Oxidative Stress in Military Missions—Impact and Management Strategies: A Narrative Analysis
Abstract
:1. Introduction
1.1. Contextualization
1.2. Central Issue
1.3. Purpose of the Review
2. Materials and Methods
Research Questions and Hypotheses
3. Theoretical Foundations
3.1. Physical Exercise and Oxidative Stress
3.2. Diet and Oxidative Stress
3.3. Antioxidants
3.4. Antioxidant Supplements: Panacea, Harmful, or Neutral?
3.5. Nutritional Supplements: Synergies and Divergences with Antioxidants
4. Discussion
4.1. The Impact of Physical Exercise on Oxidative Stress in Military Missions
4.2. The Influence of the Ketogenic Diet in Optimizing Performance and Regulating Oxidative Stress in Military Missions
4.3. Efficacy and Limitations of Antioxidants in a Military Context
4.4. Antioxidant Supplements: A Critical Analysis of the Benefits and Risks for Military Personnel
4.5. Interactions and Implications of Nutritional Supplements in Combating Military Oxidative Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaisman, M.; Ponce, T.; Barros, T.R.D.; Salerno, V.P.; Mainenti, M.R.M. A systematic review of hormone levels, biomarkers of cellular injury and oxidative stress in multi-stressor military field training exercises. Arch. Endocrinol. Metab. 2022, 66, 382–392. [Google Scholar]
- Różański, P.; Jówko, E.; Tomczak, A. Assessment of the Levels of Oxidative Stress, Muscle Damage, and Psychomotor Abilities of Special Force Soldiers during Military Survival Training. Int. J. Environ. Res. Public Health 2020, 17, 4886. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, S.J.; Vermetten, E.; Elbert, G. Long-term development of post-traumatic stress symptoms and associated risk factors in military service members deployed to Afghanistan: Results from the PRISMO 10-year follow-up. Eur. Psychiatry 2020, 64, e10. [Google Scholar] [CrossRef] [PubMed]
- Chinoy, E.D.; Carey, F.R.; Kolaja, C.A.; Jacobson, I.G.; Cooper, A.D.; Markwald, R.R. The bi-directional relationship between post-traumatic stress disorder and obstructive sleep apnea and/or insomnia in a large U.S. military cohort. Sleep Health 2022, 8, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of oxidative stress in retinal disease and the early intervention strategies: A review. Oxid. Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative stress and vascular dysfunction in the retina: Therapeutic strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef]
- Shukla, S.; Mbingwa, G.; Khanna, S.; Dalal, J.; Sankhyan, D.; Malik, A.; Badhwar, N. Environment and health hazards due to military metal pollution: A review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100857. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Xia, B.; Xiong, Z.; Zhang, S.; Tang, W. Health Effects of Particulate Uranium Exposure. Toxics 2022, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Darchini-Maragheh, E.; Balali-Mood, M.; Malaknezhad, M.; Mousavi, S.R. Progressive delayed respiratory complications of sulfur mustard poisoning in 43 iranian veterans, three decades after exposure. Hum. Exp. Toxicol. 2018, 37, 175–184. [Google Scholar] [CrossRef]
- Kiani, B.; Hashemi Amin, F.; Bagheri, N.; Bergquist, R.; Mohammadi, A.A.; Yousefi, M.; Faraji, H.; Roshandel, G.; Beirami, S.; Rahimzadeh, H.; et al. Association between heavy metals and colon cancer: An ecological study based on geographical information systems in North-Eastern Iran. BMC Cancer 2021, 21, 414. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc Essentiality, Toxicity, and its Bacterial Bioremediation: A Comprehensive Insight. Front. Microbiol. 2022, 13, 900740. [Google Scholar] [CrossRef]
- Lisi, V.; Senesi, G.; Balbi, C. Converging protective pathways: Exploring the linkage between physical exercise, extracellular vesicles and oxidative stress. Free Radic. Biol. Med. 2023, 208, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Kang, D.O.; Park, Y.; Lee, J.; Kim, W.; Choi, J.Y.; Roh, S.Y.; Jang, Y.; Park, S.H.; Kim, W.S.; et al. Validation of FRIEND and ACSM equations for cardiorespiratory fitness: Comparison to direct measurement in CAD patients. J. Clin. Med. 2020, 9, 1889. [Google Scholar] [CrossRef]
- Helgerud, J.; Haglo, H.; Hoff, J. Prediction of VO2max from submaximal exercise using the smartphone application myworkout GO: Validation study of a digital health method. JMIR Cardio 2022, 6, e38570. [Google Scholar] [CrossRef]
- Schroeder, E.C.; Franke, W.D.; Sharp, R.L.; Lee, D.C. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomized controlled trial. PLoS ONE 2019, 14, e0210292. [Google Scholar] [CrossRef]
- Haverkamp, B.F.; Wiersma, R.; Vertessen, K.; van Ewijk, H.; Oosterlaan, J.; Hartman, E. Effects of physical activity interventions on cognitive outcomes and academic performance in adolescents and young adults: A meta-analysis. J. Sports Sci. 2020, 38, 2637–2660. [Google Scholar] [CrossRef]
- Lake, S.L.; Guadagni, V.; Kendall, K.D.; Chadder, M.; Anderson, T.J.; Leigh, R.; Rawling, J.M.; Hogan, D.B.; Hill, M.D.; Poulin, M.J. Aerobic exercise training in older men and women—Cerebrovascular responses to submaximal exercise: Results from the Brain in Motion study. Phys. Rep. 2022, 10, e15158. [Google Scholar] [CrossRef]
- Caplin, A.; Chen, F.S.; Beauchamp, M.R.; Puterman, E. The effects of exercise intensity on the cortisol response to a subsequent acute psychosocial stressor. Psychoneuroendocrinology 2021, 131, 105336. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; Van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A.; et al. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018, 159, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Yamada, Y.; Maeda, A.; Izumo, T.; Rogi, T.; Shibata, H.; Fukuda, M.; Arimitsu, T.; Miyamoto, N.; Hashimoto, T. Effects of resistance training intensity on muscle quantity/quality in middle-aged and older people: A randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Sailani, M.R.; Halling, J.F.; Møller, H.D.; Lee, H.; Plomgaard, P.; Pilegaard, H.; Snyder, M.P.; Regenberg, B. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci. Rep. 2019, 9, 3272. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical exercise: A novel tool to protect mitochondrial health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef]
