik, K.M.; Waris, O.; Soveri, A.; Lehtonen, M.; Laine, M. The Relationship of Anxiety and Stress with Working Memory Performance in a Large Non-depressed Sample. Front. Psychol. 2019, 10, 4. [Google Scholar] [CrossRef]
- Rakshasa-Loots, A.M.; Bakewell, N.; Sharp, D.J.; Gisslén, M.; Zetterberg, H.; Alagaratnam, J.; Wit, F.W.N.M.; Kootstra, N.A.; Winston, A.; Reiss, P.; et al. Biomarkers of central and peripheral inflammation mediate the association between HIV and depressive symptoms. Transl. Psychiatry 2023, 13, 190. [Google Scholar] [CrossRef]
- Banerjee, N.; Goodman, Z.T.; McIntosh, R.; Ironson, G. Cognition, Coping, and Psychological Distress in HIV. AIDS Behav. 2022, 26, 1074–1083. [Google Scholar] [CrossRef]
- Ncitakalo, N.; Sigwadhi, L.N.; Mabaso, M.; Joska, J.; Simbayi, L. Exploring HIV status as a mediator in the relationship of psychological distress with socio-demographic and health related factors in South Africa: Findings from the 2012 nationally representative population-based household survey. AIDS Res. Ther. 2023, 20, 6. [Google Scholar] [CrossRef]
- Alford, K.; Banerjee, S.; Nixon, E.; O’brien, C.; Pounds, O.; Butler, A.; Elphick, C.; Henshaw, P.; Anderson, S.; Vera, J.H. Assessment and management of HIV-associated cognitive impairment: Experience from a multidisciplinary memory service for people living with HIV. Brain Sci. 2019, 9, 37. [Google Scholar] [CrossRef]
- Gisslén, M.; Price, R.W.; Nilsson, S. The definition of HIV-associated neurocognitive disorders: Are we overestimating the real prevalence? BMC Infect. Dis. 2011, 11, 356. [Google Scholar] [CrossRef]
- De Francesco, D.; on behalf of the POPPY study group; Underwood, J.; Post, F.A.; Vera, J.H.; Williams, I.; Boffito, M.; Sachikonye, M.; Anderson, J.; Mallon, P.W.G.; et al. Defining cognitive impairment in people-living-with-HIV: The POPPY study. BMC Infect. Dis. 2016, 16, 617. [Google Scholar] [CrossRef]
- Rosca, E.C.; Tadger, P.; Cornea, A.; Tudor, R.; Oancea, C.; Simu, M. International HIV Dementia Scale for HIV-Associated Neurocognitive Disorders: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1124. [Google Scholar] [CrossRef]
- The Mind Exchange Working Group; Antinori, A.; Arendt, G.; Grant, I.; Letendre, S.; Chair; Muñoz-Moreno, J.A.; Eggers, C.; Brew, B.; Brouillette, M.-J.; et al. Assessment, diagnosisand treatment of Human Immunodeficiency Virus (HIV)-associated neurocognitive disorders (HAND): A consensus report of the mind exchange program. Clin. Infect. Dis. 2013, 56, 1004–1017. [Google Scholar] [CrossRef]
- Deist, M.; Suliman, S.; Kidd, M.; Franklin, D.; Cherner, M.; Heaton, R.K.; Spies, G.; Seedat, S. Neuropsychological Test Norms for the Assessment of HIV-Associated Neurocognitive Impairment Among South African Adults. AIDS Behav. 2023, 27, 3080–3097. [Google Scholar] [CrossRef]
- Power, C.; Selnes, O.A.; Grim, J.A.; McArthur, J.C. HIV Dementia Scale: A Rapid Screening Test. J. Acquir. Immune Defic. Syndr. Hum. Retrovirology 1995, 8, 273–278. [Google Scholar] [CrossRef]
- Sacktor, N.C.; Wong, M.; Nakasujja, N.; Skolasky, R.L.; A Selnes, O.; Musisi, S.; Robertson, K.; McArthur, J.C.; Ronald, A.; Katabira, E. The International HIV Dementia Scale: A new rapid screening test for HIV dementia. AIDS 2005, 19, 1367–1374. [Google Scholar]
- Robbins, R.N.; Scott, T.M.; Gouse, H.; Marcotte, T.D.; Rourke, S.B. Screening for HIV-Associated Neurocognitive Disorders: Sensitivity and Specificity. In Neurocognitive Complications of HIV-Infection; Springer: Cham, Switzerland, 2019; pp. 429–478. [Google Scholar] [CrossRef]
- Haddow, L.J.; Floyd, S.; Copas, A.; Gilson, R.J.C. A systematic review of the screening accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PLoS ONE 2013, 8, e61826. [Google Scholar] [CrossRef]
- Kami-Onaga, K.; Tateyama, M.; Kinjo, T.; Parrott, G.; Tominaga, D.; Takahashi-Nakazato, A.; Nakamura, H.; Tasato, D.; Miyagi, K.; Maeda, S.; et al. Comparison of two screening tests for HIV-Associated Neurocognitive Disorder suspected Japanese patients with respect to cART usage. PLoS ONE 2018, 13, e0199106. [Google Scholar] [CrossRef]
- Aita, S.L.; Kaewpoowat, Q.; Yasri, S.; Rerkasem, A.; Rerkasem, K.; Choovuthayakorn, J.; Ausayakhun, S.; Robertson, K.; Roth, R.M.; Robbins, N.M. Psychometric utility of the international HIV dementia scale and Montreal Cognitive Assessment in HIV-associated asymptomatic neurocognitive impairment. J. Neurovirol. 2021, 27, 568–578. [Google Scholar] [CrossRef]
- Nightingale, S.; Dreyer, A.J.; Saylor, D.; Gisslén, M.; Winston, A.; Joska, J.A. Moving on From HAND: Why We Need New Criteria for Cognitive Impairment in Persons Living With Human Immunodeficiency Virus and a Proposed Way Forward. Clin. Infect. Dis 2021, 73, 1113–1118. [Google Scholar] [CrossRef]
- Masters, M.C.; Ances, B.M. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin. Neurol. 2014, 34, 89–102. [Google Scholar] [CrossRef]
- O’connor, E.; Sullivan, E.V.; Chang, L.; A Hammoud, D.; Wilson, T.W.; Ragin, A.B.; Meade, C.S.; Coughlin, J.; Ances, B.M. Imaging of Brain Structural and Functional Effects in People With Human Immunodeficiency Virus. J. Infect. Dis. 2023, 227, S16–S29. [Google Scholar] [CrossRef]
- Thompson, P.M.; Jahanshad, N. Novel Neuroimaging Methods to Understand How HIV Affects the Brain. Curr. HIV/AIDS Rep. 2015, 12, 289–298. [Google Scholar] [CrossRef]
- Thompson, P.M.; Dutton, R.A.; Hayashi, K.M.; Toga, A.W.; Lopez, O.L.; Aizenstein, H.J.; Becker, J.T. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc. Natl. Acad. Sci. USA 2005, 102, 15647–15652. [Google Scholar] [CrossRef]
