Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders
Abstract
:1. Introduction
2. Neurosteroids and Neuroimmune Regulation
2.1. Neurosteroids: An Overview and Classification
- Pregnane steroids, derived from progesterone, such as allopregnanolone, pregnanolone and 3α,5α-THDOC;
- Androstane steroids, derived from DHEA and testosterone, such as ADIOL and androstanediol;
- Sulfated steroids, such as pregnenolone sulfate (PS) and dehydroepiandrosterone sulfate (DHEAS) (Figure 1).
2.2. Toll-like Receptor Signaling and Neuroimmune Regulation
2.3. Mechanisms of Action of Pregnane Neuroactive Steroids on Toll-like Receptors
2.4. Mechanisms of Action of Androstane Neuroactive Steroids on Toll-like Receptors
3. Benefits of Neurosteroid-Mediated Neuroimmune Modulation in Neuropsychiatric Disorders
3.1. Depression
3.2. Substance Use Disorders
3.3. Pain and Neurological Injuries
3.4. Seizure Disorders
3.5. Neurodegenerative Diseases
3.6. Neurodevelopmental Disorders and Autism Spectrum Disorder (ASD)
4. Limitations and Challenges
4.1. Constraints and Potential Side Effects of Neurosteroid Therapy
4.2. Ethical and Regulatory Considerations for Neuropsychiatric Treatment
5. Future Directions in Neurosteroid Research
6. Conclusions
7. Patents
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lurie, D.I. An Integrative Approach to Neuroinflammation in Psychiatric disorders and Neuropathic Pain. J. Exp. Neurosci. 2018, 12, 1179069518793639. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Loftis, J.M.; Sullivan, E.L. Neuroinflammation in psychiatric disorders: An introductory primer. Pharmacol. Biochem. Behav. 2020, 196, 172981. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef]
- Millett, C.E.; Burdick, K.E.; Kubicki, M.R. The Effects of Peripheral Inflammation on the Brain—A Neuroimaging Perspective. Harv. Rev. Psychiatry 2022, 30, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary between Brain and Periphery. Antioxid. Redox Signal. 2020, 33, 191–210. [Google Scholar] [CrossRef]
- Paul, S.M.; Purdy, R.H. Neuroactive steroids. FASEB J. 1992, 6, 2311–2322. [Google Scholar] [CrossRef]
- Purdy, R.H.; Moore, P.H.; Morrow, A.L.; Paul, S.M. Neurosteroids and GABAA receptor function. In GABAergic Synatic Transmission; Biggio, G., Concas, A., Costa, E., Eds.; Raven Press: New York, NY, USA, 1992; pp. 87–92. [Google Scholar]
- Purdy, R.H.; Morrow, A.L.; Blinn, J.R.; Paul, S.M. Synthesis, metabolism, and pharmacological activity of 3 alpha-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J. Med. Chem. 1990, 33, 1572–1581. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Robel, P.; Vatier, O.; Haug, A.; Le Goascogne, C.; Bourreau, E. Neurosteroids: Pregnenolone and Dehydroepiandrosterone in the rat brain. In Receptor-Receptor Interactions: A New Intramembrane Integrative Mechanism; Macmillan: London, UK, 1987; pp. 89–104. [Google Scholar]
- Boero, G.; Porcu, P.; Morrow, A.L. Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol. Stress 2020, 12, 100203. [Google Scholar] [CrossRef]
- Morrow, A.L.; Boero, G.; Porcu, P. A Rationale for Allopregnanolone Treatment of Alcohol Use Disorders: Basic and Clinical Studies. Alcohol. Clin. Exp. Res. 2020, 44, 320–339. [Google Scholar] [CrossRef]
- Morrow, A.L.; Balan, I.; Boero, G. Mechanisms Underlying Recovery From Postpartum Depression Following Brexanolone Therapy. Biol. Psychiatry 2022, 91, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Ukena, K.; Takase, M.; Kohchi, C.; Lea, R.W. Neurosteroid biosynthesis in vertebrate brains. Comp. Biochem. Physiol.—Part C Toxicol. 1999, 124, 121–129. [Google Scholar] [CrossRef]
- Tsutsui, K. Neurosteroids in the Purkinje cell: Biosynthesis, mode of action and functional significance. Mol. Neurobiol. 2008, 37, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. Int. Rev. Neurobiol. 2001, 46, 1–32. [Google Scholar] [CrossRef]
- Agis-Balboa, R.; Pinna, G.; Zhubi, A.; Veldic, M.; Costa, E.; Guidotti, A. Location and expression of brain enzymes catalyzing neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed]
- Agis-Balboa, R.C.; Guidotti, A.; Pinna, G. 5α-reductase type i expression is downregulated in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients. Psychopharmacology 2014, 231, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.B.; Dumitru, A.M.; O’Buckley, T.K.; Morrow, A.L. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol. Clin. Exp. Res. 2014, 38, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Germelli, L.; Da Pozzo, E.; Giacomelli, C.; Tremolanti, C.; Marchetti, L.; Wetzel, C.H.; Barresi, E.; Taliani, S.; Da Settimo, F.; Martini, C.; et al. De novo Neurosteroidogenesis in Human Microglia: Involvement of the 18 kDa Translocator Protein. Int. J. Mol. Sci. 2021, 22, 3115. [Google Scholar] [CrossRef] [PubMed]
- Tuem, K.B.; Atey, T.M. Neuroactive Steroids: Receptor Interactions and Responses. Front. Neurol. 2017, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef]
- Lloyd-Evans, E.; Waller-Evans, H. Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays Biochem. 2020, 64, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.J.; Belelli, D.; Peden, D.R.; Vardy, A.W.; Peters, J.A. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 2003, 71, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.J.; Cooper, M.A.; Simmons, R.D.; Weir, C.J.; Belelli, D. Neurosteroids: Endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S48–S58. [Google Scholar] [CrossRef]
- Herd, M.B.; Belelli, D.; Lambert, J.J. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol. Ther. 2007, 116, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Kokate, T.G.; Svensson, B.E.; Rogawski, M.A. Anticonvulsant activity of neurosteroids: Correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J. Pharmacol. Exp. Ther. 1994, 270, 1223–1229. [Google Scholar] [PubMed]
- Puia, G.; Vicini, S.; Seeburg, P.H.; Costa, E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol. Pharmacol. 1991, 39, 691–696. [Google Scholar] [PubMed]
- Reddy, D.S.; Rogawski, M.A. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J. Neurosci. 2002, 22, 3795–3805. [Google Scholar] [CrossRef]
- Modgil, A.; Parakala, M.L.; Ackley, M.A.; Doherty, J.J.; Moss, S.J.; Davies, P.A. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology 2017, 113, 314–322. [Google Scholar] [CrossRef]
- Stell, B.M.; Brickley, S.G.; Tang, C.Y.; Farrant, M.; Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 14439–14444. [Google Scholar] [CrossRef]
- Pinna, G. Allopregnanolone (1938–2019): A trajectory of 80 years of outstanding scientific achievements. Neurobiol. Stress 2020, 13, 100246. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G. Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Front. Endocrinol. 2020, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Antonoudiou, P.; Colmers, P.L.W.; Walton, N.L.; Weiss, G.L.; Smith, A.C.; Nguyen, D.P.; Lewis, M.; Quirk, M.C.; Barros, L.; Melon, L.C.; et al. Allopregnanolone Mediates Affective Switching Through Modulation of Oscillatory States in the Basolateral Amygdala. Biol. Psychiatry 2022, 91, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Beattie, M.C.; O’Buckley, T.K.; Aurelian, L.; Morrow, A.L. Endogenous Neurosteroid (3⍺,5⍺)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Sci. Rep. 2019, 9, 1220. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Aurelian, L.; Williams, K.S.; Campbell, B.; Meeker, R.B.; Morrow, A.L. Inhibition of human macrophage activation via pregnane neurosteroid interactions with toll-like receptors: Sex differences and structural requirements. Front. Immunol. 2022, 13, 940095. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Gale, S.E.; Frolov, A.; Mohri, I.; Suzuki, K.; Mellon, S.H.; Walkley, S.U.; Covey, D.F.; Schaffer, J.E.; Ory, D.S. Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA 2006, 103, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Biggio, G.; Concas, A.; Follesa, P.; Sanna, E.; Serra, M. Stress, ethanol, and neuroactive steroids. Pharmacol. Ther. 2007, 116, 140–171. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Pisu, M.G.; Mostallino, M.C.; Sanna, E.; Biggio, G. Changes in neuroactive steroid content during social isolation stress modulate GABAA receptor plasticity and function. Brain Res. Rev. 2008, 57, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Janis, G.C.; Devaud, L.L.; Mitsuyama, H.; Morrow, A.L. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol. Clin. Exp. Res. 1998, 22, 2055–2061. [Google Scholar] [CrossRef]
- Khisti, R.T.; Boyd, K.N.; Kumar, S.; Morrow, A.L. Systemic ethanol administration elevates deoxycorticosterone levels and chronic ethanol exposure attenuates this response. Brain Res. 2005, 1049, 104–111. [Google Scholar] [CrossRef]
- Brown, E.S.; Park, J.; Marx, C.E.; Hynan, L.S.; Gardner, C.; Davila, D.; Nakamura, A.; Sunderajan, P.; Lo, A.; Holmes, T. A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology 2014, 39, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Girdler, S.S.; Klatzkin, R. Neurosteroids in the context of stress: Implications for depressive disorders. Pharmacol. Ther. 2007, 116, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Osuji, I.J.; Vera-Bolanos, E.; Carmody, T.J.; Brown, E.S. Pregnenolone for cognition and mood in dual diagnosis patients. Psychiatry Res. 2010, 178, 309–312. [Google Scholar] [CrossRef]
- Girdler, S.S.; Lindgren, M.; Porcu, P.; Rubinow, D.R.; Johnson, J.L.; Morrow, A.L. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology 2012, 37, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Brancati, A.; De Lorenzo, A.; Fucci, P.; Furnari, C.; Pompili, E.; Sasso, G.F.; Spalletta, G.; Troisi, A.; Pasini, A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin. Neuropharmacol. 1996, 19, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Besheer, J.; Lindsay, T.G.; O’Buckley, T.K.; Hodge, C.W.; Morrow, A.L. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol. Clin. Exp. Res. 2010, 34, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.B.; Werner, D.F.; Maldonado-Devincci, A.M.; Leonard, M.N.; Fisher, K.R.; O’Buckley, T.K.; Porcu, P.; McCown, T.J.; Besheer, J.; Hodge, C.W.; et al. Overexpression of the steroidogenic enzyme cytochrome P450 side chain cleavage in the ventral tegmental area increases 3alpha,5alpha-THP and reduces long-term operant ethanol self-administration. J. Neurosci. 2014, 34, 5824–5834. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, L.C.; Boero, G.; Van Voorhies, K.; O’Buckley, T.K.; Besheer, J.; Morrow, A.L. Pharmacological administration of 3alpha,5alpha-THP into the nucleus accumbens core increases 3alpha,5alpha-THP expression and reduces alcohol self-administration. Alcohol 2023, 47, 459–469. [Google Scholar] [CrossRef]
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.Y.; He, X.Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J. Neuroendocrinol. 2016, 28, 12351. [Google Scholar] [CrossRef]
- He, J.; Evans, C.O.; Hoffman, S.W.; Oyesiku, N.M.; Stein, D.G. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 2004, 189, 404–412. [Google Scholar] [CrossRef]
- He, J.; Hoffman, S.W.; Stein, D.G. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor. Neurol. Neurosci. 2004, 22, 19–31. [Google Scholar] [PubMed]
- Noorbakhsh, F.; Baker, G.B.; Power, C. Allopregnanolone and neuroinflammation: A focus on multiple sclerosis. Front. Cell. Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Guennoun, R.; Stein, D.G.; De Nicola, A.F. Progesterone: Therapeutic opportunities for neuroprotection and myelin repair. Pharmacol. Ther. 2007, 116, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D. Neurosteroids as regenerative agents in the brain: Therapeutic implications. Nat. Rev. Endocrinol. 2013, 9, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.W.; Kellermann, A.L.; Hertzberg, V.S.; Clark, P.L.; Frankel, M.; Goldstein, F.C.; Salomone, J.P.; Dent, L.L.; Harris, O.A.; Ander, D.S.; et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007, 49, 391–402.e2. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; Sofuoglu, M.; Morgan, P.T.; Tuit, K.L.; Sinha, R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinology 2013, 38, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, V.; Covault, J.; Angarita, G.A.; Siedlarz, K.; Sinha, R. Neuroactive steroid levels and cocaine use chronicity in men and women with cocaine use disorder receiving progesterone or placebo. Am. J. Addict. 2019, 28, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, V.; Fox, H.C.; Sofuoglu, M.; Covault, J.; Sinha, R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology 2016, 65, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, V.; Sullivan, L.; Tiber, J.; Fogelman, N.; Simpson, C.; Hermes, G.; Sinha, R. Pregnenolone effects on provoked alcohol craving, anxiety, HPA axis, and autonomic arousal in individuals with alcohol use disorder. Psychopharmacology 2023, 240, 101–114. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemson, C.; Schacterle, A.; et al. Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
- Kanes, S.; Colquhoun, H.; Gunduz-Bruce, H.; Raines, S.; Arnold, R.; Schacterle, A.; Doherty, J.; Epperson, C.N.; Deligiannidis, K.M.; Riesenberg, R.; et al. Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. Lancet 2017, 390, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Patterson, R.; Boero, G.; Krohn, H.; O’Buckley, T.K.; Meltzer-Brody, S.; Morrow, A.L. Brexanolone therapeutics in post-partum depression involves inhibition of systemic inflammatory pathways. eBioMedicine 2023, 89, 104473. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Krohn, H.; Richardson, E.; Kimmel, M.; Meltzer-Brody, S. A Brexanolone Treatment Program at an Academic Medical Center: Patient Selection, 90-Day Posttreatment Outcomes, and Lessons Learned. J. Acad. Consult. Liaison Psychiatry 2022, 63, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Vallée, M.; Fabre, S.; Collins Reed, S.; Zanese, M.; Campistron, G.; Arout, C.A.; Foltin, R.W.; Cooper, Z.D.; Kearney-Ramos, T.; et al. Signaling-specific inhibition of the CB1 receptor for cannabis use disorder: Phase 1 and phase 2a randomized trials. Nat. Med. 2023, 29, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Aurelian, L.; Schleicher, R.; Boero, G.; O’Buckley, T.; Morrow, A.L. Neurosteroid allopregnanolone (3alpha,5alpha-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl. Psychiatry 2021, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.; Jakka, P.; Namani, S.; Mujumdar, V.; Radhakrishnan, G. The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J. Biol. Chem. 2019, 294, 4596–4607. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Sun, Y.; Ma, F.; Lü, P.; Huang, H.; Zhou, J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol. Lett. 2009, 125, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Anthony, J.P.; Henriquez, F.L.; Lyons, R.E.; Nickdel, M.B.; Carter, K.C.; Alexander, J.; Roberts, C.W. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology 2008, 125, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, M.; Wu, C.Y.; Xia, G.Q. Role of progesterone in TLR4-MyD88-dependent signaling pathway in pre-eclampsia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 730–734. [Google Scholar] [CrossRef]