- Matta, L.; de Faria, C.C.; De Oliveira, D.F.; Andrade, I.S.; Lima-Junior, N.C.; Gregório, B.M.; Takiya, C.M.; Ferreira, A.C.F.; Nascimento, J.H.M.; de Carvalho, D.P.; et al. Exercise improves redox homeostasis and mitochondrial function in white adipose tissue. Antioxidants 2022, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, K.; Jamurtas, A.Z.; Poulios, A.; Tsimeas, P.; Draganidis, D.; Margaritelis, N.V.; Baloyiannis, I.; Papadopoulos, C.; Sovatzidis, A.; Deli, C.K.; et al. Skeletal muscle and erythrocyte redox status is associated with dietary cysteine intake and physical fitness in healthy young physically active men. Eur. J. Nutr. 2023, 62, 1767–1782. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Militello, R.; Amoresano, A.; Modesti, P.A.; Modesti, A.; Luti, S. Relationships between sex and adaptation to physical exercise in young athletes: A pilot study. Healthcare 2022, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Reis, A.; Maté-Muñoz, J.L.; Hernández-Lougedo, J.; Vilches-Sáez, S.; Benet, M.; García-Fernández, P.; Pleguezuelos, E.; Carbonell, T.; Alva, N.; Garnacho-Castaño, M.V. Aerobic dance on an air dissipation platform improves cardiorespiratory, muscular and cellular fitness in the overweight and obese elderly. Biology 2022, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: A review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Gruenberg, J. Life in the lumen: The multivesicular endosome. Traffic 2020, 21, 76–93. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metabol. 2018, 27, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Alberro, A.; Iparraguirre, L.; Fernandes, A.; Otaegui, D. Extracellular vesicles in blood: Sources, effects, and applications. Int. J. Mol. Sci. 2021, 22, 8163. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef] [PubMed]
- Szivak, T.K.; Kraemer, W.J. Physiological Readiness and Resilience: Pillars of Military Preparedness. J. Strength Cond. Res. 2015, 29 (Suppl. S11), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Wu, Y.C.; Lai, C.H.; Chen, P.C.; Guo, Y.L. Effects of physical fitness training on metabolic syndrome among military personnel in Taiwan. BMJ Mil. Health 2023, 169, e15–e19. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.; Börjesson, M.; Österberg, J. Team Learning in a Multinational Military Staff Exercise. Small Group. Res. 2015, 46, 179–203. [Google Scholar] [CrossRef]
- Manning, F.J.; Fullerton, T.D. Health and well-being in highly cohesive units of the U.S. Army. J. Appl. Soc. Psychol. 1988, 18, 503–519. [Google Scholar] [CrossRef]
- Adler, A.B.; Williams, J.; McGurk, D.; Moss, A.; Bliese, P.D. Resilience training with soldiers during basic combat training: Randomisation by platoon. Appl. Psychol. Health Well Being 2015, 7, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Kyröläinen, H.; Pihlainen, K.; Vaara, J.P.; Ojanen, T.; Santtila, M. Optimising training adaptations and performance in military environment. J. Sci. Med. Sport 2018, 21, 1131–1138. [Google Scholar] [CrossRef]
- Vantarakis, A.; Chatzinikolaou, A.; Avloniti, A.; Vezos, N.; Douroudos, I.I.; Draganidis, D.; Jamurtas, A.Z.; Kambas, A.; Kalligeros, S.; Fatouros, I.G. A 2-Month Linear Periodized Resistance Exercise Training Improved Musculoskeletal Fitness and Specific Conditioning of Navy Cadets. J. Strength Cond. Res. 2017, 31, 1362–1370. [Google Scholar] [CrossRef]
- Jovanov, E.; Lords, A.O.; Raskovic, D.; Cox, P.G.; Adhami, R.; Andrasik, F. Stress monitoring using a distributed wireless intelligent sensor system. IEEE Eng. Med. Biol. Mag. 2003, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, M.M.; Uusitalo, A.L.; Kinnunen, H.; Häkkinen, K.; Kyröläinen, H.; Atalay, M. Association of military training with oxidative stress and overreaching. Med. Sci. Sports Exerc. 2011, 43, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.S.; Morey, M.C.; Beckham, J.C.; Bosworth, H.B.; Sloane, R.; Pieper, C.F.; Pebole, M.M. Warrior Wellness: A Randomized Controlled Pilot Trial of the Effects of Exercise on Physical Function and Clinical Health Risk Factors in Older Military Veterans With PTSD. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.G.; Bhatia, S.K.; Buatti, J.M.; Brandt, K.E.; Lindholm, K.E.; Button, A.M.; Szweda, L.I.; Smith, B.J.; Spitz, D.R.; Fath, M.A. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res. 2013, 19, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based effects of high-intensity interval training on exercise capacity and health: A review with historical perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: New perspectives for neuroprotection in Alzheimer’s disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Kodydkova, J.; Vavrova, L.; Stankova, B.; Macasek, J.; Krechler, T.; Zak, A. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas 2013, 42, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, G.; Biondi, F.; et al. Inflammaging and Cancer: A challenge for the mediterranean diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef]
- Susan, P. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Bone 2005, 23, 1–7. [Google Scholar]
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’Agostino, D.P. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS ONE 2013, 8, e65522. [Google Scholar] [CrossRef] [PubMed]
- Nazarewicz, R.R.; Ziolkowski, W.; Vaccaro, P.S.; Ghafourifar, P. Effect of short-term ketogenic diet on redox status of human blood. Rejuvenation Res. 2007, 10, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Rippy, N.A.; Dorenbos, K.; Concepcion, R.C.; Agarwal, A.K.; Rho, J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004, 55, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.R.; Bell, D.A. Identification of novel NRF2-regulated genes by ChiP-Seq: Influence on retinoid X receptor alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Le Moan, N.; Grueter, C.A.; Lim, H.; Laura, R.; Stevens, R.D.; Newgard, C.B.; et al. Supression of oxidative stress and β-OHB as endogenous histone deactetylase. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Milder, J.B.; Liang, L.P.; Patel, M. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008, 106, 1044–1051. [Google Scholar] [CrossRef]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in hippocampus. Front. Cell. Neurosci. 2018, 12, 263. [Google Scholar] [CrossRef]
- Stafford, P.; Abdelwahab, M.G.; Kim, D.Y.; Preul, M.C.; Rho, J.M.; Scheck, A.C. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr. Metab. 2010, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Alhamzah, S.A.; Gatar, O.M.; Alruwaili, N.W. Effects of ketogenic diet on oxidative stress and cancer: A literature review. Adv. Cancer Biol. Metastasis 2023, 7, 100093. [Google Scholar] [CrossRef]
- Xu, T.; Cao, L.; Zhou, M.; Chen, W. The influencing factors of Upin peroxination in the body. Public Health Prev. Med. 2020, 31, 122–125. [Google Scholar]