- Nir, T.M.; Fouche, J.-P.; Ananworanich, J.; Ances, B.M.; Boban, J.; Brew, B.J.; Chaganti, J.R.; Chang, L.; Ching, C.R.K.; Cysique, L.A.; et al. Association of Immunosuppression and Viral Load with Subcortical Brain Volume in an International Sample of People Living With HIV. JAMA Netw. Open 2021, 4, e2031190. [Google Scholar] [CrossRef]
- Cohen, R.A.; Harezlak, J.; Schifitto, G.; Hana, G.; Clark, U.; Gongvatana, A.; Paul, R.; Taylor, M.; Thompson, P.; Alger, J.; et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J. Neurovirol. 2010, 16, 25–32. [Google Scholar] [CrossRef]
- Kallianpur, K.J.; Shikuma, C.; Kirk, G.R.; Shiramizu, B.; Valcour, V.; Chow, D.; Souza, S.; Nakamoto, B.; Sailasuta, N. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology 2013, 80, 1792–1799. [Google Scholar] [CrossRef]
- Underwood, J.; Cole, J.H.; Caan, M.; De Francesco, D.; Leech, R.; van Zoest, R.A.; Su, T.; Geurtsen, G.J.; Schmand, B.A.; Portegies, P.; et al. Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin. Infect. Dis. 2017, 65, 422–432. [Google Scholar] [CrossRef]
- Alakkas, A.; for the CHARTER Group; Ellis, R.J.; Watson, C.W.-M.; Umlauf, A.; Heaton, R.K.; Letendre, S.; Collier, A.; Marra, C.; Clifford, D.B.; et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J. Neurovirol. 2019, 25, 32–41. [Google Scholar] [CrossRef]
- Becker, J.T.; Multicenter AIDS Cohort Study; Sanders, J.; Madsen, S.K.; Ragin, A.; Kingsley, L.; Maruca, V.; Cohen, B.; Goodkin, K.; Martin, E.; et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011, 5, 77–85. [Google Scholar] [CrossRef]
- Kamat, R.; Brown, G.G.; Bolden, K.; Fennema-Notestein, C.; Archibald, S.; Marcotte, T.D.; Letendre, S.L.; Ellis, R.J.; Woods, S.P.; Grant, I.; et al. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J. Clin. Exp. Neuropsychol. 2014, 36, 854–866. [Google Scholar] [CrossRef][Green Version]
- Nir, T.M.; Jahanshad, N.; Busovaca, E.; Wendelken, L.; Nicolas, K.; Thompson, P.M.; Valcour, V.G. Mapping white matter integrity in elderly people with HIV. Hum. Brain Mapp. 2014, 35, 975–992. [Google Scholar] [CrossRef]
- Kuhn, T.; Jin, Y.; Huang, C.; Kim, Y.; Nir, T.M.; Gullett, J.M.; Jones, J.D.; Sayegh, P.; Chung, C.; Dang, B.H.; et al. The joint effect of aging and HIV infection on microstructure of white matter bundles. Hum. Brain Mapp. 2019, 40, 4370–4380. [Google Scholar] [CrossRef]
- Wright, P.W.; Vaida, F.F.; Fernández, R.J.; Rutlin, J.; Price, R.W.; Lee, E.; Peterson, J.; Fuchs, D.; Shimony, J.S.; Robertson, K.R.; et al. Cerebral white matter integrity during primary HIV infection. AIDS 2015, 29, 433–442. [Google Scholar] [CrossRef]
- Nakamoto, B.K.; Jahanshad, N.; McMurtray, A.; Kallianpur, K.J.; Chow, D.C.; Valcour, V.G.; Paul, R.H.; Marotz, L.; Thompson, P.M.; Shikuma, C.M. Cerebrovascular risk factors and brain microstructural abnormalities on diffusion tensor images in HIV-infected individuals. J. Neurovirol. 2012, 18, 303–312. [Google Scholar] [CrossRef]
- Chang, K.; Premeaux, T.A.; Cobigo, Y.; Milanini, B.; Hellmuth, J.; Rubin, L.H.; Javandel, S.; Allen, I.; Ndhlovu, L.C.; Paul, R.; et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS 2020, 34, 203–213. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Xu, F.; Li, H. Application of Diffusion Tensor Imaging (DTI) in the Diagnosis of HIV-Associated Neurocognitive Disorder (HAND): A Meta-Analysis and a System Review. Front. Neurol. 2022, 13, 898191. [Google Scholar] [CrossRef]
- Ahmed-Leitao, F.; Du Plessis, S.; Konkiewitz, E.C.; Spies, G.; Seedat, S. Altered white matter integrity in the corpus callosum in adults with HIV: A systematic review of diffusion tensor imaging studies. Psychiatry Res. Neuroimaging 2022, 326, 111543. [Google Scholar] [CrossRef]
- Spagnolo-Allende, A.; Schnall, R.; Liu, M.; Igwe, K.C.; Laing, K.K.; Chesebro, A.G.; Brickman, A.M.; Gutierrez, J. Serum inflammation markers associated with altered brain white matter microstructure in people with HIV on antiretroviral treatment. Neurol. Sci. 2023, 44, 2159–2166. [Google Scholar] [CrossRef]
- Ann, H.W.; Jun, S.; Shin, N.-Y.; Han, S.; Ahn, J.Y.; Ahn, M.Y.; Jeon, Y.D.; Jung, I.Y.; Kim, M.H.; Jeong, W.Y.; et al. Characteristics of Resting-State Functional Connectivity in HIV-Associated Neurocognitive Disorder. PLoS ONE 2016, 11, e0153493. [Google Scholar] [CrossRef]
- Thomas, J.B.; Brier, M.R.; Snyder, A.Z.; Vaida, F.F.; Ances, B.M. Pathways to neurodegeneration: Effects of HIV and aging on resting-state functional connectivity. Neurology 2013, 80, 1186–1193. [Google Scholar] [CrossRef]
- Ernst, T.; Yakupov, R.; Nakama, H.; Crocket, G.; Cole, M.; Watters, M.; Ricardo-Dukelow, M.L.; Chang, L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann. Neurol. 2009, 65, 316–325. [Google Scholar] [CrossRef]
- Chaganti, J.; Gates, T.M.; Brew, B.J. Reversible large-scale network disruption correlates with neurocognitive improvement in HIV-associated minor neurocognitive disorder with combined anti-retroviral therapy intensification: A prospective longitudinal resting-state functional magnetic resonance imaging study. Neurol. Sci. 2023, 44, 3261–3269. [Google Scholar] [CrossRef]
- Cole, J.H.; A Caan, M.W.; Underwood, J.; De Francesco, D.; A van Zoest, R.; Wit, F.W.N.M.; Mutsaerts, H.J.M.M.; Leech, R.; Geurtsen, G.J.; Portegies, P.; et al. No Evidence for Accelerated Aging-Related Brain Pathology in Treated Human Immunodeficiency Virus: Longitudinal Neuroimaging Results From the Comorbidity in Relation to AIDS (COBRA) Project. Clin. Infect. Dis. 2018, 66, 1899–1909. [Google Scholar] [CrossRef]