- Zandieh, Z.; Amjadi, F.; Ashrafi, M.; Aflatoonian, A.; Fazeli, A.; Aflatoonian, R. The Effect of Estradiol and Progesterone on Toll Like Receptor Gene Expression in A Human Fallopian Tube Epithelial Cell Line. Cell J. 2016, 17, 678–691. [Google Scholar] [CrossRef]
- Foust-Wright, C.E.; Pulliam, S.J.; Batalden, R.P.; Berk, T.K.; Weinstein, M.M.; Wakamatsu, M.M.; Phillippe, M. Hormone Modulation of Toll-Like Receptor 5 in Cultured Human Bladder Epithelial Cells. Reprod. Sci. 2017, 24, 713–719. [Google Scholar] [CrossRef]
- Chen, G.; Shi, J.; Jin, W.; Wang, L.; Xie, W.; Sun, J.; Hang, C. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Ann. Clin. Lab. Sci. 2008, 38, 65–74. [Google Scholar]
- Tajalli-Nezhad, S.; Karimian, M.; Beyer, C.; Atlasi, M.A.; Azami Tameh, A. The regulatory role of Toll-like receptors after ischemic stroke: Neurosteroids as TLR modulators with the focus on TLR2/4. Cell Mol. Life Sci. 2019, 76, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Balan, I.; Morrow, A.L.; Meltzer-Brody, S. Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology 2024, 49, 67–72. [Google Scholar] [CrossRef]
- Naert, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology 2007, 32, 1062–1078. [Google Scholar] [CrossRef]
- Nin, M.S.; Martinez, L.A.; Pibiri, F.; Nelson, M.; Pinna, G. Neurosteroids reduce social isolation-induced behavioral deficits: A proposed link with neurosteroid-mediated upregulation of BDNF expression. Front. Endocrinol. 2011, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.B.; Nin, M.S.; Barros, H.M.T. The role of allopregnanolone in depressive-like behaviors: Focus on neurotrophic proteins. Neurobiol. Stress 2020, 12, 100218. [Google Scholar] [CrossRef]
- Balan, I.; Grusca, A.; O’Buckley, T.K.; Morrow, A.L. Neurosteroid [3α,5α]-3-hydroxy-pregnan-20-one enhances IL-10 production via endosomal TRIF-dependent TLR4 signaling pathway. Front. Endocrinol. 2023, 14, 1299420. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.D.; Brinton, R.D. Allopregnanolone: Regenerative therapeutic to restore neurological health. Neurobiol. Stress 2022, 21, 100502. [Google Scholar] [CrossRef]
- Irwin, R.W.; Brinton, R.D. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: Translational development and clinical promise. Prog. Neurobiol. 2014, 113, 40–55. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Frye, C.A.; Koonce, C.J.; Edinger, K.L.; Osborne, D.M.; Walf, A.A. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm. Behav. 2008, 54, 726–734. [Google Scholar] [CrossRef]
- Asselmann, E.; Kische, H.; Haring, R.; Hertel, J.; Schmidt, C.-O.; Nauck, M.; Beesdo-Baum, K.; Grabe, H.-J.; Pané-Farré, C.A. Prospective associations of androgens and sex hormone-binding globulin with 12-month, lifetime and incident anxiety and depressive disorders in men and women from the general population. J. Affect. Disord. 2019, 245, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Kische, H.; Pieper, L.; Venz, J.; Klotsche, J.; März, W.; Koch-Gromus, U.; Pittrow, D.; Lehnert, H.; Silber, S.; Stalla, G.K.; et al. Longitudinal change instead of baseline testosterone predicts depressive symptoms. Psychoneuroendocrinology 2018, 89, 7–12. [Google Scholar] [CrossRef]
- Frye, C.A.; Edinger, K.; Sumida, K. Androgen Administration to Aged Male Mice Increases Anti-Anxiety Behavior and Enhances Cognitive Performance. Neuropsychopharmacology 2008, 33, 1049–1061. [Google Scholar] [CrossRef]
- Cai, Z.; Li, H. An Updated Review: Androgens and Cognitive Impairment in Older Men. Front. Endocrinol. 2020, 11, 586909. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Heck, A.L.; De Guzman, R.M.; Handa, R.J. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol. Sex Differ. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Drakopoulos, P.; Bianchi-Demicheli, F.; Wenger, J.M.; Petignat, P.; Genazzani, A.R. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol. 2015, 145, 273–280. [Google Scholar] [CrossRef]
- Nenezic, N.; Kostic, S.; Strac, D.S.; Grunauer, M.; Nenezic, D.; Radosavljevic, M.; Jancic, J.; Samardzic, J. Dehydroepiandrosterone (DHEA): Pharmacological Effects and Potential Therapeutic Application. Mini Rev. Med. Chem. 2023, 23, 941–952. [Google Scholar] [CrossRef]
- Sripada, R.K.; Marx, C.E.; King, A.P.; Rajaram, N.; Garfinkel, S.N.; Abelson, J.L.; Liberzon, I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology 2013, 38, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Tracey, A.Q.; Stephen, R.R.; David, W. Chapter 3—Dehydroepiandrosterone (DHEA) and DHEA Sulfate: Roles in Brain Function and Disease. In Sex Hormones in Neurodegenerative Processes and Diseases; Gorazd, D., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- do Vale, S.; Selinger, L.; Martins, J.M.; Bicho, M.; do Carmo, I.; Escera, C. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone-sulfate (DHEAS) and emotional processing—A behavioral and electrophysiological approach. Horm. Behav. 2015, 73, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Faviana, P.; Boldrini, L.; Gronchi, L.; Galli, L.; Erba, P.; Gentile, C.; Lippolis, P.V.; Marchetti, E.; Di Stefano, I.; Sammarco, E.; et al. Steroid Hormones as Modulators of Emotional Regulation in Male Urogenital Cancers. Int. J. Behav. Med. 2023, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Chapman, D.F.; Nunez, S.Y.; Zivin, J.A. Dehydroepiandrosterone Sulfate Is Neuroprotective in a Reversible Spinal Cord Ischemia Model. Stroke 2000, 31, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Vuksan-Ćusa, B.; Šagud, M.; Radoš, I. The role of dehydroepiandrosterone (DHEA) in schizophrenia. Psychiatr. Danub. 2016, 28, 30–33. [Google Scholar] [PubMed]
- Schmidt, P.J.; Daly, R.C.; Bloch, M.; Smith, M.J.; Danaceau, M.A.; Clair, S.L.L.; Murphy, J.H.; Haq, N.; Rubinow, D.R. Dehydroepiandrosterone Monotherapy in Midlife-Onset Major and Minor Depression. Arch. Gen. Psychiatry 2005, 62, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Edinger, K.L.; Lephart, E.D.; Walf, A.A. 3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front. Aging Neurosci. 2010, 2, 15. [Google Scholar] [CrossRef]
- Brinton, R.D.; Tran, J.; Proffitt, P.; Montoya, M. 17 β-Estradiol Enhances the Outgrowth and Survival of Neocortical Neurons in Culture. Neurochem. Res. 1997, 22, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, A.; Díaz, H.; Carrer, H.; Cáceres, A. Amygdala neurons in vitro: Neurite growth and effects of estradiol. J. Neurosci. Res. 1992, 33, 418–435. [Google Scholar] [CrossRef]
- McCarthy, M.M. Estradiol and the developing brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina, L.; Bátiz, L.F. The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell. Neurosci. 2021, 15, 636176. [Google Scholar] [CrossRef] [PubMed]
- Nerattini, M.; Jett, S.; Andy, C.; Carlton, C.; Zarate, C.; Boneu, C.; Battista, M.; Pahlajani, S.; Loeb-Zeitlin, S.; Havryulik, Y.; et al. Systematic review and meta-analysis of the effects of menopause hormone therapy on risk of Alzheimer’s disease and dementia. Front. Aging Neurosci. 2023, 15, 1260427. [Google Scholar] [CrossRef]
- Abbas, N.A.T.; Hassan, H.A. The protective and therapeutic effects of 5-androstene3β, 17β-diol (ADIOL) in abdominal post-operative adhesions in rat: Suppressing TLR4/NFκB/HMGB1/TGF1 β/α SMA pathway. Int. Immunopharmacol. 2022, 109, 108801. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Asai, M.; Yoshida, M.; Nigawara, T.; Kambayashi, M.; Nakashima, N. Dehydroepiandrosterone-Sulfate Inhibits Nuclear Factor-κB-Dependent Transcription in Hepatocytes, Possibly through Antioxidant Effect. J. Clin. Endocrinol. Metab. 2004, 89, 3449–3454. [Google Scholar] [CrossRef]
- Saijo, K.; Collier, J.G.; Li, A.C.; Katzenellenbogen, J.A.; Glass, C.K. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 2011, 145, 584–595. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Fodelianaki, G.; Neuwirth, A.; Mund, C.; Kourgiantaki, A.; Ieronimaki, E.; Lyroni, K.; Troullinaki, M.; Fujii, C.; Kanczkowski, W.; et al. DHEA inhibits acute microglia-mediated inflammation through activation of the TrkA-Akt1/2-CREB-Jmjd3 pathway. Mol. Psychiatry 2018, 23, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Cao, J.; Yu, L.; Zhao, J.; Ma, H. Effect of dehydroepiandrosterone on the immune function of mice in vivo and in vitro. Mol. Immunol. 2019, 112, 283–290. [Google Scholar] [CrossRef]
- Cao, J.; Li, Q.; Shen, X.; Yao, Y.; Li, L.; Ma, H. Dehydroepiandrosterone attenuates LPS-induced inflammatory responses via activation of Nrf2 in RAW264.7 macrophages. Mol. Immunol. 2021, 131, 97–111. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, J.; Yu, L.; Ma, H. Dehydroepiandrosterone alleviates E. Coli O157:H7-induced inflammation by preventing the activation of p38 MAPK and NF-kappaB pathways in mice peritoneal macrophages. Mol. Immunol. 2019, 114, 114–122. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, J.; Yu, L.; Ma, H. Dehydroepiandrosterone resisted E. Coli O157:H7-induced inflammation via blocking the activation of p38 MAPK and NF-kappaB pathways in mice. Cytokine 2020, 127, 154955. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.D.; Voskuhl, R.R. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol. 2012, 33, 105–115. [Google Scholar] [CrossRef]
- Vegeto, E.; Pollio, G.; Ciana, P.; Maggi, A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp. Gerontol. 2000, 35, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Belcredito, S.; Etteri, S.; Ghisletti, S.; Brusadelli, A.; Meda, C.; Krust, A.; Dupont, S.; Ciana, P.; Chambon, P.; et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 2003, 100, 9614–9619. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Belcredito, S.; Ghisletti, S.; Meda, C.; Etteri, S.; Maggi, A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 2006, 147, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef]
- Czlonkowska, A.; Ciesielska, A.; Gromadzka, G.; Kurkowska-Jastrzebska, I. Estrogen and cytokines production—The possible cause of gender differences in neurological diseases. Curr. Pharm. Des. 2005, 11, 1017–1030. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef]
- Villa, A.; Rizzi, N.; Vegeto, E.; Ciana, P.; Maggi, A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015, 5, 15224. [Google Scholar] [CrossRef]
- Siani, F.; Greco, R.; Levandis, G.; Ghezzi, C.; Daviddi, F.; Demartini, C.; Vegeto, E.; Fuzzati-Armentero, M.T.; Blandini, F. Influence of Estrogen Modulation on Glia Activation in a Murine Model of Parkinson’s Disease. Front. Neurosci. 2017, 11, 306. [Google Scholar] [CrossRef]
- Heitzer, M.; Kaiser, S.; Kanagaratnam, M.; Zendedel, A.; Hartmann, P.; Beyer, C.; Johann, S. Administration of 17beta-Estradiol Improves Motoneuron Survival and Down-regulates Inflammasome Activation in Male SOD1(G93A) ALS Mice. Mol. Neurobiol. 2017, 54, 8429–8443. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.; Bolanos, J.; Nair, S.; Saad, F.; Morgentaler, A. Do Androgens Modulate the Pathophysiological Pathways of Inflammation? Appraising the Contemporary Evidence. J. Clin. Med. 2018, 7, 549. [Google Scholar] [CrossRef]
- Zahaf, A.; Kassoussi, A.; Hutteau-Hamel, T.; Mellouk, A.; Marie, C.; Zoupi, L.; Tsouki, F.; Mattern, C.; Bobé, P.; Schumacher, M.; et al. Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination. Nat. Commun. 2023, 14, 1592. [Google Scholar] [CrossRef]
- Vancolen, S.; Sebire, G.; Robaire, B. Influence of androgens on the innate immune system. Andrology 2023, 11, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.; Yang, M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 1995, 15, 4971–4979. [Google Scholar] [CrossRef]
- Kettelhut, A.; Bowman, E.; Gabriel, J.; Hand, B.; Liyanage, N.P.M.; Kulkarni, M.; Avila-Soto, F.; Lake, J.E.; Funderburg, N.T. Estrogen May Enhance Toll-Like Receptor 4-Induced Inflammatory Pathways in People with HIV: Implications for Transgender Women on Hormone Therapy. Front. Immunol. 2022, 13, 879600. [Google Scholar] [CrossRef]
- Young, N.A.; Wu, L.C.; Burd, C.J.; Friedman, A.K.; Kaffenberger, B.H.; Rajaram, M.V.; Schlesinger, L.S.; James, H.; Shupnik, M.A.; Jarjour, W.N. Estrogen modulation of endosome-associated toll-like receptor 8: An IFNalpha-independent mechanism of sex-bias in systemic lupus erythematosus. Clin. Immunol. 2014, 151, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, Z.; Amjadi, F.; Vakilian, H.; Aflatoonian, K.; Amirchaghmaghi, E.; Fazeli, A.; Aflatoonian, R. Sex hormones alter the response of Toll-like receptor 3 to its specific ligand in fallopian tube epithelial cells. Clin. Exp. Reprod. Med. 2018, 45, 154–162. [Google Scholar] [CrossRef]
- Calippe, B.; Douin-Echinard, V.; Laffargue, M.; Laurell, H.; Rana-Poussine, V.; Pipy, B.; Guéry, J.C.; Bayard, F.; Arnal, J.F.; Gourdy, P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: Involvement of the phosphatidylinositol 3-kinase pathway. J. Immunol. 2008, 180, 7980–7988. [Google Scholar] [CrossRef]
- Calippe, B.; Douin-Echinard, V.; Delpy, L.; Laffargue, M.; Lélu, K.; Krust, A.; Pipy, B.; Bayard, F.; Arnal, J.F.; Guéry, J.C.; et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 2010, 185, 1169–1176. [Google Scholar] [CrossRef]
- Brauer, V.S.; Zambuzi, F.A.; Espíndola, M.S.; Cavalcanti Neto, M.P.; Prado, M.K.B.; Cardoso, P.M.; Soares, L.S.; Galvao-Lima, L.J.; Leopoldino, A.M.; Cardoso, C.R.d.B.; et al. The influence of dehydroepiandrosterone on effector functions of neutrophils. Braz. J. Pharm. Sci. 2021, 57, e19139. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet-Hudson, Y.M.; Marriott, I. Testosterone Reduces Macrophage Expression in the Mouse of Toll-Like Receptor 4, a Trigger for Inflammation and Innate Immunity. Biol. Reprod. 2008, 78, 432–437. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Dkhil, M.A.; S Abdel-Baki, A.-A.; Araúzo-Bravo, M.J.; Delic, D.; Wunderlich, F. Testosterone persistently dysregulates hepatic expression of Tlr6 and Tlr8 induced by Plasmodium chabaudi malaria. Parasitol. Res. 2014, 113, 3609–3620. [Google Scholar] [CrossRef]
- Buendía-González, F.O.; Legorreta-Herrera, M. The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules 2022, 12, 1768. [Google Scholar] [CrossRef]
- Matsuda, A.; Furukawa, K.; Suzuki, H.; Matsutani, T.; Tajiri, T.; Chaudry, I.H. Dehydroepiandrosterone Modulates Toll-Like Receptor Expression on Splenic Macrophages of Mice after Severe Polymicrobial Sepsis. Shock 2005, 24, 364–369. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-Like Receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Hanke, M.L.; Kielian, T. Toll-like receptors in health and disease in the brain: Mechanisms and therapeutic potential. Clin. Sci. 2011, 121, 367–387. [Google Scholar] [CrossRef]