- Kong, W.; Jiang, T.; Ning, Y.; Guo, Y.; Liu, H.; Lyu, X.; Li, M. Dietary diversity, diet quality, and oxidative stress in older adults. Geriatr. Nurs. 2022, 48, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Liu, Z.; Varma, D.S.; Wan, R.; Zhao, S. Diet diversity and nutritional status among adults in southwest China. PLoS ONE 2017, 12, e0172406. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e8. [Google Scholar] [CrossRef] [PubMed]
- Greco, T.; Glenn, T.C.; Hovda, D.A.; Prins, M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 2016, 36, 1603–1613. [Google Scholar] [CrossRef]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Volek, J.S.; LaFountain, R.A.; Dituro, P. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kyparos, A.; Spanou, C.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S. Redox biology of exercise: An integrative and comparative consideration of some overlooked issues. J. Exp. Biol. 2012, 215 Pt 10, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Margaritelis, N.V.; Kyparos, A.; Nikolaidis, M.G. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic. Biol. Med. 2018, 115, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, Y.; Karagounis, L.G.; Terzis, G.; Jamurtas, A.Z.; Spengos, K.; Tsoukas, D.; Chatzinikolaou, A.; Mandalidis, D.; Stefanetti, R.J.; Papassotiriou, I.; et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013, 98, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Peternelj, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Pļaviņa, L.; Koļesova, O.; Eglīte, J.; Čakstiņš, A.; Cakstina, S.; Koļesovs, A. Antioxidative system capacity after a 10-day-long intensive training course and one-month-long recovery in military cadets. Phys. Act. Rev. 2021, 9, 62–69. [Google Scholar]
- Knapik, J.J.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Dietary Supplement Use in a Large, Representative Sample of the US Armed Forces. J. Acad. Nutr. Diet. 2018, 118, 1370–1388. [Google Scholar] [CrossRef]
- Macedo, R.C.; Vieira, A.; Marin, D.P.; Otton, R. Effects of chronic resveratrol supplementation in military firefighters undergo a physical fitness test--a placebo-controlled, double blind study. Chem. Biol. Interact. 2015, 227, 89–95. [Google Scholar] [CrossRef]
- Meydani, S.N.; Meydani, M.; Blumberg, J.B.; Leka, L.S.; Pedrosa, M.; Diamond, R.; Schaefer, E.J. Assessment of the safety of supplementation with different amounts of vitamin E in healthy older adults. Am. J. Clin. Nutr. 1998, 68, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in Personalized Nutrition and Exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Morrison, D.; McConell, G.K.; Wadley, G.D. Muscle redox signalling pathways in exercise: Role of antioxidants. Free Radic. Biol. Med. 2016, 98, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Calbet, J.A. AMPK signaling in skeletal muscle during exercise: Role of reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2016, 98, 68–77. [Google Scholar] [CrossRef]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Koutsias, S.; Panayiotou, G.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am. J. Clin. Nutr. 2011, 93, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Theodorou, A.A.; Panayiotou, G.; Zafeiridis, A.; Dipla, K.; Nikolaidis, M.G.; Vrabas, I.S. Reductive stress after exercise: The issue of redox individuality. Redox Biol. 2014, 2, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox inter-individual variability. Acta Physiol. 2018, 222, e12898. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Coltell, O.; Mattingley, G.; Sorlí, J.V.; Ordovas, J.M. Utilizing nutritional genomics to tailor diets for the prevention of cardiovascular disease: A guide for upcoming studies and implementations. Expert Rev. Mol. Diagn. 2017, 17, 495–513. [Google Scholar] [CrossRef] [PubMed]
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.J.; Walsh, M.C. The future direction of personalised nutrition: My diet, my phenotype, my genes. Proc. Nutr. Soc. 2013, 72, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hampl, J.S.; Taylor, C.A.; Johnston, C.S. Vitamin C deficiency and depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994. Am. J. Public Health 2004, 94, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Gieger, C.; Hauner, H.; Daniel, H.; Linseisen, J. Metabotyping and its application in targeted nutrition: An overview. Br. J. Nutr. 2017, 117, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: Reversal by vitamin C supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Brigelius-Flohé, R.; Flohé, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011, 15, 2335–2381. [Google Scholar] [CrossRef]
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014, 73, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Principles for integrating reactive species into in vivo biological processes: Examples from exercise physiology. Cell. Signal. 2016, 28, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.R.; Hawley, J.A. Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: Historical advances, current knowledge, and future challenges. Cold Spring Harb. Perspect. Med. 2018, 8, a029686. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; McConell, G.K. Skeletal muscle glucose uptake during exercise: A focus on reactive oxygen species and nitric oxide signaling. IUBMB Life 2009, 61, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. Role of reactive oxygen species in regulation of glucose transport in skeletal muscle during exercise. J. Physiol. 2016, 594, 2787–2794. [Google Scholar] [CrossRef]
- Pinheiro, C.H.; Silveira, L.R.; Nachbar, R.T.; Vitzel, K.F.; Curi, R. Regulation of glycolysis and expression of glucose metabolism-related genes by reactive oxygen species in contracting skeletal muscle cells. Free Radic. Biol. Med. 2010, 48, 953–960. [Google Scholar] [CrossRef]
- Koufen, P.; Stark, G. Free radical induced inactivation of creatine kinase: Sites of interaction, protection, and recovery. Biochim. Biophys. Acta 2000, 1501, 44–50. [Google Scholar] [CrossRef]
- Mitrea, D.R.; Mortazavi Moshkenani, H.; Hoteiuc, O.A.; Bidian, C.; Toader, A.M.; Clichici, S. Antioxidant protection against cosmic radiation-induced oxidative stress at commercial flight altitude. J. Physiol. Pharmacol. 2018, 69, 1–9. [Google Scholar]