- Ortega, M.; Brier, M.R.; Ances, B.M. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS 2015, 29, 703–712. [Google Scholar] [CrossRef]
- Hakkers, C.S.; Arends, J.E.; Barth, R.E.; Du Plessis, S.; Hoepelman, A.I.M.; Vink, M. Review of functional MRI in HIV: Effects of aging and medication. J. Neurovirol. 2017, 23, 20–32. [Google Scholar] [CrossRef]
- Chaganti, J.R.; Heinecke, A.; Gates, T.M.; Moffat, K.J.; Brew, B.J. Functional Connectivity in Virally Suppressed Patients with HIV-Associated Neurocognitive Disorder: A Resting-State Analysis. AJNR Am. J. Neuroradiol. 2017, 38, 1623–1629. [Google Scholar] [CrossRef]
- Thippabhotla, S.; Adeyemo, B.; A Cooley, S.; Roman, J.; Metcalf, N.; Boerwinkle, A.; Wisch, J.; Paul, R.; Ances, B.M. Comparison of Resting State Functional Connectivity in Persons with and without HIV: A Cross-sectional Study. J. Infect. Dis. 2023, 228, 751–758. [Google Scholar] [CrossRef]
- Chaganti, J.; Brew, B.J. MR spectroscopy in HIV associated neurocognitive disorder in the era of cART: A review. AIDS Res. Ther. 2021, 18, 65. [Google Scholar] [CrossRef]
- Anderson, A.M.; for the CHARTER Group; Fennema-Notestine, C.; Umlauf, A.; Taylor, M.J.; Clifford, D.B.; Marra, C.M.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; et al. CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. J. Neurovirol. 2015, 21, 559–567. [Google Scholar] [CrossRef]
- Campbell, L.M.; Fennema-Notestine, C.; Saloner, R.; Hussain, M.; Chen, A.; Franklin, D.; Umlauf, A.; Ellis, R.J.; Collier, A.C.; Marra, C.M.; et al. Use of Neuroimaging to Inform Optimal Neurocognitive Criteria for Detecting HIV-Associated Brain Abnormalities. J. Int. Neuropsychol. Soc. 2020, 26, 147–162. [Google Scholar] [CrossRef]
- Mohamed, M.; Barker, P.B.; Skolasky, R.L.; Sacktor, N. 7T brain MRS in HIV infection: Correlation with cognitive impairment and performance on neuropsychological tests. Am. J. Neuroradiol. 2018, 39, 704–712. [Google Scholar] [CrossRef]
- Chang, L.; Ernst, T.; Leonido–Yee, M.; Witt, M.; Speck, O.; Walot, I.; Miller, E. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology 1999, 53, 782–789. [Google Scholar] [CrossRef]
- Boban, J.M.; Kozic, D.B.; Brkic, S.V.; Lendak, D.F.; Thurnher, M.M. Early Introduction of cART Reverses Brain Aging Pattern in Well-Controlled HIV Infection: A Comparative MR Spectroscopy Study. Front. Aging Neurosci. 2018, 10, 329. [Google Scholar] [CrossRef]
- Chang, L.; Ernst, T.; St Hillaire, C.; Conant, K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir. Ther. 2004, 9, 431–440. [Google Scholar] [CrossRef]
- Ernst, T.; Chang, L.; Jovicich, J.; Ames, N.; Arnold, S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 2002, 59, 1343–1349. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Barker, P.B.; Skolasky, R.L.; Selnes, O.A.; Moxley, R.T.; Pomper, M.G.; Sacktor, N.C. Brain metabolism and cognitive impairment in HIV infection: A 3-T magnetic resonance spectroscopy study. Magn. Reson. Imaging 2010, 28, 1251–1257. [Google Scholar] [CrossRef]
- Hammoud, D.A.; Sinharay, S.; Steinbach, S.; Wakim, P.G.; Geannopoulos, K.; Traino, K.; Dey, A.K.; Tramont, E.; Rapoport, S.I.; Snow, J.; et al. Global and regional brain hypometabolism on FDG-PET in treated HIV-infected individuals. Neurology 2018, 91, e1591–e1601. [Google Scholar] [CrossRef]
- Wang, Z.; Manion, M.M.; Laidlaw, E.; Rupert, A.; Lau, C.-Y.; Smith, B.R.; Nath, A.; Sereti, I.; Hammoud, D.A. Redistribution of brain glucose metabolism in people with HIV after antiretroviral therapy initiation. AIDS 2021, 35, 1209–1219. [Google Scholar] [CrossRef]
- Rubin, L.H.; Sacktor, N.; Creighton, J.; Du, Y.; Endres, C.J.; Pomper, M.G.; Coughlin, J.M. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 2018, 32, 1661–1667. [Google Scholar] [CrossRef]
- Garvey, L.J.; Pavese, N.; Politis, M.; Ramlackhansingh, A.; Brooks, D.J.; Taylor-Robinson, S.D.; Winston, A. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS 2014, 28, 67–72. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Wang, Y.; Ma, S.; Yue, C.; Kim, P.K.; Adams, A.V.; Roosa, H.V.; Gage, K.L.; Stathis, M.; Rais, R.; et al. Regional brain distribution of translocator protein using [11C]DPA-713 PET in individuals infected with HIV. J. Neurovirol. 2014, 20, 219–232. [Google Scholar] [CrossRef]
- Boerwinkle, A.H.B.; Strain, J.F.; Burdo, T.; Doyle, J.B.; Christensen, J.B.; Su, Y.; Wisch, J.K.; Cooley, S.A.; Vaida, F.; Smith, M.D.; et al. Comparison of [11C]-PBR28 Binding Between Persons Living With HIV and HIV-Uninfected Individuals. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 85, 244–251. [Google Scholar] [CrossRef]
- Vera, J.H.; Guo, Q.; Cole, J.H.; Boasso, A.; Greathead, L.; Kelleher, P.; A Rabiner, E.; Kalk, N.; Bishop, C.; Gunn, R.N.; et al. Neuroinflammation in treated HIV-positive individuals. Neurology 2016, 86, 1425–1432. [Google Scholar] [CrossRef]
- Andersen, A.B.; Law, I.; Krabbe, K.S.; Bruunsgaard, H.; Ostrowski, S.R.; Ullum, H.; Højgaard, L.; Lebech, A.; Gerstoft, J.; Kjær, A. Cerebral FDG-PET scanning abnormalities in optimally treated HIV patients. J. Neuroinflamm. 2010, 7, 13. [Google Scholar] [CrossRef]
- Anderson, A.M.; Harezlak, J.; Bharti, A.; Mi, D.; Taylor, M.J.; Daar, E.S.; Schifitto, G.; Zhong, J.; Alger, J.R.; Brown, M.S.; et al. Plasma and Cerebrospinal Fluid Biomarkers Predict Cerebral Injury in HIV-Infected Individuals on Stable Combination Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2015, 69, 29–35. [Google Scholar] [CrossRef]