- Esen, N.; Kielian, T. Toll-Like Receptors in Brain Abscess. In Toll-like Receptors: Roles in Infection and Neuropathology; Kielian, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 41–61. [Google Scholar]
- Airapetov, M.I.; Eresko, S.O.; Lebedev, A.A.; Bychkov, E.R.; Shabanov, P.D. The Role of Toll-Like Receptors in Neuroimmunology of Alcoholism. Biochem. Suppl. Ser. B Biomed. Chem. 2021, 15, 71–79. [Google Scholar] [CrossRef]
- Aurelian, L.; Balan, I. GABAAR α2-activated neuroimmune signal controls binge drinking and impulsivity through regulation of the CCL2/CX3CL1 balance. Psychopharmacology 2019, 236, 3023–3043. [Google Scholar] [CrossRef]
- Balan, I.; Warnock, K.T.; Puche, A.; Gondre-Lewis, M.C.; June, H.; Aurelian, L. The GABAA Receptor α2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area that Regulates Alcohol and Nicotine Abuse. Brain Sci. 2018, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Warnock, K.T.; Puche, A.; Gondre-Lewis, M.C.; Aurelian, L. Innately activated TLR4 signal in the nucleus accumbens is sustained by CRF amplification loop and regulates impulsivity. Brain Behav. Immun. 2018, 69, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 2017, 8, 508. [Google Scholar] [CrossRef]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2021, 12, 796867. [Google Scholar] [CrossRef]
- Li, T.; Chen, H.; Xu, B.; Yu, M.; Li, J.; Shi, Y.; Xia, S.; Wu, S. Deciphering the interplay between LPS/TLR4 pathways, neurotransmitter, and deltamethrin-induced depressive-like behavior: Perspectives from the gut-brain axis. Pestic. Biochem. Physiol. 2023, 197, 105697. [Google Scholar] [CrossRef]
- Liu, J.; Buisman-Pijlman, F.; Hutchinson, M.R. Toll-like receptor 4: Innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front. Neurosci. 2014, 8, 309. [Google Scholar] [CrossRef]
- Figueroa-Hall, L.K.; Paulus, M.P.; Savitz, J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology 2020, 121, 104843. [Google Scholar] [CrossRef]
- Du, Y.; Yan, T.; Wu, B.; He, B.; Jia, Y. Research on the mechanism of antidepressive effect of Suanzaoren Decoction through TLR4/MyD88/NF-κB pathway and Wnt/β-catenin pathway. J. Ethnopharmacol. 2024, 319, 117190. [Google Scholar] [CrossRef]
- An, Q.; Xia, J.; Pu, F.; Shi, S. MCPIP1 alleviates depressive-like behaviors in mice by inhibiting the TLR4/TRAF6/NF-κB pathway to suppress neuroinflammation. Mol. Med. Rep. 2024, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Souza-Junior, F.J.C.; Cunha, L.C.; Lisboa, S.F. Toll-like receptor 4 in the interface between neuroimmune response and behavioral alterations caused by stress. Explor. Neuroprot. Ther. 2022, 2, 182–209. [Google Scholar] [CrossRef]
- Crews, F.T.; Walter, T.J.; Coleman, L.G., Jr.; Vetreno, R.P. Toll-like receptor signaling and stages of addiction. Psychopharmacology 2017, 234, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G., Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflammation 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Qin, L.; Coleman, L.G., Jr.; Crews, F.T. Increased Toll-like Receptor-MyD88-NFkappaB-Proinflammatory neuroimmune signaling in the orbitofrontal cortex of humans with alcohol use disorder. Alcohol. Clin. Exp. Res. 2021, 45, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, A.R.; Kelly, T.; Puche, A.; Esoga, C.; June, H.L., Jr.; Elnabawi, A.; Merchenthaler, I.; Sieghart, W.; June, H.L., Sr.; et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc. Natl. Acad. Sci. USA 2011, 108, 4465–4470. [Google Scholar] [CrossRef]
- Czerwińska-Błaszczyk, A.; Pawlak, E.; Pawłowski, T. The Significance of Toll-Like Receptors in the Neuroimmunologic Background of Alcohol Dependence. Front. Psychiatry 2021, 12, 797123. [Google Scholar] [CrossRef] [PubMed]
- June, H.L.; Liu, J.; Warnock, K.T.; Bell, K.A.; Balan, I.; Bollino, D.; Puche, A.; Aurelian, L. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 2015, 40, 1549–1559. [Google Scholar] [CrossRef]
- Lovelock, D.F.; Liu, W.; Langston, S.E.; Liu, J.; Van Voorhies, K.; Giffin, K.A.; Vetreno, R.P.; Crews, F.T.; Besheer, J. The Toll-like receptor 7 agonist imiquimod increases ethanol self-administration and induces expression of Toll-like receptor related genes. Addict. Biol. 2022, 27, e13176. [Google Scholar] [CrossRef]
- Aurelian, L.; Warnock, K.T.; Balan, I.; Puche, A.; June, H. TLR4 signaling in VTA dopaminergic neurons regulates impulsivity through tyrosine hydroxylase modulation. Transl. Psychiatry 2016, 6, e815. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Q.; Yu, W.H.; Hu, Y.Y.; Zhang, Z.Y.; Huang, M. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflamm. Res. 2011, 60, 533–539. [Google Scholar] [CrossRef]
- Shi, H.; Hua, X.; Kong, D.; Stein, D.; Hua, F. Role of Toll-like receptor mediated signaling in traumatic brain injury. Neuropharmacology 2019, 145, 259–267. [Google Scholar] [CrossRef] [PubMed]
- El Baassiri, M.G.; Chun, Y.H.; Rahal, S.S.; Fulton, W.B.; Sodhi, C.P.; Hackam, D.J.; Nasr, I.W. Infiltrating anti-inflammatory monocytes modulate microglial activation through toll-like receptor 4/interferon-dependent pathways following traumatic brain injury. J. Trauma Acute Care Surg. 2023, 95, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Okun, E.; Griffioen, K.J.; Lathia, J.D.; Tang, S.C.; Mattson, M.P.; Arumugam, T.V. Toll-like receptors in neurodegeneration. Brain Res. Rev. 2009, 59, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Kruger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Lehnardt, S.; Lachance, C.; Patrizi, S.; Lefebvre, S.; Follett, P.L.; Jensen, F.E.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002, 22, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Trotta, T.; Porro, C.; Calvello, R.; Panaro, M.A. Biological role of Toll-like receptor-4 in the brain. J. Neuroimmunol. 2014, 268, 1–12. [Google Scholar] [CrossRef]
- Essam, R.M.; Saadawy, M.A.; Gamal, M.; Abdelsalam, R.M.; El-Sahar, A.E. Lactoferrin averts neurological and behavioral impairments of thioacetamide-induced hepatic encephalopathy in rats via modulating HGMB1/TLR-4/MyD88/Nrf2 pathway. Neuropharmacology 2023, 236, 109575. [Google Scholar] [CrossRef]
- Caso, J.R.; Pradillo, J.M.; Hurtado, O.; Lorenzo, P.; Moro, M.A.; Lizasoain, I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 2007, 115, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Ma, J.; Ha, T.; Xia, Y.; Kelley, J.; Williams, D.L.; Kao, R.L.; Browder, I.W.; Schweitzer, J.B.; Kalbfleisch, J.H.; et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J. Neuroimmunol. 2007, 190, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Sakaguchi, R.; Suzuki, M.; Yoshimura, A. Post-ischemic inflammation in the brain. Front. Immunol. 2012, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Gesuete, R.; Kohama, S.G.; Stenzel-Poore, M.P. Toll-like receptors and ischemic brain injury. J. Neuropathol. Exp. Neurol. 2014, 73, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, L.; Jin, N.; Sha, S.; Ouyang, Y. L-F001, a multifunctional fasudil-lipoic acid dimer, antagonizes hypoxic-ischemic brain damage by inhibiting the TLR4/MyD88 signaling pathway. Brain Behav. 2023, 13, e3280. [Google Scholar] [CrossRef]
- Balaji, R.; Subbanna, M.; Shivakumar, V.; Abdul, F.; Venkatasubramanian, G.; Debnath, M. Pattern of expression of Toll like receptor (TLR)-3 and -4 genes in drug-naive and antipsychotic treated patients diagnosed with schizophrenia. Psychiatry Res. 2020, 285, 112727. [Google Scholar] [CrossRef] [PubMed]
- Weickert, T.W.; Ji, E.; Galletly, C.; Boerrigter, D.; Morishima, Y.; Bruggemann, J.; Balzan, R.; O’Donnell, M.; Liu, D.; Lenroot, R.; et al. Toll-Like Receptor mRNA Levels in Schizophrenia: Association with Complement Factors and Cingulate Gyrus Cortical Thinning. Schizophr. Bull. 2023, 50, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Y.J.; Ji, R.R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Zhu, C.; Sun, S.; Wu, S.; Wang, L.; Wang, Y.; Ge, Z. Toll-like receptors and their role in neuropathic pain and migraine. Mol. Brain 2022, 15, 73. [Google Scholar] [CrossRef]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef]
- Vezzani, A.; Ruegg, S. The pivotal role of immunity and inflammatory processes in epilepsy is increasingly recognized: Introduction. Epilepsia 2011, 52 (Suppl. S3), 1–4. [Google Scholar] [CrossRef] [PubMed]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Bianchi, M.E.; Vezzani, A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: The importance of IL-1beta and high-mobility group box 1. J. Intern. Med. 2011, 270, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Mehra, P.; Keshri, A.K.; Rawat, S.S.; Mishra, A.; Prasad, A. The Emerging Role of Toll-Like Receptor-Mediated Neuroinflammatory Signals in Psychiatric Disorders and Acquired Epilepsy. Mol. Neurobiol. 2024, 61, 1527–1542. [Google Scholar] [CrossRef]
- Purdy, R.H.; Moore, P.H.; Morrow, A.L.; Paul, S.M. The 3a-hydroxy ring-A-reduced metabolites of progesterone and deoxycorticosterone: Natural ligands of central GABAA receptors. In Neurosteroids and Brain Function; Costa, E., Paul, S.M., Eds.; Raven Press: New York, NY, USA, 1991; pp. 95–102. [Google Scholar]
- Corpechot, C.; Young, J.; Calvel, M.; Wehrey, C.; Veltz, J.N.; Touyer, G.; Mouren, M.; Prasad, V.V.K.; Banner, C.; Sjövall, J.; et al. Neurosteroids: 3a-hydroxy-5a-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology 1993, 133, 1003–1009. [Google Scholar] [CrossRef]
- Mensah-Nyagan, A.G.; Do-Rego, J.L.; Beaujean, D.; Luu-The, V.; Pelletier, G.; Vaudry, H. Neurosteroids: Expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999, 51, 63–81. [Google Scholar] [PubMed]
- Mellon, S.H.; Deschepper, C.F. Neurosteroid biosynthesis: Genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993, 629, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Testas, I.J.; Hu, Z.Y.; Baulieuf, E.E.; Robel, P. Neurosteroids: Biosynthesis of Pregnenolone and Progesterone in Primary Cultures of Rat Glial Cells. Endocrinology 1989, 125, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Liu, J.; Culty, M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol. Cell. Endocrinol. 2007, 265–266, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Mass spectrometric assay and physiological-pharmacological activity of androgenic neurosteroids. Neurochem. Int. 2008, 52, 541–553. [Google Scholar] [CrossRef]
- Jellinck, P.H.; Kaufmann, M.; Gottfried-Blackmore, A.; McEwen, B.S.; Jones, G.; Bulloch, K. Selective conversion by microglia of dehydroepiandrosterone to 5-androstenediol—A steroid with inherent estrogenic properties. J. Steroid Biochem. Mol. Biol. 2007, 107, 156–162. [Google Scholar] [CrossRef]
- Lathe, R. Steroid and sterol 7-hydroxylation: Ancient pathways. Steroids 2002, 67, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Strott, C.A. Sulfonation and molecular action. Endocr. Rev. 2002, 23, 703–732. [Google Scholar] [CrossRef]
- Le Goascogne, C.; Robel, P.; Gouézou, M.; Sananes, N.; Baulieu, E.-E.; Waterman, M. Neurosteroids: Cytochrome P-450scc in Rat Brain. Science 1987, 237, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Milano, S.; Kluth, L.; Rhodes, M.E. Synthesis and sulfatase inhibitory activities of non-steroidal estrone sulfatase inhibitors. J. Steroid Biochem. Mol. Biol. 1996, 59, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003, 28, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Slater, E.P.; Hesse, H.; Beato, M. Regulation of transcription by steroid hormones. Ann. N. Y. Acad. Sci. 1994, 733, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Vitiello, S.; Bellocchio, L.; Hébert-Chatelain, E.; Monlezun, S.; Martin-Garcia, E.; Kasanetz, F.; Baillie, G.L.; Panin, F.; Cathala, A.; et al. Pregnenolone can protect the brain from cannabis intoxication. Science 2014, 343, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Fellous, A.; Baulieu, E.E.; Robel, P. Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly. Proc. Natl. Acad. Sci. USA 2000, 97, 3579–3584. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Koonce, C.J.; Walf, A.A. Role of pregnane xenobiotic receptor in the midbrain ventral tegmental area for estradiol- and 3alpha,5alpha-THP-facilitated lordosis of female rats. Psychopharmacology 2014, 231, 3365–3374. [Google Scholar] [CrossRef]
- Frye, C.A.; Koonce, C.J.; Walf, A.A. Novel receptor targets for production and action of allopregnanolone in the central nervous system: A focus on pregnane xenobiotic receptor. Front. Cell. Neurosci. 2014, 8, 106. [Google Scholar] [CrossRef]
- Sever, R.; Glass, C.K. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef]
- Arnal, J.F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, Y.; Su, W.; Xing, L.; Shen, Y.; He, X.; Li, L.; Yuan, Y.; Tang, X.; Chen, G. 17β-Estradiol Enhances Schwann Cell Differentiation via the ERβ-ERK1/2 Signaling Pathway and Promotes Remyelination in Injured Sciatic Nerves. Front. Pharmacol. 2018, 9, 1026. [Google Scholar] [CrossRef]
- Sieghart, W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006, 54, 231–263. [Google Scholar] [CrossRef]
- Hosie, A.M.; Wilkins, M.E.; da Silva, H.M.; Smart, T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 2006, 444, 486–489. [Google Scholar] [CrossRef]
- Puia, G.; Ducic, I.; Vicini, S.; Costa, E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Recept. Channels 1993, 1, 135–142. [Google Scholar]
- Mihalek, R.M.; Banerjee, P.K.; Korpi, E.R.; Quinlan, J.J.; Firestone, L.L.; Mi, Z.P.; Lagenaur, C.; Tretter, V.; Sieghart, W.; Anagnostaras, S.G.; et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12905–12910. [Google Scholar] [CrossRef]
- Spigelman, I.; Li, Z.; Liang, J.; Cagetti, E.; Samzadeh, S.; Mihalek, R.M.; Homanics, G.E.; Olsen, R.W. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J. Neurophysiol. 2003, 90, 903–910. [Google Scholar] [CrossRef]
- Lambert, J.J.; Belelli, D.; Hill-Venning, C.; Peters, J.A. Neurosteroids and GABAA receptor function. Trends Pharmacol. Sci. 1995, 16, 295–303. [Google Scholar] [CrossRef]
- Purdy, R.H.; Moore, P.H.; Rao, P.N.; Hagino, N.; Yamaguchi, T.; Schmidt, P.; Rubinow, D.; Morrow, A.L.; Paul, S.M. Radioimmunoassay of 3 alpha-hydroxy-5 alpha-pregnan-20-one in rat and human plasma. Steroids 1990, 55, 290–296. [Google Scholar] [CrossRef]
- Majewska, M.D.; Schwartz, R.D. Pregnenolone-sulfate: An endogenous antagonist of the g-aminobutyric acid receptor complex in brain. Brain Res. 1987, 404, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Demirgören, S.; London, E.D. Binding of pregnenolone sulfate to rat brain membranes suggests multiple sites of steroid action at the GABAA receptor. Eur. J. Pharmacol. Mol. Pharmacol. 1990, 189, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Demirgören, S.; Spivak, C.E.; London, E.D. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990, 526, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Park-Chung, M.; Malayev, A.; Purdy, R.H.; Gibbs, T.T.; Farb, D.H. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999, 830, 72–87. [Google Scholar] [CrossRef]