- Pfeiffer, J.M.; Askew, E.W.; Roberts, D.E.; Wood, S.M.; Benson, J.E.; Johnson, S.C.; Freedman, M.S. Effect of antioxidant supplementation on urine and blood markers of oxidative stress during extended moderate-altitude training. Wilderness Environ. Med. 1999, 10, 66–74. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Åkerström, T.; Nielsen, S.; Nielsen, A.; Mounier, R.; Mortensen, O.; Lykkesfeldt, J.; Rose, A.; Fischer, C.; Pedersen, B. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Fezeu, L.; Jeandel, C.; Ferry, M.; Andreeva, V.; Amieva, H.; Hercberg, S.; Galan, P. French adults’ cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: A post hoc analysis of the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) trial. Am. J. Clin. Nutr. 2011, 94, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kang, S.W.; Jeong, W.; Chang, T.S.; Yang, K.S.; Woo, H.A. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005, 17, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Many tocopherols, one vitamin, E. Mol. Asp. Med. 2018, 61, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Maras, J.E.; Bermudez, O.I.; Qiao, N.; Bakun, P.J.; Boody-Alter, E.L.; Tucker, K.L. Intake of alpha-tocopherol is limited among US adults. J. Am. Diet. Assoc. 2004, 104, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E inadequacy in humans: Causes and consequences. Adv. Nutr. 2014, 5, 503–514. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.T.; Neuzil, J.; Zingg, J.M.; Azzi, A. The European perspective on vitamin E: Current knowledge and future research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- McDougall, M.; Choi, J.; Kim, H.K.; Bobe, G.; Stevens, J.F.; Cadenas, E.; Tanguay, R.; Traber, M.G. Lethal dysregulation of energy metabolism during embryonic vitamin E deficiency. Free Radic. Biol. Med. 2017, 104, 324–332. [Google Scholar] [CrossRef] [PubMed]
- McDougall, M.; Choi, J.; Truong, L.; Tanguay, R.; Traber, M.G. Vitamin E deficiency during embryogenesis in zebrafish causes lasting metabolic and cognitive impairments despite refeeding adequate diets. Free Radic. Biol. Med. 2017, 110, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Abner, E.L.; Schmitt, F.A.; Mendiondo, M.S.; Marcum, L.; Kryscio, R.J. Vitamin E and all-cause mortality: A meta-analysis. Curr. Aging Sci. 2011, 4, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.K.; Hill, C.L.; Shanahan, E.M.; Taylor, A.W.; Appleton, S.L.; Grant, J.F.; Shi, Z.; Dal Grande, E.; Price, K.; Adams, R.J. Vitamin D levels in an Australian population. BMC Public Health 2014, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Fraser, W.D.; Close, G.L. Vitamin D and the athlete: Emerging insights. Eur. J. Sport Sci. 2015, 15, 73–84. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1019–E1031. [Google Scholar] [CrossRef]
- Owens, D.J.; Tang, J.C.; Bradley, W.J.; Sparks, A.S.; Fraser, W.D.; Morton, J.P.; Close, G.L. Efficacy of high-dose vitamin D supplements for elite athletes. Med. Sci. Sports Exerc. 2017, 49, 349–356. [Google Scholar] [CrossRef]
- Noble, D.; Jablonka, E.; Joyner, M.J.; Müller, G.B.; Omholt, S.W. Evolution evolves: Physiology returns to centre stage. J. Physiol. 2014, 592, 2237–2244. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Stavinoha, T.B.; McGraw, S.M.; White, A.; Hadden, L.S.; Marriott, B.P. Use of dietary supplements among active-duty US Army soldiers. Am. J. Clin. Nutr. 2010, 92, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Attipoe, S.; Costello, R.; Kohlmeier, M.; Deuster, P. Dietary supplement education for the military: An education module for healthcare providers. FASEB J. 2013, 27, 1064.5. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Gramignano, G.; Lusso, M.R.; Serpe, R.; Massa, E.; Astara, G.; Deiana, L. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Weeks, S.R.; McAuliffe, C.L.; Durussel, D.; Pasquina, P.F. Physiological and psychological fatigue in extreme conditions: The military example. PM R 2010, 2, 438–441. [Google Scholar] [CrossRef]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Liu, Y.; Liu, H.Q.; Li, J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J. Food Biochem. 2020, 44, e13140. [Google Scholar] [CrossRef]
- Parastouei, K.; Rostami, H.; Velikova, T.; Alipour, M. Role of nutritional supplements in military personnel: A review article. Int. J. Biomed. Public Health 2018, 1, 155–161. [Google Scholar]
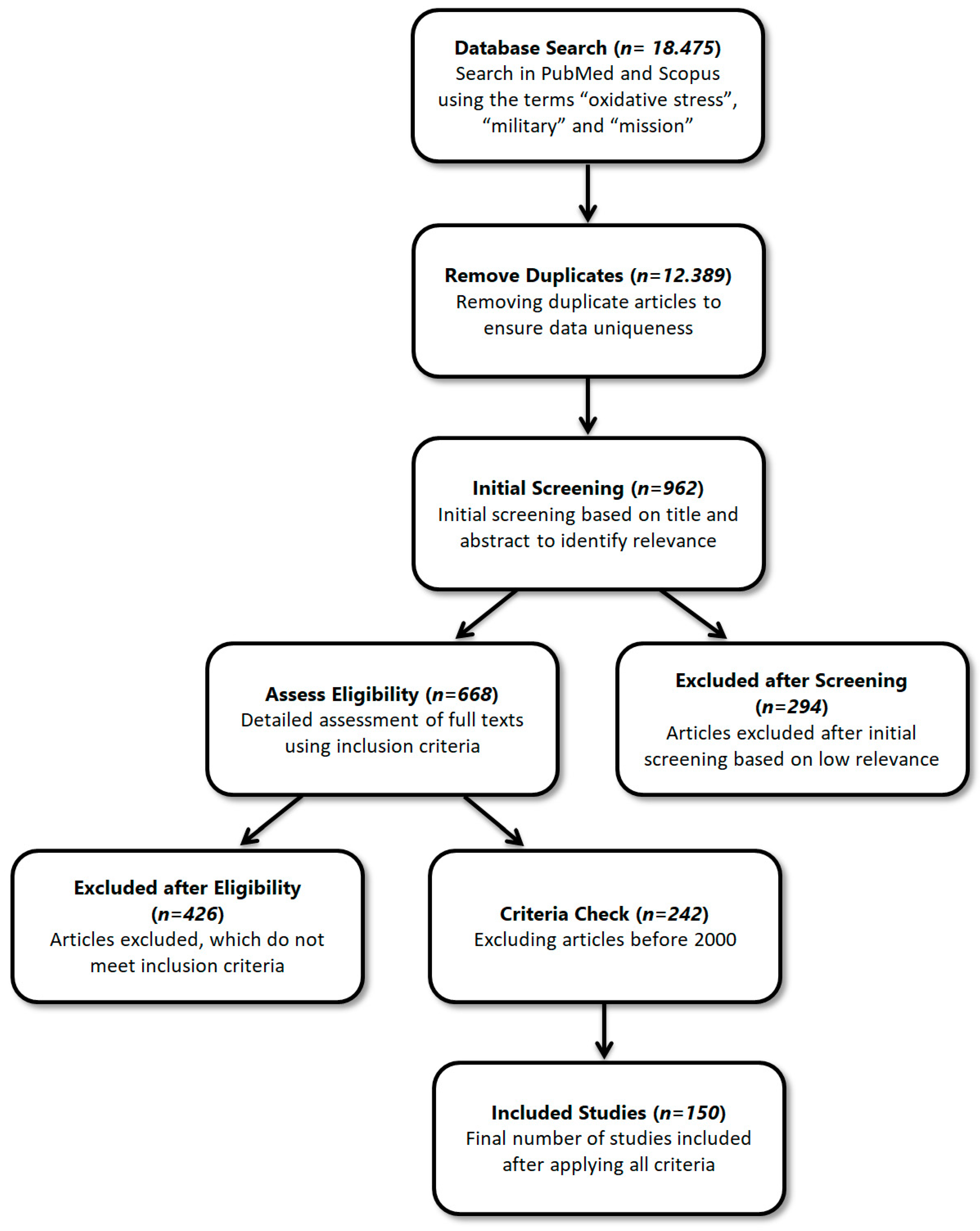
| Nr. | Question of Research | Description |
|---|---|---|
| 1 | Interindividual variability and exercise response | Investigates how individual differences affect the effectiveness of physical exercise in attenuating oxidative stress, highlighting the need for personalized training regimens. |
| 2 | Characteristics of the optimal diet | Explores the features of a diet that maximizes adaptation to extreme oxidative stress, emphasizing the identification of specific diets that offer maximum protection. |
| 3 | Synergy between exercise and nutrition | Analyzes how regular physical exercise and a personalized diet work together to combat oxidative stress, with a focus on the underlying biological mechanisms. |
| 4 | Development of personalized strategies | Proposes the design and implementation of personalized intervention strategies to efficiently respond to the diversity of military personnel needs, recognizing the complexity of individual experiences. |
| Author(s) and Year | Key Findings and Contribution to Physical Exercise |
|---|---|
| Hussain et al. (2022) [13] | Discussed the broad health benefits of physical exercise (EXR), emphasizing its role in improving cardiovascular health and cognitive function, and reducing chronic disease risks. |
| Lisi et al. (2023) [14] | Explored how physical exercise promotes adaptations in extracellular vesicles that help manage oxidative stress, highlighting a novel pathway for its beneficial effects. |
| Fragala et al. (2019) [15] | Outlined the importance of resistance training in enhancing cardiopulmonary fitness and overall health, especially in older adults. |
| Jang et al. (2020) [16] | Validated exercise equations for assessing cardiorespiratory fitness, demonstrating their practical utility in clinical settings. |
| Helgerud et al. (2022) [17] | Demonstrated how submaximal exercises can predict VO2max, crucial for evaluating cardiovascular improvements due to EXR. |
| Schroeder et al. (2019) [18] | Highlighted the cardiovascular benefits of combining different training modalities, contributing to reduced disease risk factors. |
| Haverkamp et al. (2020) [19] | Showed significant cognitive improvements from physical activity, establishing a link between aerobic capacity and cognitive health. |
| Lake et al. (2022) [20] | Discussed the cerebrovascular responses to aerobic training in older adults, emphasizing its role in enhancing brain health and function. |
| Caplin et al. (2021) [21] | Investigated the effects of exercise intensity on stress responses, indicating protective benefits against stress via cortisol regulation. |