- Schifitto, G.; Kieburtz, K.; McDermott, M.; McArthur, J.; Marder, K.; Sacktor, N.; Palumbo, D.; Selnes, O.; Stern, Y.; Epstein, L.; et al. Clinical trials in HIV-associated cognitive impairment: Cognitive and functional outcomes. Neurology 2001, 56, 415–418. [Google Scholar] [CrossRef]
- Harezlak, J.; Buchthal, S.; Taylor, M.; Schifitto, G.; Zhong, J.; Daar, E.; Alger, J.; Singer, E.; Campbell, T.; Yiannoutsos, C.; et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011, 25, 625–633. [Google Scholar] [CrossRef]
- Chang, L.; Shukla, D. Imaging studies of the HIV-infected brain. Handb. Clin. Neurol. 2018, 152, 229–264. [Google Scholar] [CrossRef]
- Haziot, M.E.J.; Barbosa Junior, S.P.; Vidal, J.E.; de Oliveira, F.T.M.; de Oliveira, A.C.P. Neuroimaging of HIV-associated neurocognitive disorders. Dement. Neuropsychol. 2015, 9, 380–384. [Google Scholar] [CrossRef]
- Wilson, T.W.; Heinrichs-Graham, E.; Becker, K.M.; Aloi, J.; Robertson, K.R.; Sandkovsky, U.; White, M.L.; O’Neill, J.; Knott, N.L.; Fox, H.S.; et al. Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum. Brain Mapp. 2015, 36, 897–910. [Google Scholar] [CrossRef]
- Stout, J.C.; Ellis, R.J.; Jernigan, T.L.; Archibald, S.L.; Abramson, I.; Wolfson, T.; McCutchan, J.A.; Wallace, M.R.; Atkinson, J.H.; Grant, I. Progressive cerebral volume loss in human immunodeficiency virus infection: A longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch. Neurol. 1998, 55, 161–168. [Google Scholar] [CrossRef]
- Ragin, A.B.; Du, H.; Ochs, R.; Wu, Y.; Sammet, C.L.; Shoukry, A.; Epstein, L.G. Structural brain alterations can be detected early in HIV infection. Neurology 2012, 79, 2328–2334. [Google Scholar] [CrossRef]
- Sanford, R.; Ances, B.M.; Meyerhoff, D.J.; Price, R.W.; Fuchs, D.; Zetterberg, H.; Spudich, S.; Collins, D.L. Longitudinal Trajectories of Brain Volume and Cortical Thickness in Treated and Untreated Primary Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2018, 67, 1697–1704. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Grant, P.E.; Gonzalez, R.G. Diffusion-weighted MR imaging of the brain. Radiology 2000, 217, 331–345. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Kato, T.; Watanabe, D.; Fukumoto, M.; Wada, K.; Oishi, N.; Nakakura, T.; Kuriyama, K.; Shirasaka, T.; Murai, T. Altered white matter microstructure and neurocognitive function of HIV-infected patients with low nadir CD4. J. Neurovirol. 2022, 28, 355–366. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Lv, J.; Jiang, X.; Guo, L.; Liu, T. Characterizing and differentiating task-based and resting state fMRI signals via two-stage sparse representations. Brain Imaging Behav. 2016, 10, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Speck, O.; Miller, E.N.; Braun, J.; Jovicich, J.; Koch, C.; Itti, L.; Ernst, T. Neural correlates of attention and working memory deficits in HIV patients. Neurology 2001, 57, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Meade, C.S.; Cordero, D.M.; Hobkirk, A.L.; Metra, B.M.; Chen, N.-K.; Huettel, S.A. Compensatory activation in fronto-parietal cortices among HIV-infected persons during a monetary decision-making task. Hum. Brain Mapp. 2016, 37, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Qiu, X.; Wang, L.; Ma, Q.; Mapstone, M.; Luque, A.; Weber, M.; Tivarus, M.; Miller, E.; Arduino, R.C.; et al. Combination antiretroviral therapy improves cognitive performance and functional connectivity in treatment-naïve HIV-infected individuals. J. Neurovirol. 2017, 23, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, S.; Cercignani, M.; Mora-Peris, B.; Underwood, J.; Alagaratnam, J.; Bozzali, M.; Boffito, M.; Nelson, M.; Winston, A.; Vera, J.H. Changes in functional connectivity in people with HIV switching antiretroviral therapy. J. Neurovirol. 2020, 26, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Abidin, A.Z.; Dsouza, A.M.; Nagarajan, M.B.; Wang, L.; Qiu, X.; Schifitto, G.; Wismüller, A. Alteration of brain network topology in HIV-associated neurocognitive disorder: A novel functional connectivity perspective. Neuroimage Clin. 2018, 17, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Henrard, S.; Trotta, N.; Rovai, A.; Coolen, T.; Slama, H.; Bertels, J.; Puttaert, D.; Goffard, J.-C.; Van Vooren, J.-P.; Goldman, S.; et al. Impact of Human Immunodeficiency Virus and Recreational Drugs on Cognitive Functions. Clin. Infect. Dis. 2023, 76, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Milanini, B.; Valcour, V. Differentiating HIV-Associated Neurocognitive Disorders From Alzheimer’s Disease: An Emerging Issue in Geriatric NeuroHIV. Curr. HIV/AIDS Rep. 2017, 14, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, A.M.; Phan, T.; Orlando, D.; Lyu, I.; Kang, H.; Landman, B.; Darby, R.R.; Alzheimer’s Disease Neuroimaging Initiative. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain 2020, 143, 1249–1260. [Google Scholar] [CrossRef]
- Nichols, M.J.; Gates, T.M.; Soares, J.R.; Moffat, K.J.; Rae, C.D.; Brew, B.J.; Cysique, L.A. Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS 2019, 33, 55–66. [Google Scholar] [CrossRef]
- Sakaie, K.; Koenig, K.; Lerner, A.; Appleby, B.; Ogrocki, P.; Pillai, J.A.; Rao, S.; Leverenz, J.B.; Lowe, M.J. Multi-shell diffusion MRI of the fornix as a biomarker for cognition in Alzheimer’s disease. Magn. Reson. Imaging 2024, 109, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.V.; Gazerani, P.; Duan, Y.; Michel, T.M.; Vafaee, M.S. The role of multimodal MRI in mild cognitive impairment and Alzheimer’s disease. J. Neuroimaging 2022, 32, 148–157. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Howdle, G.C.; Quidé, Y.; Kassem, M.S.; Johnson, K.; Rae, C.D.; Brew, B.J.; Cysique, L.A. Brain amyloid in virally suppressed HIV-associated neurocognitive disorder. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e739. [Google Scholar] [CrossRef]