- Wu, F.S.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate: A positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 1991, 40, 333–336. [Google Scholar] [PubMed]
- Shi, S.H.; Hayashi, Y.; Petralia, R.S.; Zaman, S.H.; Wenthold, R.J.; Svoboda, K.; Malinow, R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 1999, 284, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Roman, F.J.; Privat, A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to s1 receptors in the mouse forebrain. J. Neurosci. Res. 1996, 46, 734–743. [Google Scholar] [CrossRef]
- Reddy, D.S. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol. Sci. 2003, 24, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Schverer, M.; Lanfumey, L.; Baulieu, E.E.; Froger, N.; Villey, I. Neurosteroids: Non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacol. Ther. 2018, 191, 190–206. [Google Scholar] [CrossRef]
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol. Stress 2020, 12, 100215. [Google Scholar] [CrossRef]
- Milivojevic, V.; Charron, L.; Fogelman, N.; Hermes, G.; Sinha, R. Pregnenolone Reduces Stress-Induced Craving, Anxiety, and Autonomic Arousal in Individuals with Cocaine Use Disorder. Biomolecules 2022, 12, 1593. [Google Scholar] [CrossRef] [PubMed]
- Bixo, M.; Andersson, A.; Winblad, B.; Purdy, R.H.; Backstrom, T. Progesterone, 5⍺-pregnan-3,20-dione and 3⍺-hydroxy-5⍺-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997, 764, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Adinoff, B.; Junghanns, K.; Kiefer, F.; Krishnan-Sarin, S. Suppression of the HPA axis stress-response: Implications for relapse. Alcohol. Clin. Exp. Res. 2005, 29, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Adinoff, B.; Krebaum, S.R.; Chandler, P.A.; Ye, W.; Brown, M.B.; Williams, M.J. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: Adrenocortical and pituitary glucocorticoid responsiveness. Alcohol. Clin. Exp. Res. 2005, 29, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Adinoff, B.; Krebaum, S.R.; Chandler, P.A.; Ye, W.; Brown, M.B.; Williams, M.J. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: Response to ovine corticotropin-releasing factor and naloxone. Alcohol. Clin. Exp. Res. 2005, 29, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Lightman, S.L.; Pariante, C.M. The Interface of Stress and the HPA Axis in Behavioural Phenotypes of Mental Illness. Curr. Top. Behav. Neurosci. 2014, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Schule, C.; Nothdurfter, C.; Rupprecht, R. The role of allopregnanolone in depression and anxiety. Prog. Neurobiol. 2014, 113, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, A.M.; Marx, C.E.; Pineles, S.L.; Locci, A.; Scioli-Salter, E.R.; Nillni, Y.I.; Liang, J.J.; Pinna, G. Neuroactive steroids and PTSD treatment. Neurosci. Lett. 2017, 649, 156–163. [Google Scholar] [CrossRef]
- Decavel, C.; Van den Pol, A.N. GABA: A dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 1990, 302, 1019–1037. [Google Scholar] [CrossRef]
- Decavel, C.; van den Pol, A.N. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J. Comp. Neurol. 1992, 316, 104–116. [Google Scholar] [CrossRef]
- Rasmusson, A.M.; Pinna, G.; Paliwal, P.; Weisman, D.; Gottschalk, C.; Charney, D.; Krystal, J.; Guidotti, A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol. Psychiatry 2006, 60, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Pisu, M.G.; Littera, M.; Papi, G.; Sanna, E.; Tuveri, F.; Usala, L.; Purdy, R.H.; Biggio, G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J. Neurochem. 2000, 75, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Matsumoto, K.; Uzunova, V.; Sugaya, I.; Takahata, H.; Nomura, H.; Watanabe, H.; Costa, E.; Guidotti, A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. USA 2001, 98, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Pisu, M.G.; Floris, I.; Biggio, G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress 2005, 8, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Pisu, M.G.; Garau, A.; Boero, G.; Biggio, F.; Pibiri, V.; Dore, R.; Locci, V.; Paci, E.; Porcu, P.; Serra, M. Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience 2016, 320, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Boero, G.; Pisu, M.G.; Biggio, F.; Muredda, L.; Carta, G.; Banni, S.; Paci, E.; Follesa, P.; Concas, A.; Porcu, P.; et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology 2018, 133, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Blaine, S.K.; Nautiyal, N.; Hart, R.; Guarnaccia, J.B.; Sinha, R. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict. Biol. 2019, 24, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Ströhle, A.; Spalletta, G.; di Michele, F.; Hermann, B.; Holsboer, F.; Pasini, A.; Rupprecht, R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry 1998, 155, 910–913. [Google Scholar] [CrossRef]
- Uzunova, V.; Sheline, Y.; Davis, J.M.; Rasmusson, A.; Uzunov, D.P.; Costa, E.; Guidotti, A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. USA 1998, 95, 3239–3244. [Google Scholar] [CrossRef]
- Ströhle, A.; Romeo, E.; Hermann, B.; Pasini, A.; Spalletta, G.; di Michele, F.; Holsboer, F.; Rupprecht, R. Concentrations of 3a-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol. Psychiatry 1999, 45, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Deligiannidis, K.M.; Sikoglu, E.M.; Shaffer, S.A.; Frederick, B.; Svenson, A.E.; Kopoyan, A.; Kosma, C.A.; Rothschild, A.J.; Moore, C.M. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: A preliminary study. J. Psychiatr. Res. 2013, 47, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Deligiannidis, K.M.; Kroll-Desrosiers, A.R.; Mo, S.; Nguyen, H.P.; Svenson, A.; Jaitly, N.; Hall, J.E.; Barton, B.A.; Rothschild, A.J.; Shaffer, S.A. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology 2016, 70, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Magiakou, M.A.; Mastorakos, G.; Rabin, D.; Dubbert, B.; Gold, P.W.; Chrousos, G.P. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: Implications for the increase in psychiatric manifestations at this time. J. Clin. Endocrinol. Metab. 1996, 81, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S. New insights into perinatal depression: Pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin. Neurosci. 2011, 13, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.; Mody, I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron 2008, 59, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Wakefield, S.; MacKenzie, G.; Moss, S.J.; Maguire, J. Neurosteroidogenesis is required for the physiological response to stress: Role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 2011, 31, 18198–18210. [Google Scholar] [CrossRef]
- Maguire, J.; Mody, I. Behavioral Deficits in Juveniles Mediated by Maternal Stress Hormones in Mice. Neural Plast. 2016, 2016, 2762518. [Google Scholar] [CrossRef]
- Patchev, V.K.; Shoaib, M.; Holsboer, F.; Almeida, O.F.X. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994, 62, 265–271. [Google Scholar] [CrossRef]
- Patchev, V.K.; Hassan, A.H.S.; Holsboer, F.; Almeida, O.F.X. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 1996, 15, 533–540. [Google Scholar] [CrossRef]
- Owens, M.J.; Ritchie, J.C.; Nemeroff, C.B. 5 alpha-pregnane-3 alpha, 21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: Comparison with alprazolam. Brain Res. 1992, 573, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kendall-Tackett, K. A new paradigm for depression in new mothers: The central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int. Breastfeed. J. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Uematsu, S.; Hoshino, K.; Kaisho, T.; Takeuchi, O.; Takeda, K.; Akira, S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 2003, 4, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- L’Episcopo, F.; Tirolo, C.; Serapide, M.F.; Caniglia, S.; Testa, N.; Leggio, L.; Vivarelli, S.; Iraci, N.; Pluchino, S.; Marchetti, B. Microglia Polarization, Gene-Environment Interactions and Wnt/β-Catenin Signaling: Emerging Roles of Glia-Neuron and Glia-Stem/Neuroprogenitor Crosstalk for Dopaminergic Neurorestoration in Aged Parkinsonian Brain. Front. Aging Neurosci. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D.; Woo, D.H.; Basser, P.J. Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication. Neuron 2015, 86, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Sur, M.; Araque, A. Neuron-glia networks: Integral gear of brain function. Front. Cell. Neurosci. 2014, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Maroso, M.; Balosso, S.; Ravizza, T.; Iori, V.; Wright, C.I.; French, J.; Vezzani, A. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 2011, 8, 304–315. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Akyuz, E.; Piperi, C.; Othman, I.; Shaikh, M.F. Role of Innate Immune Receptor TLR4 and its endogenous ligands in epileptogenesis. Pharmacol. Res. 2020, 160, 105172. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Benninger, F.; Madar, R.; Illouz, T.; Griffioen, K.; Steiner, I.; Offen, D.; Okun, E. Toll-like receptor 3 deficiency decreases epileptogenesis in a pilocarpine model of SE-induced epilepsy in mice. Epilepsia 2017, 58, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, D.F.; Randall, P.A.; Van Voorhies, K.; Vetreno, R.P.; Crews, F.T.; Besheer, J. Increased alcohol self-administration following repeated Toll-like receptor 3 agonist treatment in male and female rats. Pharmacol. Biochem. Behav. 2022, 216, 173379. [Google Scholar] [CrossRef]
- Tanga, F.Y.; Nutile-McMenemy, N.; DeLeo, J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. USA 2005, 102, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tanga, F.Y.; DeLeo, J.A. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience 2009, 158, 896–903. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z. Upregulated TLR3 Promotes Neuropathic Pain by Regulating Autophagy in Rat with L5 Spinal Nerve Ligation Model. Neurochem. Res. 2017, 42, 634–643. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Han, G.; Wu, S.; Du, S.; Zhang, Y.; Liu, W.; Jiang, B.; Zhang, L.; Xia, S.; Jia, S.; et al. Toll-like receptor 7 contributes to neuropathic pain by activating NF-κB in primary sensory neurons. Brain Behav. Immun. 2020, 87, 840–851. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Guo, J.-S.; Li, S.-S.; Wu, X.-B.; Cao, D.-L.; Jiang, B.-C.; Jing, P.-B.; Bai, X.-Q.; Li, C.-H.; Wu, Z.-H.; et al. TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J. Exp. Med. 2018, 215, 3019–3037. [Google Scholar] [CrossRef]
- Yang, H.; Wu, L.; Deng, H.; Chen, Y.; Zhou, H.; Liu, M.; Wang, S.; Zheng, L.; Zhu, L.; Lv, X. Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia. J. Neuroinflammation 2020, 17, 154. [Google Scholar] [CrossRef]
- Colleselli, K.; Stierschneider, A.; Wiesner, C. An Update on Toll-like Receptor 2, Its Function and Dimerization in Pro- and Anti-Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 12464. [Google Scholar] [CrossRef] [PubMed]
- Siegemund, S.; Sauer, K. Balancing pro- and anti-inflammatory TLR4 signaling. Nat. Immunol. 2012, 13, 1031–1033. [Google Scholar] [CrossRef]
- Aksoy, E.; Taboubi, S.; Torres, D.; Delbauve, S.; Hachani, A.; Whitehead, M.A.; Pearce, W.P.; Berenjeno, I.M.; Nock, G.; Filloux, A.; et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat. Immunol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef]
- Dasgupta, S.; Erturk-Hasdemir, D.; Ochoa-Reparaz, J.; Reinecker, H.C.; Kasper, D.L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 2014, 15, 413–423. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Chávez-Arroyo, A.; Cheng, M.I.; Krasilnikov, M.; Louie, A.; Portnoy, D.A. TLR2 and endosomal TLR-mediated secretion of IL-10 and immune suppression in response to phagosome-confined Listeria monocytogenes. PLoS Pathog. 2020, 16, e1008622. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Dembek, A.; Linnenberger, R.; Dahlem, C.; Barghash, A.; Fecher-Trost, C.; Fuhrmann, G.; Koch, M.; Kraegeloh, A.; Huwer, H.; et al. Toll-Like Receptor 2 Release by Macrophages: An Anti-inflammatory Program Induced by Glucocorticoids and Lipopolysaccharide. Front. Immunol. 2019, 10, 1634. [Google Scholar] [CrossRef]
- Zamora-Pineda, J.; Kalinina, O.; Sperling, A.I.; Knight, K.L. Mechanism of TLR4-Mediated Anti-Inflammatory Response Induced by Exopolysaccharide from the Probiotic Bacillus subtilis. J. Immunol. 2023, 211, 1232–1239. [Google Scholar] [CrossRef]
- Leibler, C.; John, S.; Elsner, R.A.; Thomas, K.B.; Smita, S.; Joachim, S.; Levack, R.C.; Callahan, D.J.; Gordon, R.A.; Bastacky, S.; et al. Genetic dissection of TLR9 reveals complex regulatory and cryptic proinflammatory roles in mouse lupus. Nat. Immunol. 2022, 23, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, X. TLR9 triggers MyD88-independent anti-inflammatory signaling in lupus. Trends Immunol. 2023, 44, 153–155. [Google Scholar] [CrossRef]
- Verma, R.; Kim, J.Y. 1,25-Dihydroxyvitamin D3 Facilitates M2 Polarization and Upregulates TLR10 Expression on Human Microglial Cells. Neuroimmunomodulation 2016, 23, 75–80. [Google Scholar] [CrossRef]
- Lei, B.; Mace, B.; Dawson, H.N.; Warner, D.S.; Laskowitz, D.T.; James, M.L. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS ONE 2014, 9, e103969. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Brevé, J.J.; Wierinckx, A.; van der Jagt, S.; Bristow, A.F.; Leysen, J.E.; Tilders, F.J.; Van Dam, A.M. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur. J. Neurosci. 2002, 16, 1175–1185. [Google Scholar] [CrossRef]
- Balasingam, V.; Yong, V.W. Attenuation of astroglial reactivity by interleukin-10. J. Neurosci. 1996, 16, 2945–2955. [Google Scholar] [CrossRef]
- Norden, D.M.; Fenn, A.M.; Dugan, A.; Godbout, J.P. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 2014, 62, 881–895. [Google Scholar] [CrossRef]
- Makar, T.K.; Bever, C.T.; Singh, I.S.; Royal, W.; Sahu, S.N.; Sura, T.P.; Sultana, S.; Sura, K.T.; Patel, N.; Dhib-Jalbut, S.; et al. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. J. Neuroimmunol. 2009, 210, 40–51. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, N.; Zhu, J.; Lu, T.; Chen, Z.; Xu, G.; Liu, X. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediat. Inflamm. 2010, 2010, 372423. [Google Scholar] [CrossRef]
- Xu, D.; Lian, D.; Wu, J.; Liu, Y.; Zhu, M.; Sun, J.; He, D.; Li, L. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Neuroinflamm. 2017, 14, 156. [Google Scholar] [CrossRef]