| Ryan et al. (2020) [22] | Demonstrated similar benefits of moderate and high-intensity training on insulin sensitivity, relevant for metabolic health. |
| Bobinski et al. (2018) [23] | Discussed the analgesic effects of low-intensity exercise in neuropathic pain management, mediated by interleukin-4. |
| Otsuka et al. (2022) [24] | Reported positive effects of resistance training intensity on muscle quality in older individuals, important for maintaining physical function. |
| Sailani et al. (2019) [25] | Linked lifelong physical activity to beneficial genetic expressions related to metabolism and oxidative stress resistance in aged muscle. |
| Hargreaves and Spriet (2020) [26] | Examined how exercise influences skeletal muscle energy metabolism, crucial for enhancing endurance and performance. |
| Sorriento et al. (2021) [27] | Highlighted the protective role of physical exercise on mitochondrial health, a key factor in cellular energy management and longevity. |
| Steinbacher and Eckl (2015) [28] | Discussed the impact of oxidative stress on exercising muscle, underlining the dual role of exercise in managing oxidative balance. |
| Powers et al. (2020) [29] | Analyzed exercise-induced oxidative stress as both a beneficial and challenging aspect of regular physical activity. |
| Henríquez-Olguin et al. (2019) [30] | Showed how exercise-induced ROS influences glucose uptake during physical activity, linking to metabolic health improvements. |
| Matta et al. (2022) [31] | Investigated the effects of exercise on white adipose tissue, showing improvements in redox homeostasis and mitochondrial function. |
| Baird and Yamamoto (2020) [32] | Explored the molecular mechanisms of the KEAP1-NRF2 pathway in oxidative stress regulation, pivotal for cellular defense during exercise. |
| Papanikolaou et al. (2023) [33] | Connected dietary intake, physical fitness, and redox status, illustrating the holistic impact of exercise and nutrition on health. |
| Pinto et al. (2022) [34] | Investigated gender differences in response to exercise training, important for tailored physical education and training programs. |
| Moreira-Reis et al. (2022) [35] | Demonstrated improvements in cardiovascular and muscular fitness through aerobic dance, emphasizing its benefits for the elderly. |
| Gonzalez-Gil and Elizondo-Montemayor (2020) [36] | Reviewed the complex interactions between different body-produced factors during exercise, enhancing understanding of its systemic benefits. |
| Chow et al. (2022) [37] | Highlighted the role of exerkines in mediating the health benefits of exercise, expanding the understanding of inter-organ communication. |
| Thyfault and Bergouignan (2020) [38] | Discussed the broader metabolic health impacts of exercise, beyond just muscle adaptation, showing its influence on overall metabolic health. |
| Yáñez-Mó et al. (2015) [39] | Described the physiological functions of extracellular vesicles, which play significant roles in cellular communication during exercise. |
| Gruenberg (2020) [40] | Explored the role of multivesicular bodies in cellular processes, relevant to understanding how exercise affects cell biology. |
| Whitham et al. (2018) [41] | Elaborated on how extracellular vesicles mediate tissue crosstalk during exercise, facilitating the systemic health benefits of physical activity. |
| Alberro et al. (2021) [42] | Discussed the sources and effects of extracellular vesicles in blood, highlighting their applications in health and disease contexts influenced by exercise. |
| Hink et al. (2001) [43] | Examined the mechanisms of endothelial dysfunction in diabetes, showing how exercise can positively influence vascular health. |
| Szivak and Kraemer (2015) [44] ** | Emphasized the role of physical exercise in enhancing physiological readiness and resilience, crucial for military preparedness. |
| Chang et al. (2023) [45] ** | Demonstrated that physical fitness programs significantly reduce risk factors for metabolic syndrome among military personnel, underscoring the health benefits of regular exercise in the military. |
| Hedlund et al. (2015) [46] ** | Showed that structured workouts improve communication and cooperation among military units, enhancing team cohesion and effectiveness in operations. |
| Manning and Fullerton (1988) [47] ** | Found that high cohesion in military units, facilitated through joint physical training, leads to improved well-being and satisfaction in military careers. |
| Adler et al. (2015) [48] ** | Reported that resilience training during Basic Combat Training reduces psychological stress and enhances stress management, promoting positive morale among soldiers. |
| Kyröläinen et al. (2018) [49] ** | Highlighted the importance of integrating endurance and strength training to optimize physical performance in military environments. |
| Vantarakis et al. (2017) [50] ** | Found that linear periodized resistance training significantly improves musculoskeletal fitness and specific conditioning needs of naval cadets, emphasizing tailored training benefits. |
| Jovanov et al. (2003) [51] ** | Discussed the use of wireless sensor systems for continuous monitoring of physiological responses during intense military training, aiding in personalized training adjustments. |
| Tanskanen et al. (2011) [52] ** | Studied the association of military training with oxidative stress, highlighting the need for monitoring and managing oxidative stress in training programs. |
| Hall et al. (2020) [53] ** | Demonstrated that physical exercise significantly enhances physical function and reduces clinical risk factors for chronic diseases in older military veterans with PTSD. |
| Author(s) and Year | Key Findings and Contribution to Ketogenic Diet and Oxidative Stress |
|---|---|
| Allen et al. (2013) [54] | Demonstrated that the ketogenic diet enhances oxidative stress responses and therapy outcomes in lung cancer, underscoring its potential for improving mitochondrial metabolism. |
| Atakan et al. (2021) [55] | Identified high-intensity interval training’s enhancement of exercise capacity and health, illustrating how such exercise, potentially alongside ketogenic diets, optimizes mitochondrial metabolism and reduces oxidative stress. |
| Pinto et al. (2018) [56] | Highlighted the antioxidant and anti-inflammatory activities of the ketogenic diet, showing its neuroprotective potential in Alzheimer’s disease through reducing oxidative stress. |
| Reuter et al. (2010) [57] | Explored the links between oxidative stress, inflammation, and cancer, suggesting that the ketogenic diet could mitigate these pathways by efficiently managing oxidative stress. |