- Mohamed, M.; Skolasky, R.L.; Zhou, Y.; Ye, W.; Brasic, J.R.; Brown, A.; Pardo, C.A.; Barker, P.B.; Wong, D.F.; Sacktor, N. Beta-amyloid (Aβ) uptake by PET imaging in older HIV+ and HIV- individuals. J. Neurovirol. 2020, 26, 382–390. [Google Scholar] [CrossRef]
- Vera, J.H.; Eftychiou, N.; Schuerer, M.; Rullmann, M.; Barthel, H.; Sabri, O.; Gisslen, M.; Zetterberg, H.; Blennow, K.; O’Brien, C.; et al. Clinical Utility of β-Amyloid PET Imaging in People Living With HIV With Cognitive Symptoms. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 87, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, V.; Troili, F.; Giubilei, F. Vascular dementia. Diagnosis and Management in Dementia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–32. [Google Scholar] [CrossRef]
- Cornea, A.; Lata, I.; Simu, M.; Rosca, E.C. Assessment and Diagnosis of HIV-Associated Dementia. Viruses 2023, 15, 378. [Google Scholar] [CrossRef]
- Cysique, L.A.; Brew, B.J. Vascular cognitive impairment and HIV-associated neurocognitive disorder: A new paradigm. J. Neurovirol. 2019, 25, 710–721. [Google Scholar] [CrossRef]
- Murray, K.D.; Uddin, N.; Tivarus, M.E.; Sahin, B.; Wang, H.Z.; Singh, M.V.; Qiu, X.; Wang, L.; Spincemaille, P.; Wang, Y.; et al. Increased risk for cerebral small vessel disease is associated with quantitative susceptibility mapping in HIV infected and uninfected individuals. Neuroimage Clin. 2021, 32, 102786. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr Opin HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Gisslen, M.; Keating, S.M.; Spudich, S.; Arechiga, V.; Stephenson, S.; Zetterberg, H.; Di Germanio, C.; Blennow, K.; Fuchs, D.; Hagberg, L.; et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS ONE 2021, 16, e0250987. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Gianella, S.; Burdo, T.H.; Cinque, P.; Gisslen, M.; Letendre, S.; Nath, A.; Morgello, S.; Ndhlovu, L.C.; Spudich, S. Biotypes of Central Nervous System Complications in People with Human Immunodeficiency Virus: Virology, Immunology, and Neuropathology. J. Infect. Dis. 2023, 227, S3–S15. [Google Scholar] [CrossRef]
- Hellmuth, J.; Slike, B.M.; Sacdalan, C.; Best, J.; Kroon, E.; Phanuphak, N.; Fletcher, J.L.K.; Prueksakaew, P.; Jagodzinski, L.L.; Valcour, V.; et al. Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated With Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J. Infect. Dis. 2019, 220, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Price, R.W.; Spudich, S. Antiretroviral therapy and central nervous system HIV type 1 infection. J. Infect. Dis. 2008, 197, S294–S306. [Google Scholar] [CrossRef] [PubMed]
- Edén, A.; Marcotte, T.D.; Heaton, R.K.; Nilsson, S.; Zetterberg, H.; Fuchs, D.; Franklin, D.; Price, R.W.; Grant, I.; Letendre, S.L.; et al. Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS ONE 2016, 11, e0157160. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, R.T.; Williams, D.W.; Shirk, E.N.; Abreu, C.M.; Ferreira, E.A.; Coughlin, J.M.; Brown, T.T.; Maki, P.M.; Anastos, K.; Berman, J.W.; et al. Higher circulating intermediate monocytes are associated with cognitive function in women with HIV. JCI Insight 2021, 6, e146215. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Cinque, P.; Gisslen, M.; Brew, B.J.; Spudich, S.; Bestetti, A.; Price, R.W.; Fuchs, D. Cerebrospinal fluid neopterin: An informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010, 7, 15. [Google Scholar] [CrossRef]
- Bandera, A.; Taramasso, L.; Bozzi, G.; Muscatello, A.; Robinson, J.A.; Burdo, T.H.; Gori, A. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front. Aging Neurosci. 2019, 11, 187. [Google Scholar] [CrossRef]
- Amirayan-Chevillard, N.; Tissot-Dupont, H.; Obadia, Y.; Gallais, H.; Mege, J.L.; Capo, C. Highly active antiretroviral therapy (HAART) and circulating markers of immune activation: Specific effect of HAART on neopterin. Clin. Diagn. Lab. Immunol. 2000, 7, 832–834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brew, B.J.; Dunbar, N.; Pemberton, L.; Kaldor, J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J. Infect. Dis. 1996, 174, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.S.; Lyles, R.H.; Graham, N.M.H.; Tassoni, C.J.; Margolick, J.B.; Phair, J.P.; Rinaldo, C.; Saah, R.D.A.; Bilello, J.; Detels, R.; et al. Predicting clinical progression or death in subjects with early-stage human immunodeficiency virus (HIV) infection: A comparative analysis of quantification of HIV RNA, soluble tumor necrosis factor type II receptors, neopterin, and beta2-microglobulin. J. Infect. Dis. 1997, 176, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Yiannoutsos, C.T.; Fuchs, D.; Price, R.W.; Crozier, K.; Hagberg, L.; Spudich, S.; Gisslén, M. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J. Neuroinflamm. 2013, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, D.A.; Arfanakis, K.; Leurgans, S.; Keating, S.M.; Lamar, M.; Bennett, D.A.; Adeyemi, O.M.; Barnes, L.L. Neopterin is associated with hippocampal subfield volumes and cognition in HIV. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e467. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; A Robinson, J.; Cooley, S.; Smith, M.D.; Flynn, J.; Petersen, K.J.; Nelson, B.; Westerhaus, E.; Wisch, J.; Ances, B.M. Increased peripheral inflammation is associated with structural brain changes and reduced blood flow in virologically controlled people with HIV. J. Infect. Dis. 2023, 18, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Monnig, M.A.; Kahler, C.W.; Cioe, P.A.; Monti, P.M.; Mayer, K.H.; Pantalone, D.W.; Cohen, R.A.; Ramratnam, B. Markers of Microbial Translocation and Immune Activation Predict Cognitive Processing Speed in Heavy-Drinking Men Living with HIV. Microorganisms 2017, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; Uno, H.; Ancuta, P.; Kamat, A.; Moore, D.J.; Singer, E.J.; Morgello, S.; Gabuzda, D. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J. Acquir. Immune Defic. Syndr. 2011, 57, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.; Lyons, J.L.; Misra, V.; Uno, H.; Morgello, S.; Singer, E.J.; Gabuzda, D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J. Acquir. Immune Defic. Syndr. 2012, 60, 234–243. [Google Scholar] [CrossRef]
- Schrier, R.D.; Hong, S.; Crescini, M.; Ellis, R.; Pérez-Santiago, J.; Spina, C.; Letendre, S.; for the HNRP Group. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS ONE 2015, 10, e0116526. [Google Scholar] [CrossRef]
- Jumare, J.M.; Akolo, C.M.; Ndembi, N.; Bwala, S.M.F.; Alabi, P.M.F.; Okwuasaba, K.M.; Adebiyi, R.; Umlauf, A.; Cherner, M.; Abimiku, A.; et al. Elevated Plasma Levels of sCD14 and MCP-1 Are Associated with HIV Associated Neurocognitive Disorders Among Antiretroviral-Naive Individuals in Nigeria. J. Acquir. Immune Defic. Syndr. 2020, 84, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Lo, J.; Abbara, S.; Wei, J.; DeLelys, M.E.; Preffer, F.; Rosenberg, E.S.; Williams, K.C.; Grinspoon, S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 2011, 204, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.J.; Nielsen, M.J.; Maniecki, M.B.; Madsen, M.; Moestrup, S.K. Soluble macrophage-derived CD163, A homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology 2010, 215, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Bryant, C.; Montero, M.; Creegan, M.; Slike, B.; Krebs, S.J.; Ratto-Kim, S.; Valcour, V.; Sithinamsuwan, P.; Chalermchai, T.; et al. Monocyte and CD4+ T-cell antiviral and innate responses associated with HIV-1 inflammation and cognitive impairment. AIDS 2020, 34, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Weiffenbach, A.; Woods, S.P.; Letendre, S.; Ellis, R.J.; Williams, K.C. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013, 27, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Avison, M.J.; Nath, A.; Greene-Avison, R.; A Schmitt, F.; A Bales, R.; Ethisham, A.; Greenberg, R.N.; Berger, J.R. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J. Neurovirol. 2004, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kelder, W.; McArthur, J.C.; Nance-Sproson, T.; McClernon, D.; Griffin, D.E. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann. Neurol. 1998, 44, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, T.D.; for the CHARTER Group; Deutsch, R.; Michael, B.D.; Franklin, D.; Cookson, D.R.; Bharti, A.R.; Grant, I.; Letendre, S.L. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J. Neuroimmune Pharmacol. 2013, 8, 1123–1135. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, A.; Qiao, L.; Sheng, B.; Xu, M.; Li, W.; Chen, D. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. BioMed Res. Int. 2015, 2015, 506872. [Google Scholar] [CrossRef]
- Hong, S.; Banks, W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2015, 45, 1–12. [Google Scholar] [CrossRef]
- Williams, D.W.; Byrd, D.; Rubin, L.H.; Anastos, K.; Morgello, S.; Berman, J.W. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e36. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, P.; He, J.J. HIV/neuroAIDS biomarkers. Prog. Neurobiol. 2017, 157, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Abdulle, S.; Mellgren, A.; Brew, B.J.; Cinque, P.; Hagberg, L.; Price, R.W.; Rosengren, L.; Gisslén, M. CSF neurofilament protein (NFL)—A marker of active HIV-related neurodegeneration. J. Neurol. 2007, 254, 1026–1032. [Google Scholar] [CrossRef]
- McGuire, J.L.; Gill, A.J.; Douglas, S.D.; Kolson, D.L. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J. Neurovirol. 2015, 21, 439–448. [Google Scholar] [CrossRef]
- Alagaratnam, J.; von Widekind, S.; De Francesco, D.; Underwood, J.; Edison, P.; Winston, A.; Zetterberg, H.; Fidler, S. Correlation between CSF and blood neurofilament light chain protein: A systematic review and meta-analysis. BMJ Neurol. Open 2021, 3, e000143. [Google Scholar] [CrossRef]
- Gisslén, M.; Price, R.W.; Andreasson, U.; Norgren, N.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Spudich, S.; Blennow, K.; Zetterberg, H. Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3, 135–140. [Google Scholar] [CrossRef]
- Anderson, A.M.; Easley, K.A.; Kasher, N.; Franklin, D.; Heaton, R.K.; Zetterberg, H.; Blennow, K.; Gisslen, M.; Letendre, S.L. Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J. Neurovirol. 2018, 24, 695–701. [Google Scholar] [CrossRef]
- Krut, J.J.; Mellberg, T.; Price, R.W.; Hagberg, L.; Fuchs, D.; Rosengren, L.; Nilsson, S.; Zetterberg, H.; Gisslén, M. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS ONE 2014, 9, e88591. [Google Scholar] [CrossRef]
- Anesten, B.; Yilmaz, A.; Hagberg, L.; Zetterberg, H.; Nilsson, S.; Brew, B.J.; Fuchs, D.; Price, R.W.; Gisslén, M. Blood-brain barrier integrity, intrathecal immunoactivation, and neuronal injury in HIV. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e300. [Google Scholar] [CrossRef]