- Morrow, A.L.; Boero, G.; Balan, I. Emerging Evidence for Endogenous Neurosteroid Modulation of Pro-Inflammatory and Anti-Inflammatory Pathways that Impact Neuropsychiatric Disease. Neurosci. Biobehav. Rev. 2024, 158, 105558. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sipthorp, J.; Mahata, B.; Pramanik, J.; Hennrich, M.L.; Gavin, A.C.; Ley, S.V.; Teichmann, S.A. CLICK-enabled analogues reveal pregnenolone interactomes in cancer and immune cells. iScience 2021, 24, 102485. [Google Scholar] [CrossRef]
- Roy, S.; Roy, S.; Mahata, B.; Pramanik, J.; Hennrich, M.L.; Gavin, A.C.; Teichmann, S.A. CLICK-chemoproteomics and molecular dynamics simulation reveals pregnenolone targets and their binding conformations in Th2 cells. Front. Immunol. 2023, 14, 1229703. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.L. Interaction between Cannabinoid System and Toll-Like Receptors Controls Inflammation. Mediat. Inflamm. 2016, 2016, 5831315. [Google Scholar] [CrossRef]
- Duncan, M.; Galic, M.A.; Wang, A.; Chambers, A.P.; McCafferty, D.M.; McKay, D.M.; Sharkey, K.A.; Pittman, Q.J. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R224–R231. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef] [PubMed]
- Jitprasertwong, P.; Charadram, N.; Kumphune, S.; Pongcharoen, S.; Sirisinha, S. Female sex hormones modulate Porphyromonas gingivalis lipopolysaccharide-induced Toll-like receptor signaling in primary human monocytes. J. Periodontal Res. 2016, 51, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Guignard, L.; Reymond, M.K.; Perreau, M.; Roth-Kleiner, M.; Calandra, T.; Roger, T. Estradiol and Progesterone Strongly Inhibit the Innate Immune Response of Mononuclear Cells in Newborns. Infect. Immun. 2011, 79, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.; Kerschbaum, H.H. Progesterone and its metabolites 5-dihydroprogesterone and 5-3-tetrahydroprogesterone decrease LPS-induced NO release in the murine microglial cell line, BV-2. Neuro Endocrinol. Lett. 2006, 27, 675–678. [Google Scholar] [PubMed]
- Salama, R.M.; Tadros, M.G.; Schaalan, M.F.; Bahaa, N.; Abdel-Tawab, A.M.; Khalifa, A.E. Potential neuroprotective effect of androst-5-ene-3β, 17β-diol (ADIOL) on the striatum, and substantia nigra in Parkinson’s disease rat model. J. Cell. Physiol. 2018, 233, 5981–6000. [Google Scholar] [CrossRef]
- Auci, D.; Nicoletti, F.; Mangano, K.; Pieters, R.; Nierkens, S.; Morgan, L.; Offner, H.; Frincke, J.; Reading, C. Anti-inflammatory and Immune Regulatory Properties of 5-Androsten-3β, 17β-Diol (HE2100), and Synthetic Analogue HE3204: Implications for Treatment of Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2005, 1051, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Kalakh, S.; Mouihate, A. Androstenediol Reduces Demyelination-Induced Axonopathy in the Rat Corpus Callosum: Impact on Microglial Polarization. Front. Cell. Neurosci. 2017, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, N.; Khanlou, N.; Clare, R.; Jiang, Q.; Reed-Geaghan, E.G.; Landreth, G.E.; Vinters, H.V.; Tontonoz, P. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 10601–10606. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.M.; Tadros, M.G.; Khalifa, A.E. ADIOL protects against 3-NP-induced neurotoxicity in rats: Possible impact of its anti-oxidant, anti-inflammatory and anti-apoptotic actions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 36–51. [Google Scholar] [CrossRef]
- Nicoletti, F.; Auci, D.L.; Mangano, K.; Flores-Riveros, J.; Villegas, S.; Frincke, J.M.; Reading, C.L.; Offner, H. 5-Androstenediol Ameliorates Pleurisy, Septic Shock, and Experimental Autoimmune Encephalomyelitis in Mice. Autoimmune Dis. 2010, 2010, 757432. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Benedusi, V.; Maggi, A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol. 2008, 29, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yang, D.; Yin, C.; Zhang, J. Androgen Receptor (AR)-TLR4 Crosstalk Mediates Gender Disparities in Hepatocellular Carcinoma Incidence and Progression. J. Cancer 2020, 11, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Ainola, M.; Porola, P.; Takakubo, Y.; Przybyla, B.; Kouri, V.P.; Tolvanen, T.A.; Hänninen, A.; Nordström, D.C. Activation of plasmacytoid dendritic cells by apoptotic particles—Mechanism for the loss of immunological tolerance in Sjögren’s syndrome. Clin. Exp. Immunol. 2017, 191, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Huang, H.M.; Chen, H.L.; Kuo, J.S.; Jeng, K.C. Dehydroepiandrosterone inhibits lipopolysaccharide-induced nitric oxide production in BV-2 microglia. J. Neurochem. 2001, 77, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Schurr, M.J.; Fabian, T.C.; Croce, M.A.; Varnavas, L.E.; Proctor, K.G. Dehydroepiandrosterone, an endogenous immune modulator, after traumatic shock. Shock 1997, 7, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Luborsky, L.; McKay, J.R.; Rosenthal, R.; Houldin, A.; Tax, A.; McCorkle, R.; Seligman, D.A.; Schmidt, K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav. Immun. 2001, 15, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Derecki, N.C.; Lovenberg, T.W.; Drevets, W.C. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology 2016, 233, 1623–1636. [Google Scholar] [CrossRef]
- Vichaya, E.G.; Laumet, G.; Christian, D.L.; Grossberg, A.J.; Estrada, D.J.; Heijnen, C.J.; Kavelaars, A.; Dantzer, R. Motivational changes that develop in a mouse model of inflammation-induced depression are independent of indoleamine 2,3 dioxygenase. Neuropsychopharmacology 2019, 44, 364–371. [Google Scholar] [CrossRef]
- Bullmore, E. The art of medicine: Inflamed depression. Lancet 2018, 392, 1189–1190. [Google Scholar] [CrossRef]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020, 83, 239–247. [Google Scholar] [CrossRef]
- Sha, Q.; Madaj, Z.; Keaton, S.; Escobar Galvis, M.L.; Smart, L.; Krzyzanowski, S.; Fazleabas, A.T.; Leach, R.; Postolache, T.T.; Achtyes, E.D.; et al. Cytokines and tryptophan metabolites can predict depressive symptoms in pregnancy. Transl. Psychiatry 2022, 12, 35. [Google Scholar] [CrossRef]
- Kim, T.D.; Lee, S.; Yoon, S. Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants 2020, 9, 107. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Hajebrahimi, B.; Bagheri, M.; Hassanshahi, G.; Nazari, M.; Bidaki, R.; Khodadadi, H.; Arababadi, M.K.; Kennedy, D. The adapter proteins of TLRs, TRIF and MYD88, are upregulated in depressed individuals. Int. J. Psychiatry Clin. Pract. 2014, 18, 41–44. [Google Scholar] [CrossRef]
- Wu, M.K.; Huang, T.L.; Huang, K.W.; Huang, Y.L.; Hung, Y.Y. Association between toll-like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatr. Dis. Treat. 2015, 11, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.Y.; Kang, H.Y.; Huang, K.W.; Huang, T.L. Association between toll-like receptors expression and major depressive disorder. Psychiatry Res. 2014, 220, 283–286. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Fareed, J.; Hoppensteadt, D.A.; Roberts, R.C.; Conley, R.R.; Dwivedi, Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res. 2012, 46, 57–63. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Bhaumik, R.; Ren, X. Innate immunity in the postmortem brain of depressed and suicide subjects: Role of Toll-like receptors. Brain Behav. Immun. 2019, 75, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Corwin, E.J.; Johnston, N.; Pugh, L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol. Res. Nurs. 2008, 10, 128–133. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Peters, R.M.; Johnson, D.A.; Templin, T.N. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J. Reprod. Immunol. 2012, 94, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Yurashevich, M.; Cooter Wright, M.; Sims, S.C.; Tan, H.S.; Berger, M.; Ji, R.R.; Habib, A.S. Inflammatory changes in the plasma and cerebrospinal fluid of patients with persistent pain and postpartum depression after elective Cesarean delivery: An exploratory prospective cohort study. Can. J. Anaesth. 2023, 70, 1917–1927. [Google Scholar] [CrossRef]
- Boufidou, F.; Lambrinoudaki, I.; Argeitis, J.; Zervas, I.M.; Pliatsika, P.; Leonardou, A.A.; Petropoulos, G.; Hasiakos, D.; Papadias, K.; Nikolaou, C. CSF and plasma cytokines at delivery and postpartum mood disturbances. J. Affect. Disord. 2009, 115, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Lin, A.H.; Ombelet, W.; Stevens, K.; Kenis, G.; De Jongh, R.; Cox, J.; Bosmans, E. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology 2000, 25, 121–137. [Google Scholar] [CrossRef]
- Fransson, E.; Dubicke, A.; Byström, B.; Ekman-Ordeberg, G.; Hjelmstedt, A.; Lekander, M. Negative emotions and cytokines in maternal and cord serum at preterm birth. Am. J. Reprod. Immunol. 2012, 67, 506–514. [Google Scholar] [CrossRef]
- Picard, K.; Bisht, K.; Poggini, S.; Garofalo, S.; Golia, M.T.; Basilico, B.; Abdallah, F.; Ciano Albanese, N.; Amrein, I.; Vernoux, N.; et al. Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice. Brain Behav. Immun. 2021, 97, 423–439. [Google Scholar] [CrossRef]
- Sierra, A.; Gottfried-Blackmore, A.; Milner, T.A.; McEwen, B.S.; Bulloch, K. Steroid hormone receptor expression and function in microglia. Glia 2008, 56, 659–674. [Google Scholar] [CrossRef]
- Hermoso, M.A.; Matsuguchi, T.; Smoak, K.; Cidlowski, J.A. Glucocorticoids and tumor necrosis factor alpha cooperatively regulate toll-like receptor 2 gene expression. Mol. Cell. Biol. 2004, 24, 4743–4756. [Google Scholar] [CrossRef]
- Busillo, J.M.; Azzam, K.M.; Cidlowski, J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef]
- Jones, K.A.; Thomsen, C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013, 53, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Marsh, S.E.; Stevens, B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020, 41, 820–835. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Charles-Messance, H.; Blot, G.; Couturier, A.; Vignaud, L.; Touhami, S.; Beguier, F.; Siqueiros, L.; Forster, V.; Barmo, N.; Augustin, S.; et al. IL-1β induces rod degeneration through the disruption of retinal glutamate homeostasis. J. Neuroinflammation 2020, 17, 1. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Liu, Y.; Li, Y.Y.; Chen, C.; Liu, L.M.; Wu, Y.M.; Li, S.; Yang, C. Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med. Sci. Monit. 2014, 20, 499–512. [Google Scholar] [CrossRef]
- Meyerhoff, D.J.; Mon, A.; Metzler, T.; Neylan, T.C. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 2014, 37, 893–900. [Google Scholar] [CrossRef]
- Nie, H.; Peng, Z.; Lao, N.; Wang, H.; Chen, Y.; Fang, Z.; Hou, W.; Gao, F.; Li, X.; Xiong, L.; et al. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 16–22. [Google Scholar] [CrossRef]
- Porcu, P.; Lallai, V.; Locci, A.; Catzeddu, S.; Serra, V.; Pisu, M.G.; Serra, M.; Dazzi, L.; Concas, A. Changes in stress-stimulated allopregnanolone levels induced by neonatal estradiol treatment are associated with enhanced dopamine release in adult female rats: Reversal by progesterone administration. Psychopharmacology 2017, 234, 749–760. [Google Scholar] [CrossRef]
- Kanes, S.J.; Colquhoun, H.; Doherty, J.; Raines, S.; Hoffmann, E.; Rubinow, D.R.; Meltzer-Brody, S. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum. Psychopharmacol. 2017, 32, e2576. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Howard, L.M.; Bergink, V.; Vigod, S.; Jones, I.; Munk-Olsen, T.; Honikman, S.; Milgrom, J. Postpartum psychiatric disorders. Nat. Rev. Dis. Primers 2018, 4, 18022. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Meltzer-Brody, S.; Gunduz-Bruce, H.; Doherty, J.; Jonas, J.; Li, S.; Sankoh, A.J.; Silber, C.; Campbell, A.D.; Werneburg, B.; et al. Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 951–959. [Google Scholar] [CrossRef]
- Gunduz-Bruce, H.; Silber, C.; Kaul, I.; Rothschild, A.J.; Riesenberg, R.; Sankoh, A.J.; Li, H.; Lasser, R.; Zorumski, C.F.; Rubinow, D.R.; et al. Trial of SAGE-217 in Patients with Major Depressive Disorder. N. Engl. J. Med. 2019, 381, 903–911. [Google Scholar] [CrossRef]
- Rasmusson, A.M.; Marx, C.E.; Jain, S.; Farfel, G.M.; Tsai, J.; Sun, X.; Geracioti, T.D.; Hamner, M.B.; Lohr, J.; Rosse, R.; et al. A randomized controlled trial of ganaxolone in posttraumatic stress disorder. Psychopharmacology 2017, 234, 2245–2257. [Google Scholar] [CrossRef]
- Walther, A.; Breidenstein, J.; Miller, R. Association of Testosterone Treatment with Alleviation of Depressive Symptoms in Men: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 31–40. [Google Scholar] [CrossRef]
- Espallergues, J.; Mamiya, T.; Vallée, M.; Koseki, T.; Nabeshima, T.; Temsamani, J.; Laruelle, C.; Maurice, T. The antidepressant-like effects of the 3β-hydroxysteroid dehydrogenase inhibitor trilostane in mice is related to changes in neuroactive steroid and monoamine levels. Neuropharmacology 2012, 62, 492–502. [Google Scholar] [CrossRef]
- Koonce, C.J.; Walf, A.A.; Frye, C.A. Trilostane exerts antidepressive effects among wild-type, but not estrogen receptor [beta] knockout mice. Neuroreport 2009, 20, 1047–1050. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflamm. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Syed, W.A.; Minter, S.C.; Bowers, M.S. Differential response of glial fibrillary acidic protein-positive astrocytes in the rat prefrontal cortex following ethanol self-administration. Alcohol. Clin. Exp. Res. 2015, 39, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Fu, M.; Kumar, S.; Kumar, A. Methamphetamine potentiates HIV-1 gp120-mediated autophagy via Beclin-1 and Atg5/7 as a pro-survival response in astrocytes. Cell Death Dis. 2016, 7, e2425. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, P.M.; Hauser, K.F. Glial modulators as potential treatments of psychostimulant abuse. Adv. Pharmacol. 2014, 69, 1–69. [Google Scholar] [CrossRef]
- Kane, C.J.; Phelan, K.D.; Douglas, J.C.; Wagoner, G.; Johnson, J.W.; Xu, J.; Phelan, P.S.; Drew, P.D. Effects of ethanol on immune response in the brain: Region-specific changes in adolescent versus adult mice. Alcohol. Clin. Exp. Res. 2014, 38, 384–391. [Google Scholar] [CrossRef]
- Pascual, M.; Balino, P.; Aragon, C.M.; Guerri, C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology 2015, 89, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Calvo-Rodriguez, M.; Nunez, L.; Villalobos, C.; Urena, J.; Guerri, C. Toll-like receptors in neuroinflammation, neurodegeneration, and alcohol-induced brain damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.J.; Fuller, B.E.; Huckans, M.; Loftis, J.M. Peripheral immune factors are elevated in women with current or recent alcohol dependence and associated with altered mood and memory. Drug Alcohol Depend. 2017, 176, 71–78. [Google Scholar] [CrossRef]