| Kodydkova et al. (2013) [58] | Investigated oxidative stress markers in chronic conditions, reinforcing how ketogenic diet-induced adjustments in oxidative balance could protect against cellular damage. |
| Lin and Karin (2007) [59] | Discussed cytokine-mediated links between inflammation and cancer, relevant to how ketogenic diets might modulate inflammation and oxidative stress in clinical settings. |
| Ostan et al. (2015) [60] | Reviewed the impact of diet on inflammaging and cancer, emphasizing the role of ketogenic diets in reducing inflammation-driven oxidative stress. |
| Susan (2005) [61] | Examined how ketogenic diets modulate oxidative stress and mitochondrial function, providing a protective mechanism against tumor development and oxidative damage. |
| Poff et al. (2013) [62] | Showed that the ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer, highlighting the role of ketosis in oxidative stress management. |
| Nazarewicz et al. (2007) [63] | Analyzed the redox status of human blood on a short-term ketogenic diet, noting improvements in antioxidant capacity without inducing oxidative stress. |
| Sullivan et al. (2004) [64] | Demonstrated that the ketogenic diet increases levels and activity of mitochondrial uncoupling proteins, potentially reducing ROS and enhancing mitochondrial health. |
| Tieu et al. (2003) [65] | Discussed the neuroprotective effects of β-Hydroxybutyrate, a ketone body, showing its role in enhancing mitochondrial respiration and reducing oxidative stress through the NRF2 pathway. |
| Chorley et al. (2012) [66] | Identified novel NRF2-regulated genes, indicating how the ketogenic diet might influence antioxidant pathways and detoxification. |
| Shimazu et al. (2013) [67] | Explored the role of β-Hydroxybutyrate in suppressing oxidative stress and acting as a histone deacetylase inhibitor, facilitating protective gene transcription. |
| Yang et al. (2016) [68] | Highlighted how the ketogenic diet protects mitochondrial DNA against oxidative damage, supporting mitochondrial health in an animal study. |
| Jarrett et al. (2008) [69] | Found that the ketogenic diet increases mitochondrial glutathione levels, suggesting its role in enhancing the body’s internal antioxidant system and protecting cells from oxidative stress. |
| Elamin et al. (2018) [70] | Demonstrated that the ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in the hippocampus, indicating its potential protective effects against oxidative and metabolic damage in malignant brain tissue. |
| Stafford et al. (2010) [71] | Showed that the ketogenic diet reverses gene expression patterns and reduces levels of reactive oxygen species in glioma, highlighting its ability to modulate oxidative stress beneficially. |
| Alhamzah et al. (2023) [72] | Reviewed the effects of the ketogenic diet on oxidative stress and cancer, underscoring the need for further research to confirm its anticancer benefits due to the current limitations in controlled studies. |
| Xu et al. (2020) [73] | Explored the direct association between diet and oxidative stress development, pointing out that dietary factors significantly influence oxidative stress, a common pathogenic mechanism in chronic diseases. |
| Kong et al. (2022) [74] | Investigated the link between dietary diversity and oxidative stress in older adults, finding that a higher-quality diet is associated with improved Total Antioxidant Capacity (T-AOC), which indicates a better balance of oxidative stress. |
| Aleksandrova et al. (2021) [75] | Conducted a systematic review of dietary patterns and their impacts on biomarkers of oxidative stress and inflammation, reinforcing the connection between diet diversity and nutritional status in managing oxidative stress. |
| Zhang et al. (2017) [76] | Highlighted the beneficial effects of cherry juice on inflammation and oxidative stress biomarkers, supporting the diet’s capacity to modulate these processes effectively. |
| Newman et al. (2017) [78] | Found that the ketogenic diet reduces mid-life mortality and enhances memory performance in aging mice through mechanisms that include decreased insulin levels and improved mitochondrial efficiency. |
| Greco et al. (2016) [79] | Showed that the ketogenic diet improves mitochondrial function and reduces oxidative stress, contributing to better energy efficiency and the maintenance of muscular integrity under prolonged physical stress. |
| LaFountain et al. (2019) [80] ** | Observed significant health improvements among military personnel on a ketogenic diet, noting reductions in weight, body fat, and enhanced physical performance necessary for training, suggesting overall health and readiness benefits. |
| Volek et al. (2019) [81] ** | Discussed how a ketogenic diet supports the maintenance of physical and cognitive function in challenging conditions, providing a stable and efficient energy source derived from ketone bodies, valuable in scenarios where food access is limited. |
| Author(s) and Year | Key Findings and Contribution to Antioxidants |
|---|---|
| Tan and Norhaizan (2019) [82] | Initially viewed antioxidants as crucial for combating exercise-induced oxidative stress due to their role in managing reactive oxygen species. |
| Nikolaidis et al. (2012) [83] | Highlighted a shift in perspective, suggesting antioxidant supplements could inhibit beneficial adaptations to exercise, such as molecular and physiological improvements. |
| Merry and Ristow (2016) [84] | Observed considerable interindividual variability in redox responses to exercise, influencing the effectiveness of antioxidants as ergogenic aids. |
| Paschalis et al. (2018) [85] | Found that antioxidant benefits, such as improved exercise performance and reduced oxidative stress, were primarily evident in individuals with initially low antioxidant levels. |
| Michailidis et al. (2013) [86] | Proposed a stratified approach to antioxidant supplementation, personalizing it based on individual redox profiles to optimize benefits. |
| Peternelj and Coombes (2011) [87] ** | Discussed the dual role of antioxidants in potentially reducing oxidative stress while possibly interfering with training adaptations like endogenous antioxidant capacity enhancement. |
| Plavina et al. (2021) [88] ** | Demonstrated that antioxidants help manage oxidative stress and aid recovery post-intensive training, emphasizing the importance of recovery periods. |
| Merry and Ristow (2016) [84] ** | Reinforced the notion that while antioxidants can reduce oxidative stress markers, they might also impede desired adaptations such as mitochondrial biogenesis. |