- Mellgren, A.; Price, R.W.; Hagberg, L.; Rosengren, L.; Brew, B.J.; Gisslén, M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology 2007, 69, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Bogerts, B.; Schroeter, M.L.; Bernstein, H.-G. S100B protein in neurodegenerative disorders. Clin. Chem. Lab. Med. 2011, 49, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wei, F.; Zhang, X.; Guo, X.; Lu, X.; Su, B.; Zhang, T.; Wu, H.; Chen, D. Intercellular Adhesion Molecular-5 as Marker in HIV Associated Neurocognitive Disorder. Aging Dis. 2017, 8, 250–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Green, A.J.; Giovannoni, G.; Miller, R.F.; Harrison, M.J.; Thompson, E.J. Cerebrospinal fluid S-100b concentrations in patients with HIV infection. AIDS 1999, 13, 139–140. [Google Scholar]
- Guha, D.; Mukerji, S.S.; Chettimada, S.; Misra, V.; Lorenz, D.R.; Morgello, S.; Gabuzda, D. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS 2019, 33, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Abassi, M.; Morawski, B.M.; Nakigozi, G.; Nakasujja, N.; Kong, X.; Meya, D.B.; Robertson, K.; Gray, R.; Wawer, M.J.; Sacktor, N.; et al. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J. Neurovirol. 2017, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Limpruttidham, N.; Mitchell, B.I.; Kallianpur, K.J.; Nakamoto, B.K.; Souza, S.A.; Shiramizu, B.; Ndhlovu, L.C.; Chow, D.C.; Shikuma, C.M. S100B and its association with HIV-associated neurocognitive disorders. J. Neurovirol. 2019, 25, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal fluid immune markers and HIV-associated neurocognitive impairments: A systematic review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef]
- Williams, M.E.; Ipser, J.C.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. The Association of Immune Markers with Cognitive Performance in South African HIV-Positive Patients. J. Neuroimmune Pharmacol. 2019, 14, 679–687. [Google Scholar] [CrossRef]
- Bloch, M.; John, M.; Smith, D.; Rasmussen, T.; Wright, E. Managing HIV-associated inflammation and ageing in the era of modern ART. HIV Med. 2020, 21, 2–16. [Google Scholar] [CrossRef]
- Longino, A.A.; Paul, R.; Wang, Y.; Lama, J.R.; Brandes, P.; Ruiz, E.; Correa, C.; Keating, S.; Spudich, S.S.; Pilcher, C.; et al. HIV Disease Dynamics and Markers of Inflammation and CNS Injury During Primary HIV Infection and Their Relationship to Cognitive Performance. JAIDS J. Acquir. Immune Defic. Syndr. 2022, 89, 183–190. [Google Scholar] [CrossRef]
- Ruhanya, V.; Jacobs, G.B.; Naidoo, S.; Paul, R.H.; Joska, J.A.; Seedat, S.; Nyandoro, G.; Engelbrecht, S.; Glashoff, R.H. Impact of Plasma IP-10/CXCL10 and RANTES/CCL5 Levels on Neurocognitive Function in HIV Treatment-Naive Patients. AIDS Res. Hum. Retroviruses 2021, 37, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, R.; Umlauf, A.; Marcotte, T.D.; Soontornniyomkij, B.; Diaconu, C.C.; Bulacu-Talnariu, A.; Temereanca, A.; Ruta, S.M.; Letendre, S.; Ene, L.; et al. Plasma CXCL10 correlates with HAND in HIV-infected women. J. Neurovirol. 2020, 26, 23–31. [Google Scholar] [CrossRef]
- Chilunda, V.; Calderon, T.M.; Martinez-Aguado, P.; Berman, J.W. The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era. Brain Res. 2019, 1724, 146426. [Google Scholar] [CrossRef] [PubMed]
- Saloner, R.; Sun-Suslow, N.; Morgan, E.E.; Lobo, J.; Cherner, M.; Ellis, R.J.; Heaton, R.K.; Grant, I.; Letendre, S.L.; Iudicello, J.E. Plasma biomarkers of vascular dysfunction uniquely relate to a vascular-risk profile of neurocognitive deficits in virally-suppressed adults with HIV. Brain Behav. Immun. Health 2022, 26, 100560. [Google Scholar] [CrossRef]
- Raghav, A.; Singh, M.; Jeong, G.-B.; Giri, R.; Agarwal, S.; Kala, S.; Gautam, K.A. Extracellular vesicles in neurodegenerative diseases: A systematic review. Front. Mol. Neurosci. 2022, 15, 1061076. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Mocchetti, I. Extracellular Vesicles and HIV-Associated Neurocognitive Disorders: Implications in Neuropathogenesis and Disease Diagnosis. Neurotox Res. 2021, 39, 2098–2107. [Google Scholar] [CrossRef]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef]
- de Menezes, E.G.M.; Liu, J.S.; Bowler, S.A.; Giron, L.B.; D’antoni, M.L.; Shikuma, C.M.; Abdel-Mohsen, M.; Ndhlovu, L.C.; Norris, P.J. Circulating brain-derived extracellular vesicles expressing neuroinflammatory markers are associated with HIV-related neurocognitive impairment. Front. Immunol. 2022, 13, 1033712. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Sun, B.; Dalvi, P.; Abadjian, L.; Tang, N.; Pulliam, L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 2017, 31, F9–F17. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, D.; Bukrinsky, M. Neuro-HIV—New insights into pathogenesis and emerging therapeutic targets. FASEB J. 2023, 37, e23301. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.; For the German Association of Neuro-AIDS und Neuro-Infectiology (DGNANI); Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; et al. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, K.; Evering, T.H.; Anderson, A.M.; Ragin, A.; Monaco, C.L.; Gavegnano, C.; Avery, R.J.; Rourke, S.B.; Cysique, L.A.; Brew, B.J. The comorbidity of depression and neurocognitive disorder in persons with HIV infection: Call for investigation and treatment. Front. Cell Neurosci. 2023, 17, 1130938. [Google Scholar] [CrossRef] [PubMed]
- EACS Guidelines Version 12.0; European AIDS Clinical Society (EACS): Brussels, Belgium, 2023.