- Warden, A.S.; Azzam, M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Messing, R.O.; Mayfield, R.D.; Harris, R.A. Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain Behav. Immun. 2019, 77, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Randall, P.A.; Vetreno, R.P.; Makhijani, V.H.; Crews, F.T.; Besheer, J. The Toll-Like Receptor 3 Agonist Poly(I:C) Induces Rapid and Lasting Changes in Gene Expression Related to Glutamatergic Function and Increases Ethanol Self-Administration in Rats. Alcohol. Clin. Exp. Res. 2019, 43, 48–60. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Benavidez, J.M.; Geil, C.; Perra, S.; Morikawa, H.; Harris, R.A. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 2011, 25 (Suppl. S1), S92–S105. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 2013, 126, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; Zou, J.; Qin, L.; Crews, F.T. HMGB1/IL-1beta complexes regulate neuroimmune responses in alcoholism. Brain Behav. Immun. 2018, 72, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Ersche, K.D.; Hagan, C.C.; Smith, D.G.; Abbott, S.; Jones, P.S.; Apergis-Schoute, A.M.; Döffinger, R. Aberrant disgust responses and immune reactivity in cocaine-dependent men. Biol. Psychiatry 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Levandowski, M.L.; Hess, A.R.; Grassi-Oliveira, R.; de Almeida, R.M. Plasma interleukin-6 and executive function in crack cocaine-dependent women. Neurosci. Lett. 2016, 628, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.P.; Medeiros, J.R.; Lhullier, A.C.; Souza, L.D.; Jansen, K.; Portela, L.V.; Lara, D.R.; da Silva, R.A.; Wiener, C.D.; Oses, J.P. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend. 2016, 158, 181–185. [Google Scholar] [CrossRef]
- Shin, E.J.; Tran, H.Q.; Nguyen, P.T.; Jeong, J.H.; Nah, S.Y.; Jang, C.G.; Nabeshima, T.; Kim, H.C. Role of Mitochondria in Methamphetamine-Induced Dopaminergic Neurotoxicity: Involvement in Oxidative Stress, Neuroinflammation, and Pro-apoptosis—A Review. Neurochem. Res. 2018, 43, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Loftis, J.M.; Janowsky, A. Neuroimmune basis of methamphetamine toxicity. Int. Rev. Neurobiol. 2014, 118, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.H.; Wiley, C.A.; Bradberry, C.W. Psychostimulant abuse and neuroinflammation: Emerging evidence of their interconnection. Neurotox. Res. 2013, 23, 174–188. [Google Scholar] [CrossRef]
- Loftis, J.M.; Choi, D.; Hoffman, W.; Huckans, M.S. Methamphetamine causes persistent immune dysregulation: A cross-species, translational report. Neurotox. Res. 2011, 20, 59–68. [Google Scholar] [CrossRef]
- Gonçalves, J.; Martins, T.; Ferreira, R.; Milhazes, N.; Borges, F.; Ribeiro, C.F.; Malva, J.O.; Macedo, T.R.; Silva, A.P. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann. N. Y. Acad. Sci. 2008, 1139, 103–111. [Google Scholar] [CrossRef] [PubMed]
- LaVoie, M.J.; Card, J.P.; Hastings, T.G. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp. Neurol. 2004, 187, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, L.; Gran, B.; Constantinescu, C.S. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology 2010, 215, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Schwaeble, W.; Constantinescu, C.S. Relationship between cannabinoids and the immune system. Special Issue 8, 2010 Introduction. Immunobiology 2010, 215, 587. [Google Scholar] [CrossRef] [PubMed]
- Bayazit, H.; Selek, S.; Karababa, I.F.; Cicek, E.; Aksoy, N. Evaluation of Oxidant/Antioxidant Status and Cytokine Levels in Patients with Cannabis Use Disorder. Clin. Psychopharmacol. Neurosci. 2017, 15, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.M.; Donaldson, L.A.; Cherian, S.; Chilukuri, R.; White, V.A.; Sopori, M.L. Effects of cigarette smoke on the immune response. II. Chronic exposure to cigarette smoke inhibits surface immunoglobulin-mediated responses in B cells. Toxicol. Appl. Pharmacol. 1991, 111, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Sopori, M.L.; Kozak, W. Immunomodulatory effects of cigarette smoke. J. Neuroimmunol. 1998, 83, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Savage, S.M.; Johnson, L.J.; Seagrave, J.; Sopori, M.L. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol. Appl. Pharmacol. 1995, 135, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.Z.; Jin, X.D.; Guan, L.X.; Yan, H.C.; Wang, P.; Gong, Z.; Li, S.J.; Cao, X.; Xing, Y.L.; Gao, T.M. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol. Neurobiol. 2015, 51, 1480–1488. [Google Scholar] [CrossRef]
- O’Dell, L.E.; Purdy, R.H.; Covey, D.F.; Richardson, H.N.; Roberto, M.; Koob, G.F. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol. Biochem. Behav. 2005, 81, 543–550. [Google Scholar] [CrossRef]
- Szabo-Pardi, T.A.; Barron, L.R.; Lenert, M.E.; Burton, M.D. Sensory Neuron TLR4 mediates the development of nerve-injury induced mechanical hypersensitivity in female mice. Brain Behav. Immun. 2021, 97, 42–60. [Google Scholar] [CrossRef]
- Agalave, N.M.; Larsson, M.; Abdelmoaty, S.; Su, J.; Baharpoor, A.; Lundbäck, P.; Palmblad, K.; Andersson, U.; Harris, H.; Svensson, C.I. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 2014, 155, 1802–1813. [Google Scholar] [CrossRef]
- Christianson, C.A.; Dumlao, D.S.; Stokes, J.A.; Dennis, E.A.; Svensson, C.I.; Corr, M.; Yaksh, T.L. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011, 152, 2881–2891. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Stokes, J.A.; Cheung, J.; Eddinger, K.; Corr, M.; Yaksh, T.L. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J. Neuroinflamm. 2013, 10, 148. [Google Scholar] [CrossRef]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef]
- Stokes, J.A.; Corr, M.; Yaksh, T.L. Spinal toll-like receptor signaling and nociceptive processing: Regulatory balance between TIRAP and TRIF cascades mediated by TNF and IFNβ. Pain 2013, 154, 733–742. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; Villar, M.J.; De Nicola, A.F.; González, S.L. Progesterone Prevents Allodynia After Experimental Spinal Cord Injury. J. Pain 2011, 12, 71–83. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; Roig, P.; Villar, M.J.; De Nicola, A.F.; González, S.L. Progesterone prevents nerve injury-induced allodynia and spinal NMDA receptor upregulation in rats. Pain Med. 2011, 12, 1249–1261. [Google Scholar] [CrossRef]
- Cutler, S.M.; Cekic, M.; Miller, D.M.; Wali, B.; VanLandingham, J.W.; Stein, D.G. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma 2007, 24, 1475–1486. [Google Scholar] [CrossRef]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef]
- Pettus, E.H.; Wright, D.W.; Stein, D.G.; Hoffman, S.W. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005, 1049, 112–119. [Google Scholar] [CrossRef]
- O’Connor, C.A.; Cernak, I.; Vink, R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005, 1062, 171–174. [Google Scholar] [CrossRef]
- Sarkaki, A.R.; Khaksari Haddad, M.; Soltani, Z.; Shahrokhi, N.; Mahmoodi, M. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J. Neurotrauma 2013, 30, 47–54. [Google Scholar] [CrossRef]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Lesnak, J.B.; Inoue, S.; Lima, L.; Rasmussen, L.; Sluka, K.A. Testosterone protects against the development of widespread muscle pain in mice. Pain 2020, 161, 2898–2908. [Google Scholar] [CrossRef]
- Naylor, J.C.; Kilts, J.D.; Strauss, J.L.; Szabo, S.T.; Dunn, C.E.; Wagner, H.R.; Hamer, R.M.; Shampine, L.J.; Zanga, J.R.; Marx, C.E. An exploratory pilot investigation of neurosteroids and self-reported pain in female Iraq/Afghanistan-era Veterans. J. Rehabil. Res. Dev. 2016, 53, 499–510. [Google Scholar] [CrossRef]
- Naylor, J.C.; Kilts, J.D.; Szabo, S.T.; Dunn, C.E.; Keefe, F.J.; Tupler, L.A.; Shampine, L.J.; Morey, R.A.; Strauss, J.L.; Hamer, R.M.; et al. Allopregnanolone Levels Are Inversely Associated with Self-Reported Pain Symptoms in U.S. Iraq and Afghanistan-Era Veterans: Implications for Biomarkers and Therapeutics. Pain Med. 2016, 17, 25–32. [Google Scholar] [CrossRef]
- Marx, C.E.; Naylor, J.C.; Kilts, J.D.; Dunn, C.E.; Tupler, L.A.; Szabo, S.T.; Capehart, B.P.; Morey, R.A.; Shampine, L.J.; Acheson, S.K. Neurosteroids and Traumatic Brain Injury: Translating Biomarkers to Therapeutics; Overview and Pilot Investigations in Iraq and Afghanistan Era Veterans. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; Frontiers in Neuroscience; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Naylor, J.C.; Kilts, J.D.; Shampine, L.J.; Parke, G.J.; Wagner, H.R.; Szabo, S.T.; Smith, K.D.; Allen, T.B.; Telford-Marx, E.G.; Dunn, C.E.; et al. Effect of Pregnenolone vs Placebo on Self-reported Chronic Low Back Pain among US Military Veterans: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200287. [Google Scholar] [CrossRef]
- Ludvigsson, P.; Hesdorffer, D.; Olafsson, E.; Kjartansson, O.; Hauser, W.A. Migraine with aura is a risk factor for unprovoked seizures in children. Ann. Neurol. 2006, 59, 210–213. [Google Scholar] [CrossRef]
- Hillbom, M.; Pieninkeroinen, I.; Leone, M. Seizures in alcohol-dependent patients: Epidemiology, pathophysiology and management. CNS Drugs 2003, 17, 1013–1030. [Google Scholar] [CrossRef]
- Berg, A.T.; Plioplys, S. Epilepsy and autism: Is there a special relationship? Epilepsy Behav. 2012, 23, 193–198. [Google Scholar] [CrossRef]
- Seidenberg, M.; Pulsipher, D.T.; Hermann, B. Association of epilepsy and comorbid conditions. Future Neurol. 2009, 4, 663–668. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kulkarni, S.K. Proconvulsant effects of neurosteroids pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. Eur. J. Pharmacol. 1998, 345, 55–59. [Google Scholar] [CrossRef]
- Williamson, J.; Mtchedlishvili, Z.; Kapur, J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology 2004, 46, 856–864. [Google Scholar] [CrossRef]
- Bäckström, T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol. Scand. 1976, 54, 321–347. [Google Scholar] [CrossRef]
- Herzog, A.G.; Klein, P.; Ransil, B.J. Three patterns of catamenial epilepsy. Epilepsia 1997, 38, 1082–1088. [Google Scholar] [CrossRef]
- Najafi, M.; Sadeghi, M.M.; Mehvari, J.; Zare, M.; Akbari, M. Progesterone therapy in women with intractable catamenial epilepsy. Adv. Biomed. Res. 2013, 2, 8. [Google Scholar] [CrossRef]
- Kokate, T.G.; Banks, M.K.; Magoo, T.; Yamaguchi, S.-I.; Rogawski, M.A. Finasteride, a 5a-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J. Pharmacol. Exp. Ther. 1999, 288, 679–684. [Google Scholar]
- Bäckström, T.; Gee, K.W.; Lan, N.; Sörensen, M.; Wahlström, G. Steroids in relation to epilepsy and anaesthesia. In Ciba Foundation Symposium 153—Steroids and Neuronal Activity; Wiley: Hoboken, NJ, USA, 1990; pp. 225–230. [Google Scholar] [CrossRef]
- Nucera, B.; Rinaldi, F.; Dono, F.; Lanzone, J.; Evangelista, G.; Consoli, S.; Tappatà, M.; Narducci, F.; Troisi, S.; Trinka, E.; et al. Progesterone and its derivatives for the treatment of catamenial epilepsy: A systematic review. Seizure 2023, 109, 52–59. [Google Scholar] [CrossRef]
- Reddy, D.S. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front. Endocrinol. 2011, 2, 38. [Google Scholar] [CrossRef]
- Meng, J.; Yan, Z.; Tao, X.; Wang, W.; Wang, F.; Xue, T.; Liu, Y.; Wang, Z. The efficacy and safety of ganaxolone for the treatment of refractory epilepsy: A meta-analysis from randomized controlled trials. Epilepsia Open 2023, 8, 90–99. [Google Scholar] [CrossRef]
- Knight, E.M.P.; Amin, S.; Bahi-Buisson, N.; Benke, T.A.; Cross, J.H.; Demarest, S.T.; Olson, H.E.; Specchio, N.; Fleming, T.R.; Aimetti, A.A.; et al. Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: Results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2022, 21, 417–427. [Google Scholar] [CrossRef]
- Olson, H.E.; Amin, S.; Bahi-Buisson, N.; Devinsky, O.; Marsh, E.D.; Pestana-Knight, E.; Rajaraman, R.R.; Aimetti, A.A.; Rybak, E.; Kong, F.; et al. Long-term treatment with ganaxolone for seizures associated with cyclin-dependent kinase-like 5 deficiency disorder: Two-year open-label extension follow-up. Epilepsia 2024, 65, 37–45. [Google Scholar] [CrossRef]
- Costa, A.M.; Gol, M.; Lucchi, C.; Biagini, G. Antiepileptogenic effects of trilostane in the kainic acid model of temporal lobe epilepsy. Epilepsia 2023, 64, 1376–1389. [Google Scholar] [CrossRef]
- Gol, M.; Costa, A.M.; Biagini, G.; Lucchi, C. Seizure progression is slowed by enhancing neurosteroid availability in the brain of epileptic rats. Epilepsia 2024, 65, e41–e46. [Google Scholar] [CrossRef]
- Vezzani, A.; Aronica, E.; Mazarati, A.; Pittman, Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013, 244, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sánchez, K.; Mogilevskaya, M.; Rodríguez-Pérez, J.; Rubiano, M.G.; Javela, J.J.; González-Reyes, R.E. Astroglial role in the pathophysiology of status epilepticus: An overview. Oncotarget 2018, 9, 26954–26976. [Google Scholar] [CrossRef]
- Wilcox, K.S.; Gee, J.M.; Gibbons, M.B.; Tvrdik, P.; White, J.A. Altered structure and function of astrocytes following status epilepticus. Epilepsy Behav. 2015, 49, 17–19. [Google Scholar] [CrossRef]
- Shen, Y.; Qin, H.; Chen, J.; Mou, L.; He, Y.; Yan, Y.; Zhou, H.; Lv, Y.; Chen, Z.; Wang, J.; et al. Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J. Cell Biol. 2016, 215, 719–734. [Google Scholar] [CrossRef]
- Henneberger, C.; Steinhäuser, C. Astrocytic TLR4 at the crossroads of inflammation and seizure susceptibility. J. Cell Biol. 2016, 215, 607–609. [Google Scholar] [CrossRef]
- Yu, C.; Deng, X.-j.; Xu, D. Microglia in epilepsy. Neurobiol. Dis. 2023, 185, 106249. [Google Scholar] [CrossRef]
- Sano, F.; Shigetomi, E.; Shinozaki, Y.; Tsuzukiyama, H.; Saito, K.; Mikoshiba, K.; Horiuchi, H.; Cheung, D.L.; Nabekura, J.; Sugita, K.; et al. Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus. JCI Insight 2021, 6, e135391. [Google Scholar] [CrossRef]
- Çarçak, N.; Onat, F.; Sitnikova, E. Astrocytes as a target for therapeutic strategies in epilepsy: Current insights. Front. Mol. Neurosci. 2023, 16, 1183775. [Google Scholar] [CrossRef]
- Cartmell, T.; Luheshi, G.N.; Rothwell, N.J. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J. Physiol. 1999, 518 Pt 2, 585–594. [Google Scholar] [CrossRef]
- Heida, J.G.; Pittman, Q.J. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia 2005, 46, 1906–1913. [Google Scholar] [CrossRef]
- Auvin, S.; Porta, N.; Nehlig, A.; Lecointe, C.; Vallée, L.; Bordet, R. Inflammation in rat pups subjected to short hyperthermic seizures enhances brain long-term excitability. Epilepsy Res. 2009, 86, 124–130. [Google Scholar] [CrossRef]
- Vezzani, A.; Baram, T.Z. New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr. 2007, 7, 45–50. [Google Scholar] [CrossRef]
- Vezzani, A.; Maroso, M.; Balosso, S.; Sanchez, M.A.; Bartfai, T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav. Immun. 2011, 25, 1281–1289. [Google Scholar] [CrossRef]
- von Rüden, E.-L.; Gualtieri, F.; Schönhoff, K.; Reiber, M.; Wolf, F.; Baumgärtner, W.; Hansmann, F.; Tipold, A.; Potschka, H. Molecular alterations of the TLR4-signaling cascade in canine epilepsy. BMC Vet. Res. 2020, 16, 18. [Google Scholar] [CrossRef]