| Knapik et al. (2018) [89] ** | Reported widespread use of antioxidant supplements among military personnel, stressing the need for proper education on their benefits and risks. |
| Macedo et al. (2015) [90] ** | Investigated the effects of resveratrol on military firefighters, noting anti-inflammatory benefits but limited impact on other oxidative stress biomarkers. |
| Meydani et al. (1998) [91] | Explored long-term antioxidant supplementation, finding benefits such as improved immune function and reduced atherosclerosis risk, relevant to military personnel’s long-term health. |
| Author(s) and Year | Key Findings and Contributions on the Role of Antioxidants: Beneficial, Harmful, or Neutral |
|---|---|
| Margaritelis et al. (2018) [92] | Highlighted the beneficial role of reactive oxygen species in promoting adaptations like mitochondrial biogenesis and neurogenesis, thereby enhancing physical performance. |
| Mason et al. (2016) [93] | Discussed the essential signaling roles of reactive oxygen species in exercise, contributing to positive adaptations such as angiogenesis and improved muscle function. |
| Morales-Alamo and Calbet (2016) [94] | Concluded that antioxidant supplementation could potentially obstruct beneficial exercise adaptations by reducing the signaling role of reactive species, leading to a suboptimal redox state. |
| Theodorou et al. (2011) [95] | Found no effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training, suggesting possible interference with beneficial exercise adaptations. |
| Radak et al. (2017) [96] | Linked excessive antioxidant supplementation to the potential progression of diseases like cancer and diabetes and even increased mortality, contributing to its negative reputation. |
| Sayin et al. (2014) [97] | Revealed significant interindividual variability in redox responses to oxidative stress, suggesting that baseline antioxidant levels influence the effectiveness of supplementation. |
| Margaritelis et al. (2014) [98] | Proposed that individual redox profiles could predict trainability and that antioxidant supplementation should be tailored based on these profiles to enhance exercise adaptations. |
| Margaritelis et al. (2017) [99] | Showed that antioxidant benefits, such as enhanced exercise adaptations, are more likely in individuals with specific deficiencies or high levels of oxidative stress. |
| Zeevi et al. (2015) [100] | Emphasized the need for personalized nutrition based on individual glycemic responses, highlighting the role of genetic factors in the effectiveness of nutritional interventions. |
| Wang and Hu (2018) [101] | Discussed the importance of precision nutrition in the management of type 2 diabetes, advocating for dietary adjustments based on individual genetic responses. |
| Corella et al. (2017) [102] | Underlined the influence of genetic factors on dietary responses, suggesting that nutritional genomics should guide diet customization to prevent cardiovascular diseases. |
| de Toro-Martín et al. (2017) [103] | Reviewed personalized nutritional approaches for metabolic syndrome, stressing the importance of considering individual dietary responses and metabolic profiles. |
| Gibney et al. (2013) [104] | Predicted the future direction of personalized nutrition, focusing on individual dietary needs based on specific phenotypic and genotypic profiles. |
| Hampl et al. (2004) [105] | Discussed the prevalence of vitamin C deficiency and its implications for health, suggesting targeted supplementation could correct specific nutrient deficiencies. |
| Nimni et al. (2007) [106] | Questioned the adequacy of dietary sulfur, pointing out its importance in human nutrition and the potential need for targeted supplementation in deficient populations. |
| Riedl et al. (2017) [107] | Introduced the concept of metabotyping for targeted nutrition, proposing that identifying metabolic profiles could guide more effective personalized nutrition strategies. |
| Maughan et al. (2018) [108] | Discussed the potential of a clinical tool to identify individual antioxidant deficiencies, proposing a threshold of F2-isoprostane for identifying candidates who might benefit from supplementation. |
| Paschalis et al. (2016) [109] | Highlighted significant interindividual variability in redox responses to exercise, showing that people with low levels of antioxidants initially perform worse physically but may benefit more from targeted supplementation. |
| Paschalis et al. (2018) [85] | Found that antioxidant supplementation can disrupt the GSH pathway, leading to broader disruptions in the antioxidative system, affecting systemic oxidative stress and physical performance. |
| Halliwell et al. (2015) [110] | Discussed how antioxidant enzymes play critical roles in regulating cellular signaling and energy metabolism, highlighting their selectivity and efficiency over other antioxidants. |
| Brigelius-Flohé et al. (2011) [111] | Explored the emerging concepts in the redox control of transcription factors, illustrating the complexity of redox regulation in cellular functions. |
| Forman et al. (2014) [112] | Provided an overview of mechanisms of redox signaling, emphasizing the nuanced role of antioxidants in cellular processes. |
| Margaritelis et al. (2016) [113] | Integrated reactive species into biological processes, particularly in exercise physiology, to explain variations in individual responses to physical activity. |
| Thomas et al. (2008) [114] | Discussed the chemical biology of nitric oxide, highlighting its role in cellular signaling related to redox states. |
| Cobley et al. (2015) [115] | Investigated the influence of vitamins C and E on redox signaling, suggesting potential interference with exercise adaptations due to antioxidant supplementation. |
| Perry and Hawley (2017) [116] | Discussed the molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis, crucial for understanding the impact of antioxidants on this process. |
| Richter and Hargreaves (2013) [117] | Explored the role of GLUT4 in glucose uptake during exercise, emphasizing how reactive oxygen species can influence this pathway. |
| Merry and McConell (2009) [118] | Reviewed the influence of reactive oxygen and nitrogen species on skeletal muscle glucose uptake, underlining the complex role of these species in exercise. |
| Katz (2016) [119] | Highlighted the regulatory role of reactive oxygen species in glucose transport in skeletal muscle, indicating potential targets for antioxidant intervention. |
| Pinheiro et al. (2010) [120] | Examined how the redox state affects enzymes like creatine kinase, essential for energy production in muscle cells. |