- Boerwinkle, A.H.; Meeker, K.L.; Luckett, P.; Ances, B.M. Neuroimaging the Neuropathogenesis of HIV. Curr. HIV/AIDS Rep. 2021, 18, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.; Spudich, S. Cognitive disorders in people living with HIV. Lancet HIV 2020, 7, e504–e513. [Google Scholar] [CrossRef] [PubMed]
- Swanta, N.; Aryal, S.; Nejtek, V.; Shenoy, S.; Ghorpade, A.; Borgmann, K. Blood-based inflammation biomarkers of neurocognitive impairment in people living with HIV. J. Neurovirol. 2020, 26, 358–370. [Google Scholar] [CrossRef]
- Yilmaz, A.; Blennow, K.; Hagberg, L.; Nilsson, S.; Price, R.W.; Schouten, J.; Spudich, S.; Underwood, J.; Zetterberg, H.; Gisslén, M. Neurofilament light chain protein as a marker of neuronal injury: Review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev. Mol. Diagn. 2017, 17, 761–770. [Google Scholar] [CrossRef]
| HAND Category | NP Criteria | Functioning |
|---|---|---|
| ANI | ≥1 SD below adjusted norms in ≥2 cognitive domains | No impairment |
| MND | ≥1 SD below adjusted norms in ≥2 cognitive domains | Mild impairment in everyday functioning |
| HAD | ≥2 SD below adjusted norms in ≥2 cognitive domains | Severe impairment in everyday functioning |
| NP evaluation: assessment of ≥5 domains, preferably by two tests each, including: attention/working memory, language/verbal fluency, abstraction/executive skills, motor skills, memory (learning/recall), visuospatial perception, speed of information processing | ||
| Functioning: self-report, proxy report from a significant other or caregiver, performance-based assessment of everyday functioning [17] | ||
| Exclusion criteria: delirium, psychiatric illness, psychoactive substance use, alcohol use disorder, opportunistic CNS infections | ||
| Frascati Criteria (HAND) (2007) [22] | Nightingale et al. (2023) [11] | |
|---|---|---|
| Purpose | Research | Research and clinical |
| NCI classification | Not impaired, ANI, MND, HAD. Based on neuropsychological criteria and exclusion of confounding conditions. | ANI is changed to ‘low cognitive performance’ and is not considered NCI per se. Clinical history and/or neurological investigations in addition to low cognitive performance are required for an NCI diagnosis. |
| Symptom definition | Interference with daily functioning. | Changes in cognition, self-reported or by proxy, independently of daily functioning. |
| Pathogenesis attributed to HIV | In relation to incidental, contributing, and confounding factors. Presence of a confounding condition precludes HAND diagnosis. | Defines HIV-associated brain injury (HABI) for direct HIV neuropathogenesis. HABI is defined in relation to HIV suppression. HABI in individuals with plasma HIV-RNA suppression may be legacy (pretreatment) or active. |
| NP data interpretation | Based on quantitative deviation from normative NP data. | Favors use of NP performance as a continuous variable, assessed longitudinally, rather than a binary cut-off. False-classification rate of NP battery should be considered. |
| Imaging Modality | Findings | Clinical/Biological Correlates | References |
|---|---|---|---|
| MRI | [60,61,62,63,64,65,66,67] | ||
| Morphometry/Volumetry | Reduced size of basal ganglia, caudate nucleus, corpus callosum, hippocampus Reduced size in sensorimotor and supplementary motor cortex expansion of lateral ventricles | Correlates with NP performance, especially MND and HAD GM reduction correlates with nadir CD4+ hippocampal and thalamic volumes correlate with current CD4+ Regional atrophy correlates with HIV RNA, CSF HIV RNA, PBMC HIV DNA | |
| DTI | Lower FA, higher MD (especially WM: subcortex, corpus callosum [splenium and/or genu], centrum hemiovale) | Correlation with severity of dementia and specific cognitive domains, apathy, differentiation from aging brain Correlation with BBB disruption Correlation with plasma VEGF, MIP-1α, MIP-1β, MCP-1, sCD14 | [68,69,70,71,72,73,74,75,76] |
| fMRI | [77,78,79,80,81,82,83,84,85] | ||
| task-based | Reduced activity of attention networks and Increased recruitment of adjacent areas to meet attention task demands Reduced cerebral blood flow during visual tasks | Differentiation HAND vs. non-HAND vs. seronegative, correlation with NP assessment | |
| resting-state | Decreased FC in cortico-striatal networks, precuneus, prefrontal region Decreased FC in executive, salient, and default mode networks | Improves with cART initiation | |
| MRS | Increased choline and MI in frontal WM and basal ganglia due to neuroinflammation and microglia activation (early) Reduced NAA due to loss of neuron integrity (later stages) Reduced Glx due to excitotoxicity | Correlation with NP assessment Correlation with MCP-1, sCD14, IP-10 Improvement with cART initiation, CD4+ Increase is associated with improvement of MRS findings | [86,87,88,89,90,91,92,93,94] |
| PET | [95,96,97,98,99,100,101,102] | ||
| FDG-PET | Increased glucose uptake in basal ganglia and thalamus, reversed with cART, eventually hypometabolism (legacy effect) | FDG PET improves with cART initiation, overlaps with CVD effects | |
| TSPO PET | Conflicting results: higher binding of ligand in diverse brain regions, others found no differences between HIV+ and HIVȒ | TSPO PET correlates with NP assessment in specific domains |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschopoulos, C.D.; Stanitsa, E.; Protopapas, K.; Kavatha, D.; Papageorgiou, S.G.; Antoniadou, A.; Papadopoulos, A. Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review. Life 2024, 14, 508. https://doi.org/10.3390/life14040508
Moschopoulos CD, Stanitsa E, Protopapas K, Kavatha D, Papageorgiou SG, Antoniadou A, Papadopoulos A. Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review. Life. 2024; 14(4):508. https://doi.org/10.3390/life14040508
Chicago/Turabian StyleMoschopoulos, Charalampos D., Evangelia Stanitsa, Konstantinos Protopapas, Dimitra Kavatha, Sokratis G. Papageorgiou, Anastasia Antoniadou, and Antonios Papadopoulos. 2024. "Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review" Life 14, no. 4: 508. https://doi.org/10.3390/life14040508
APA StyleMoschopoulos, C. D., Stanitsa, E., Protopapas, K., Kavatha, D., Papageorgiou, S. G., Antoniadou, A., & Papadopoulos, A. (2024). Multimodal Approach to Neurocognitive Function in People Living with HIV in the cART Era: A Comprehensive Review. Life, 14(4), 508. https://doi.org/10.3390/life14040508