- Liu, J.; Ke, P.; Guo, H.; Gu, J.; Liu, Y.; Tian, X.; Wang, X.; Xiao, F. Activation of TLR7-mediated autophagy increases epileptic susceptibility via reduced KIF5A-dependent GABA(A) receptor transport in a murine model. Exp. Mol. Med. 2023, 55, 1159–1173. [Google Scholar] [CrossRef]
- Wang, N.; Han, X.; Liu, H.; Zhao, T.; Li, J.; Feng, Y.; Mi, X.; Zhang, Y.; Chen, Y.; Wang, X. Myeloid differentiation factor 88 is up-regulated in epileptic brain and contributes to experimental seizures in rats. Exp. Neurol. 2017, 295, 23–35. [Google Scholar] [CrossRef]
- Wang, F.X.; Yang, X.L.; Ma, Y.S.; Wei, Y.J.; Yang, M.H.; Chen, X.; Chen, B.; He, Q.; Yang, Q.W.; Yang, H.; et al. TRIF contributes to epileptogenesis in temporal lobe epilepsy during TLR4 activation. Brain Behav. Immun. 2018, 67, 65–76. [Google Scholar] [CrossRef]
- di Biase, L.; Summa, S.; Tosi, J.; Taffoni, F.; Marano, M.; Cascio Rizzo, A.; Vecchio, F.; Formica, D.; Di Lazzaro, V.; Di Pino, G.; et al. Quantitative Analysis of Bradykinesia and Rigidity in Parkinson’s Disease. Front. Neurol. 2018, 9, 121. [Google Scholar] [CrossRef]
- Dzamko, N.; Gysbers, A.; Perera, G.; Bahar, A.; Shankar, A.; Gao, J.; Fu, Y.; Halliday, G.M. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017, 133, 303–319. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; St-Amour, I.; Saint-Pierre, M.; Lamontagne-Proulx, J.; Kriz, J.; Barker, R.A.; Cicchetti, F. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2014, 18, pyu103. [Google Scholar] [CrossRef]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural Transm. Suppl. 2000, 8, 277–290. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Zirra, A.; Rao, S.C.; Bestwick, J.; Rajalingam, R.; Marras, C.; Blauwendraat, C.; Mata, I.F.; Noyce, A.J. Gender Differences in the Prevalence of Parkinson’s Disease. Mov. Disord. Clin. Pract. 2023, 10, 86–93. [Google Scholar] [CrossRef]
- Reekes, T.H.; Higginson, C.I.; Sigvardt, K.A.; King, D.S.; Levine, D.; Wheelock, V.L.; Disbrow, E.A. Sex differences in Parkinson disease-associated episodic memory and processing speed deficits. J. Int. Neuropsychol. Soc. 2023, 29, 813–820. [Google Scholar] [CrossRef]
- Shulman, L.M. Is there a connection between estrogen and Parkinson’s disease? Park. Relat. Disord. 2002, 8, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Saunders-Pullman, R.; Gordon-Elliott, J.; Parides, M.; Fahn, S.; Saunders, H.R.; Bressman, S. The effect of estrogen replacement on early Parkinson’s disease. Neurology 1999, 52, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- di Michele, F.; Longone, P.; Romeo, E.; Lucchetti, S.; Brusa, L.; Pierantozzi, M.; Bassi, A.; Bernardi, G.; Stanzione, P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson’s disease. Neurol. Sci. 2003, 24, 172–173. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Hou, X.; Jiao, Y.; Zheng, B.; Henry, S.; Hill, R.; He, Z.; Pani, A.; Kyle, P.; Ou, X.; et al. Allopregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor performance in an MPTP-lesioned mouse model of Parkinson’s disease. PLoS ONE 2012, 7, e50040. [Google Scholar] [CrossRef]
- Nezhadi, A.; Sheibani, V.; Esmaeilpour, K.; Shabani, M.; Esmaeili-Mahani, S. Neurosteroid allopregnanolone attenuates cognitive dysfunctions in 6-OHDA-induced rat model of Parkinson’s disease. Behav. Brain Res. 2016, 305, 258–264. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P. Progesterone exerts a neuroprotective action in a Parkinson’s disease human cell model through membrane progesterone receptor alpha (mPRalpha/PAQR7). Front. Endocrinol. 2023, 14, 1125962. [Google Scholar] [CrossRef] [PubMed]
- Litim, N.; Morissette, M.; Di Paolo, T. Effects of progesterone administered after MPTP on dopaminergic neurons of male mice. Neuropharmacology 2017, 117, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.A.; An, G.J.; Koo, B.S.; Jeon, S. Effect of DHEA on recovery of muscle atrophy induced by Parkinson’s disease. J. Korean Acad. Nurs. 2011, 41, 834–842. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef]
- Bolmont, T.; Haiss, F.; Eicke, D.; Radde, R.; Mathis, C.A.; Klunk, W.E.; Kohsaka, S.; Jucker, M.; Calhoun, M.E. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008, 28, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Yee, K.L.; Sumbria, R.K. Tumor necrosis factor alpha Inhibition for Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517709278. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; Diniz, B.S.; Talib, L.L.; Mendonca, V.A.; Ojopi, E.B.; Gattaz, W.F.; Teixeira, A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Blum-Degen, D.; Muller, T.; Kuhn, W.; Gerlach, M.; Przuntek, H.; Riederer, P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995, 202, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Djordjevic, J.; Sabbir, M.G.; Albensi, B.C. Traumatic Brain Injury as a Risk Factor for Alzheimer’s Disease: Is Inflammatory Signaling a Key Player? Curr. Alzheimer Res. 2016, 13, 730–738. [Google Scholar] [CrossRef]
- Sheng, J.G.; Zhu, S.G.; Jones, R.A.; Griffin, W.S.; Mrak, R.E. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp. Neurol. 2000, 163, 388–391. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.M.; Irwin, R.W.; Yao, J.; Liu, L.; Brinton, R.D. Allopregnanolone promotes regeneration and reduces beta-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS ONE 2011, 6, e24293. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Singh, C.; Liu, L.; Irwin, R.W.; Chen, S.; Chung, E.J.; Thompson, R.F.; Brinton, R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2010, 107, 6498–6503. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Liu, L.; Wang, J.M.; Irwin, R.W.; Yao, J.; Chen, S.; Henry, S.; Thompson, R.F.; Brinton, R.D. Allopregnanolone restores hippocampal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol. Aging 2012, 33, 1493–1506. [Google Scholar] [CrossRef]
- Hernandez, G.D.; Solinsky, C.M.; Mack, W.J.; Kono, N.; Rodgers, K.E.; Wu, C.Y.; Mollo, A.R.; Lopez, C.M.; Pawluczyk, S.; Bauer, G.; et al. Safety, tolerability, and pharmacokinetics of allopregnanolone as a regenerative therapeutic for Alzheimer’s disease: A single and multiple ascending dose phase 1b/2a clinical trial. Alzheimers Dement. 2020, 6, e12107. [Google Scholar] [CrossRef]
- Paganini-Hill, A.; Henderson, V.W. Estrogen deficiency and risk of Alzheimer’s disease in women. Am. J. Epidemiol. 1994, 140, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kawas, C.; Resnick, S.; Morrison, A.; Brookmeyer, R.; Corrada, M.; Zonderman, A.; Bacal, C.; Lingle, D.D.; Metter, E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology 1997, 48, 1517–1521. [Google Scholar] [CrossRef]
- Carroll, J.C.; Rosario, E.R.; Chang, L.; Stanczyk, F.Z.; Oddo, S.; LaFerla, F.M.; Pike, C.J. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007, 27, 13357–13365. [Google Scholar] [CrossRef]
- Pan, X.; Wu, X.; Kaminga, A.C.; Wen, S.W.; Liu, A. Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2019, 11, 61. [Google Scholar] [CrossRef]
- Kim, S.B.; Hill, M.; Kwak, Y.T.; Hampl, R.; Jo, D.H.; Morfin, R. Neurosteroids: Cerebrospinal fluid levels for Alzheimer’s disease and vascular dementia diagnostics. J. Clin. Endocrinol. Metab. 2003, 88, 5199–5206. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Kramer, J.H.; Reus, V.I.; Costa, M.M.; Yaffe, K.; Walton, P.; Raskind, M.; Peskind, E.; Newhouse, P.; Sack, D.; et al. DHEA treatment of Alzheimer’s disease: A randomized, double-blind, placebo-controlled study. Neurology 2003, 60, 1071–1076. [Google Scholar] [CrossRef]
- Ouanes, S.; Clark, C.; Richiardi, J.; Marechal, B.; Lewczuk, P.; Kornhuber, J.; Kirschbaum, C.; Popp, J. Cerebrospinal Fluid Cortisol and Dehydroepiandrosterone Sulfate, Alzheimer’s Disease Pathology, and Cognitive Decline. Front. Aging Neurosci. 2022, 14, 892754. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Sadeghi, L. Investigation of THDOC effects on pathophysiological signs of Alzheimer’s disease as an endogenous neurosteroid: Inhibition of acetylcholinesterase and plaque deposition. Bratisl. Lek. Listy 2019, 120, 148–154. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013.
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sherer, M.L.; Posillico, C.K.; Schwarz, J.M. An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period. Brain Behav. Immun. 2017, 66, 201–209. [Google Scholar] [CrossRef]
- Hirst, J.J.; Palliser, H.K.; Yates, D.M.; Yawno, T.; Walker, D.W. Neurosteroids in the fetus and neonate: Potential protective role in compromised pregnancies. Neurochem. Int. 2008, 52, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.A.; Hirst, J.J.; Palliser, H.K. Changes in neuroactive steroid concentrations after preterm delivery in the Guinea pig. Reprod. Sci. 2013, 20, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Mujuni, B.M.; Martinello, K.A.; Webb, E.L.; Nalwoga, A.; Ssekyewa, J.; Musoke, M.; Kurinczuk, J.J.; Sewegaba, M.; Cowan, F.M.; et al. Elevated serum IL-10 is associated with severity of neonatal encephalopathy and adverse early childhood outcomes. Pediatr. Res. 2022, 92, 180–189. [Google Scholar] [CrossRef]
- Shaw, J.C.; Dyson, R.M.; Palliser, H.K.; Gray, C.; Berry, M.J.; Hirst, J.J. Neurosteroid replacement therapy using the allopregnanolone-analogue ganaxolone following preterm birth in male guinea pigs. Pediatr. Res. 2019, 85, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, H.; Liu, S.; Luo, W.; Jiang, Y.; Gao, J. Association of Peripheral Blood Levels of Cytokines with Autism Spectrum Disorder: A Meta-Analysis. Front. Psychiatry 2021, 12, 670200. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, B.; Bonaccorso, C.M.; Aloisi, E.; D’Antoni, S.; Catania, M.V. Neuro-Inflammatory Mechanisms in Developmental Disorders Associated with Intellectual Disability and Autism Spectrum Disorder: A Neuro- Immune Perspective. CNS Neurol. Disord. Drug Targets 2016, 15, 448–463. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Chew, L.; Sun, K.L.; Sun, W.; Wang, Z.; Rajadas, J.; Flores, R.E.; Arnold, E.; Jo, B.; Fung, L.K. Association of serum allopregnanolone with restricted and repetitive behaviors in adult males with autism. Psychoneuroendocrinology 2021, 123, 105039. [Google Scholar] [CrossRef]
- Ayatollahi, A.; Bagheri, S.; Ashraf-Ganjouei, A.; Moradi, K.; Mohammadi, M.R.; Akhondzadeh, S. Does Pregnenolone Adjunct to Risperidone Ameliorate Irritable Behavior in Adolescents with Autism Spectrum Disorder: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial? Clin. Neuropharmacol. 2020, 43, 139–145. [Google Scholar] [CrossRef]
- Porcu, P.; O’Buckley, T.K.; Alward, S.E.; Marx, C.E.; Shampine, L.J.; Girdler, S.S.; Morrow, A.L. Simultaneous quantification of GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in human and rat serum. Steroids 2009, 74, 463–473. [Google Scholar] [CrossRef] [PubMed]
- VanDoren, M.J.; Matthews, D.B.; Janis, G.C.; Grobin, A.C.; Devaud, L.L.; Morrow, A.L. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 2000, 20, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Sogliano, C.; Cinus, M.; Purdy, R.H.; Biggio, G.; Concas, A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol. Biochem. Behav. 2003, 74, 683–690. [Google Scholar] [CrossRef]
- Concas, A.; Sogliano, C.; Porcu, P.; Marra, C.; Brundu, A.; Biggio, G. Neurosteroids in nicotine and morphine dependence. Psychopharmacology 2006, 186, 281–292. [Google Scholar] [CrossRef]
- Janak, P.H.; Redfern, J.E.M.; Samson, H.H. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol. Clin. Exp. Res. 1998, 22, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.M.; Nickel, J.D.; Phillips, T.J.; Finn, D.A. Neurosteroid modulators of GABA(A) receptors differentially modulate Ethanol intake patterns in male C57BL/6J mice. Alcohol. Clin. Exp. Res. 2005, 29, 1630–1640. [Google Scholar] [CrossRef]
- Ramaker, M.J.; Ford, M.M.; Fretwell, A.M.; Finn, D.A. Alteration of ethanol drinking in mice via modulation of the GABA(A) receptor with ganaxolone, finasteride, and gaboxadol. Alcohol. Clin. Exp. Res. 2011, 35, 1994–2007. [Google Scholar] [CrossRef]
- Ramaker, M.J.; Ford, M.M.; Phillips, T.J.; Finn, D.A. Differences in the reinstatement of ethanol seeking with ganaxolone and gaboxadol. Neuroscience 2014, 272, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Boero, G.; McFarland, M.H.; Tyler, R.E.; O’Buckley, T.K.; Chery, S.L.; Robinson, D.L.; Besheer, J.; Morrow, A.L. Deleterious Interaction between the Neurosteroid (3alpha,5alpha)3-Hydroxypregnan-20-One (3alpha,5alpha-THP) and the Mu-Opioid System Activation during Forced Swim Stress in Rats. Biomolecules 2023, 13, 1205. [Google Scholar] [CrossRef]
- Zamora-Sanchez, C.J.; Hansberg-Pastor, V.; Salido-Guadarrama, I.; Rodriguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone promotes proliferation and differential gene expression in human glioblastoma cells. Steroids 2017, 119, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sánchez, C.J.; Bello-Alvarez, C.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone Promotes Migration and Invasion of Human Glioblastoma Cells through the Protein Tyrosine Kinase c-Src Activation. Int. J. Mol. Sci. 2022, 23, 4996. [Google Scholar] [CrossRef]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and Breast Cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Bello-Alvarez, C.; Camacho-Arroyo, I. Impact of sex in the prevalence and progression of glioblastomas: The role of gonadal steroid hormones. Biol. Sex. Differ. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Pelegrina, L.T.; de Los Angeles Sanhueza, M.; Ramona Caceres, A.R.; Cuello-Carrion, D.; Rodriguez, C.E.; Laconi, M.R. Effect of progesterone and first evidence about allopregnanolone action on the progression of epithelial human ovarian cancer cell lines. J. Steroid Biochem. Mol. Biol. 2020, 196, 105492. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors delta and epsilon (mPRdelta and mPRepsilon) and mPRdelta involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.; Spence, K.T.; Smith, S.S.; ffrench-Mullen, J. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J. Neurophysiol. 1995, 74, 464–469. [Google Scholar] [CrossRef]
- Moran, M.H.; Smith, S.S. Progesterone withdrawal I: Pro-convulsant effects. Brain Res. 1998, 807, 84–90. [Google Scholar] [CrossRef]
- Moran, M.H.; Goldberg, N.; Smith, S.S. Progesterone withdrawal II: Insensitivity to the sedative effects of a benzodiazepine. Brain Res. 1998, 807, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.S.; Gong, Q.H.; Li, X.; Moran, M.H.; Bitran, D.; Frye, C.A.; Hsu, F. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J. Neurosci. 1998, 18, 5275–5284. [Google Scholar] [CrossRef]
- Azhar, Y.; Din, A.U. Brexanolone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541054 (accessed on 10 January 2024).