| Koufen and Stark (2000) [121] | Studied the effects of oxidation on enzyme functionality, showing how an imbalanced redox state could impair energy metabolism. |
| Mitrea et al. (2018) [122] ** | Examined the role of antioxidants in protecting against cosmic radiation-induced oxidative stress, relevant for individuals in high-altitude or intense solar exposure conditions. |
| Pfeiffer et al. (1999) [123] ** | Investigated the effects of antioxidant supplementation at moderate altitudes, noting variable impacts on oxidative stress markers. |
| Paulsen et al. (2014) [124] | Demonstrated that vitamin C and E supplementation might inhibit beneficial physiological adaptations such as mitochondrial biogenesis during endurance training. |
| Yfanti et al. (2010) [125] ** | Showed that supplementation with vitamins C and E did not enhance physical performance or adaptation to endurance training in healthy individuals. |
| Kesse-Guyot et al. (2011) [126] | Reported potential cognitive benefits of daily antioxidant vitamin and mineral supplementation, suggesting further evaluation in military contexts. |
| Author(s) and Year | Key Findings and Contributions on Nutritional Supplements |
|---|---|
| Rhee et al. (2005) [127] | Discussed the complex roles of hydrogen peroxide and peroxiredoxins in intracellular signaling, illustrating the intricate regulation of antioxidants within cellular metabolism. |
| Azzi (2018) [128] | Highlighted the widespread deficiency in vitamin E intake among adults, emphasizing its crucial antioxidant role and the widespread neglect in achieving recommended dietary levels. |
| Maras et al. (2004) [129] | Reported that a significant percentage of the U.S. adult population does not meet the daily intake requirements for vitamin E, associating deficiency with various health issues. |
| Traber (2014) [130] | Explored the consequences of vitamin E deficiency, including severe neurological and metabolic dysfunctions, emphasizing the importance of adequate vitamin E intake. |
| Brigelius-Flohé et al. (2002) [131] | Reviewed the essential functions of vitamin E in preventing tissue damage from free radicals, especially in lipid-rich tissues, and discussed the reversibility of deficiency effects. |
| Galli et al. (2017) [132] | Investigated the broader impacts of vitamin E deficiency, linking severe cases to embryonic mortality and discussing persistent issues despite dietary corrections. |
| McDougall et al. (2017) [133] | Revealed that vitamin E deficiency during embryonic development leads to irreversible metabolic disruptions and increased risks of embryonic mortality, emphasizing the necessity of adequate vitamin E intake. |
| McDougall et al. (2017) [134] | Studied the irreversible damage caused by prolonged vitamin E deficiency in zebrafish models, showing lasting metabolic and developmental impacts. |
| Abner et al. (2011) [135] | Examined the potential risks associated with high-dose vitamin E supplementation, suggesting a balanced approach to avoid negative outcomes. |
| Miller et al. (2005) [136] | Conducted a meta-analysis indicating that excessive vitamin E supplementation may increase mortality, highlighting the need for caution in dosage. |
| Gill et al. (2014) [137] | Reported common vitamin D deficiencies in an Australian population, underlying the widespread issue across different populations. |
| Owens et al. (2015) [138] | Reviewed the critical roles of vitamin D in muscle function and immune response, particularly in athletes, stressing the importance of maintaining optimal levels. |
| Baeke et al. (2010) [139] | Discussed vitamin D as a modulator of the immune system, pointing out the broad health impacts of its deficiency. |
| Owens et al. (2015) [140] | Investigated the molecular mechanisms by which vitamin D supports muscle repair and hypertrophy, emphasizing its importance in physical rehabilitation. |
| Owens et al. (2017) [141] | Explored the benefits of high-dose vitamin D supplements for elite athletes, indicating its potential in optimizing physical performance. |
| Noble et al. (2014) [142] | Proposed a holistic approach to understanding the role of physiological processes in health and disease, advocating for a nuanced view of antioxidant supplementation. |
| Peternelj and Coombes (2011) [87] | Reviewed the mixed effects of antioxidant supplementation during exercise, suggesting a more tailored approach to its application based on individual needs. |
| Pingitore et al. (2015) [143] ** | Emphasized the need for strategic use of antioxidants to maintain physical performance under oxidative stress, particularly in military contexts. |
| Lieberman (2010) [144] ** | Highlighted the necessity of regular nutritional assessments to tailor dietary supplements to the specific needs of military personnel. |
| Attipoe et al. (2013) [145] ** | Developed educational modules for military healthcare providers on dietary supplements, promoting informed and effective use. |
| Mantovani et al. (2006) [146] ** | Demonstrated the potential benefits of integrating antioxidants in treatment regimens for cancer patients, showing improvements in nutritional status and quality of life. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, D.; Mihai, F.-D.; Trasca, M.E.-T.; Caluianu, E.-I.; Calafeteanu, C.D.M.; Radulescu, P.-M.; Mercut, R.; Ciupeanu-Calugaru, E.D.; Marinescu, G.-A.; Siloşi, C.-A.; et al. Oxidative Stress in Military Missions—Impact and Management Strategies: A Narrative Analysis. Life 2024, 14, 567. https://doi.org/10.3390/life14050567
Radulescu D, Mihai F-D, Trasca ME-T, Caluianu E-I, Calafeteanu CDM, Radulescu P-M, Mercut R, Ciupeanu-Calugaru ED, Marinescu G-A, Siloşi C-A, et al. Oxidative Stress in Military Missions—Impact and Management Strategies: A Narrative Analysis. Life. 2024; 14(5):567. https://doi.org/10.3390/life14050567
Chicago/Turabian StyleRadulescu, Dumitru, Florina-Diana Mihai, Major Emil-Tiberius Trasca, Elena-Irina Caluianu, Captain Dan Marian Calafeteanu, Patricia-Mihaela Radulescu, Razvan Mercut, Eleonora Daniela Ciupeanu-Calugaru, Georgiana-Andreea Marinescu, Cristian-Adrian Siloşi, and et al. 2024. "Oxidative Stress in Military Missions—Impact and Management Strategies: A Narrative Analysis" Life 14, no. 5: 567. https://doi.org/10.3390/life14050567
APA StyleRadulescu, D., Mihai, F.-D., Trasca, M. E.-T., Caluianu, E.-I., Calafeteanu, C. D. M., Radulescu, P.-M., Mercut, R., Ciupeanu-Calugaru, E. D., Marinescu, G.-A., Siloşi, C.-A., Nistor, C. C. E., & Danoiu, S. (2024). Oxidative Stress in Military Missions—Impact and Management Strategies: A Narrative Analysis. Life, 14(5), 567. https://doi.org/10.3390/life14050567