- Miller, L.G.; Greenblatt, D.J.; Barnhill, J.G.; Shader, R.I. Chronic benzodiazepine administration I. Tolerance is associated with benzodiazepine receptor downregulation and decreased g-aminobutyric acidA receptor function. J. Pharmacol. Exp. Ther. 1988, 246, 170–176. [Google Scholar] [PubMed]
- Impagnatiello, F.; Pesold, C.; Longone, P.; Caruncho, H.; Fritschy, J.M.; Costa, E.; Guidotti, A. Modifications of g-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol. Pharmacol. 1996, 49, 822–831. [Google Scholar]
- Birzniece, V.; Turkmen, S.; Lindblad, C.; Zhu, D.; Johansson, I.M.; Backstrom, T.; Wahlstrom, G. GABA(A) receptor changes in acute allopregnanolone tolerance. Eur. J. Pharmacol. 2006, 535, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, S.; Backstrom, T.; Wahlstrom, G.; Andreen, L.; Johansson, I.M. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br. J. Pharmacol. 2011, 162, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Follesa, P.; Ticku, M.K. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res. Mol. Brain Res. 1996, 41, 163–168. [Google Scholar] [CrossRef]
- Cornett, E.M.; Rando, L.; Labbe, A.M.; Perkins, W.; Kaye, A.M.; Kaye, A.D.; Viswanath, O.; Urits, I. Brexanolone to Treat Postpartum Depression in Adult Women. Psychopharmacol. Bull. 2021, 51, 115–130. [Google Scholar] [PubMed]
- Wang, S.; Zhang, W.; Liu, Z.; Zhang, T.; Wang, Y.; Li, W. Efficacy and safety of zuranolone in the treatment of major depressive disorder: A meta-analysis. Front. Neurosci. 2023, 17, 1332329. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Tranter, R.; O’Donovan, C.; Chandarana, P.; Kennedy, S. Prevalence and outcome of partial remission in depression. J. Psychiatry Neurosci. 2002, 27, 241–247. [Google Scholar] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; Thomas, C.P.; Hodgkin, D.; Levit, K.R.; Mark, T.L. The diminished pipeline for medications to treat mental health and substance use disorders. Psychiatr. Serv. 2014, 65, 1433–1438. [Google Scholar] [CrossRef]
- Mullard, A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016, 15, 477. [Google Scholar] [CrossRef]
- Van Norman, G.A. Phase II Trials in Drug Development and Adaptive Trial Design. JACC Basic Transl. Sci. 2019, 4, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, G.; Grignolo, A. Phase III trial failures: Costly, but preventable. Appl. Clin. Trials 2016, 25, 36–42. [Google Scholar]
- Arora, A.; Nain, P.; Kumari, R.; Kaur, J. Major causes associated with clinial trials failure and selective strategies to reduce these consequences: A Review. Arch. Pharm. Pract. 2021, 12, 45–53. [Google Scholar] [CrossRef]
- Zhu, T. Challenges of Psychiatry Drug Development and the Role of Human Pharmacology Models in Early Development—A Drug Developer’s Perspective. Front. Psychiatry 2020, 11, 562660. [Google Scholar] [CrossRef]
- Norbury, A.; Seymour, B. Response heterogeneity: Challenges for personalised medicine and big data approaches in psychiatry and chronic pain. F1000Research 2018, 7, 55. [Google Scholar] [CrossRef]
- Shukla, A.K.; Jhaj, R.; Sadasivam, B. Brexanolone: Panacea for postpartum depression? Reply to: ‘Intravenous brexanolone for postpartum depression: What it is, how well does it work, and will it be used?’. Ther. Adv. Psychopharmacol. 2021, 11, 2045125321997293. [Google Scholar] [CrossRef]
- Nonacs, R. New Oral PPD Treatment Zuranolone Will Soon Hit the Market as Zurzuvae. 2023. Available online: https://womensmentalhealth.org/posts/zuranolone-or-zurzuvae-will-soon-hit-the-market/ (accessed on 10 January 2024).
- Kozhimannil, K.B.; Attanasio, L.B.; Hardeman, R.R.; O’Brien, M. Doula care supports near-universal breastfeeding initiation among diverse, low-income women. J. Midwifery Womens Health 2013, 58, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Penning, T.M. Molecular docking simulations of steroid substrates into human cytosolic hydroxysteroid dehydrogenases (AKR1C1 and AKR1C2): Insights into positional and stereochemical preferences. Steroids 2006, 71, 380–391. [Google Scholar] [CrossRef]
- Wang, P.; Dang, L.; Zhu, B.T. Use of computational modeling approaches in studying the binding interactions of compounds with human estrogen receptors. Steroids 2016, 105, 26–41. [Google Scholar] [CrossRef]
- Mitchell, J. Small molecule immunosensing using surface plasmon resonance. Sensors 2010, 10, 7323–7346. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; McDermott, M.T. A surface plasmon resonance based inhibition immunoassay for measurement of steroid hormones. Anal. Biochem. 2018, 557, 7–12. [Google Scholar] [CrossRef]
- Kumar, R. Cryo-EM technique and its application: Structure of steroid hormone receptors. Vitam. Horm. 2023, 123, 385–397. [Google Scholar] [CrossRef]
- Yi, P.; Yu, X.; Wang, Z.; O’Malley, B.W. Steroid receptor-coregulator transcriptional complexes: New insights from CryoEM. Essays Biochem. 2021, 65, 857–866. [Google Scholar] [CrossRef]
- Couture, J.-F.; Legrand, P.; Cantin, L.; Luu-The, V.; Labrie, F.; Breton, R. Human 20α–Hydroxysteroid Dehydrogenase: Crystallographic and Site-directed Mutagenesis Studies Lead to the Identification of an Alternative Binding Site for C21-steroids. J. Mol. Biol. 2003, 331, 593–604. [Google Scholar] [CrossRef]
- Loiarro, M.; Ruggiero, V.; Sette, C. Targeting TLR/IL-1R Signalling in Human Diseases. Mediat. Inflamm. 2010, 2010, 674363. [Google Scholar] [CrossRef] [PubMed]
- Luís, J.P.; Simões, C.J.V.; Brito, R.M.M. The Therapeutic Prospects of Targeting IL-1R1 for the Modulation of Neuroinflammation in Central Nervous System Disorders. Int. J. Mol. Sci. 2022, 23, 1731. [Google Scholar] [CrossRef]
- Song, J.; Chen, D.; Pan, Y.; Shi, X.; Liu, Q.; Lu, X.; Xu, X.; Chen, G.; Cai, Y. Discovery of a Novel MyD88 Inhibitor M20 and Its Protection Against Sepsis-Mediated Acute Lung Injury. Front. Pharmacol. 2021, 12, 775117. [Google Scholar] [CrossRef]
- Reddy, D.S.; Rogawski, M.A. Neurosteroids—Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Martinez Botella, G.; Salituro, F.G.; Harrison, B.L.; Beresis, R.T.; Bai, Z.; Blanco, M.J.; Belfort, G.M.; Dai, J.; Loya, C.M.; Ackley, M.A.; et al. Neuroactive Steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (γ-Aminobutyric Acid)(A) Receptor. J. Med. Chem. 2017, 60, 7810–7819. [Google Scholar] [CrossRef]
- Gasior, M.; Beekman, M.; Carter, R.B.; Goldberg, S.R.; Witkin, J.M. Antiepileptogenic effects of the novel synthetic neuroactive steroid, ganaxolone, against pentylenetetrazol-induced kindled seizures: Comparison with diazepam and valproate. Drug Dev. Res. 1998, 44, 21–33. [Google Scholar] [CrossRef]
- Yawno, T.; Miller, S.L.; Bennet, L.; Wong, F.; Hirst, J.J.; Fahey, M.; Walker, D.W. Ganaxolone: A New Treatment for Neonatal Seizures. Front. Cell. Neurosci. 2017, 11, 246. [Google Scholar] [CrossRef]
- Martinez Botella, G.; Salituro, F.G.; Harrison, B.L.; Beresis, R.T.; Bai, Z.; Shen, K.; Belfort, G.M.; Loya, C.M.; Ackley, M.A.; Grossman, S.J.; et al. Neuroactive Steroids. 1. Positive Allosteric Modulators of the (gamma-Aminobutyric Acid)A Receptor: Structure-Activity Relationships of Heterocyclic Substitution at C-21. J. Med. Chem. 2015, 58, 3500–3511. [Google Scholar] [CrossRef]
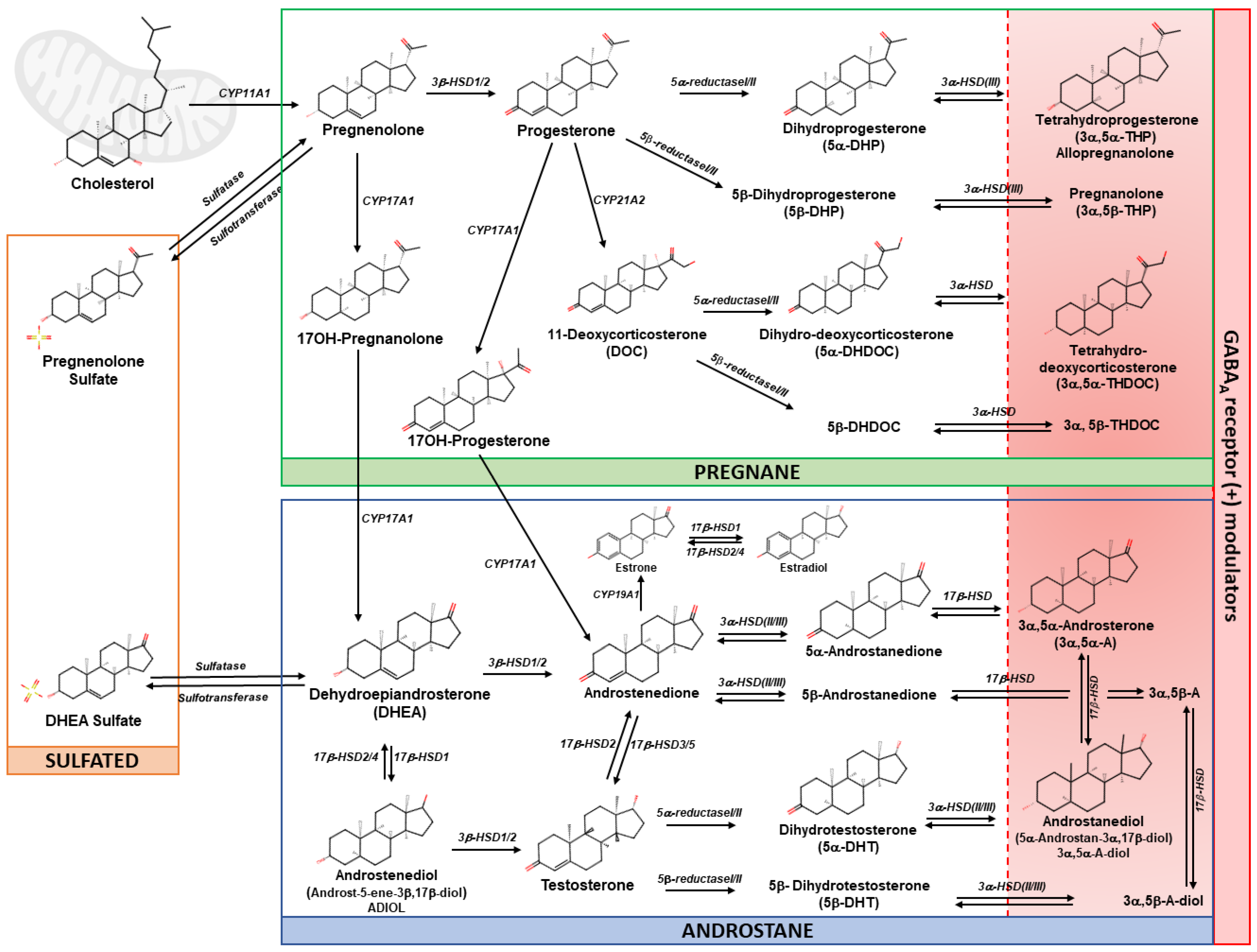


| Disease | Neurosteroid /Dose/Route of Administration | Subjects/Age/Sex | Outcomes | References |
|---|---|---|---|---|
| Postpartum Depression | Brexanolone 5 mg/mL in 250 mg/mL sulfobutylether-β-cyclo-dextrin, 60 hs continuous intravenous infusion (30 μg/kg per h (0–4 h); 60 μg/kg per h (4–24 h); 90 μg/kg per h (24–52 h); 60 μg/kg per h (52–56 h); 30 μg/kg per h (56–60 h).) | Women with PPD (n = 18), 24–41 years old (y.o.) | Brexanolone infusion reduced whole blood cell TNF-α and IL-6 and these effects were correlated with HAM-D score improvement. Brexanolone infusion prevented LPS- and imiquimod-induced elevation of TNF-α, IL-1β and IL-6 in whole blood cells in vitro, indicating inhibition of TLR4 and TLR7 responses. Inhibition of TNF-α, IL-1β and IL-6 responses to both LPS and imiquimod were correlated with HAM-D score improvements. Brexanolone infusion led to significant increases in allopregnanolone and 3α,5α-THDOC levels, while causing decreases in 3α,5α-androsterone and 3α,5α-androstandiol levels. Pregnenolone and 3α,5β-THP levels remained unchanged following infusion. However, there was no observed correlation between the percentage change in steroid levels post-infusion and the improvement in HAM-D scores. | Balan et al., 2023 [64] |
| Postpartum Depression | Brexanolone 5 mg/mL in 250 mg/mL sulfobutylether-β-cyclo-dextrin, 60 h continuous intravenous infusion (30 μg/kg per h (0–4 h); 60 μg/kg per h (4–24 h); 90 μg/kg per h (24–52 h); 60 μg/kg per h (52–56 h); 30 μg/kg per h (56–60 h). | Women with PPD (n = 21), 18–45 y.o. | Brexanolone was generally well tolerated in PPD patients. Improvement in depressive symptoms (HAM-D and MADRS scores) was observed up to 30 days after brexanolone treatment. | Kanes et al., 2017 [63] |
| Postpartum Depression | Brexanolone 5 mg/mL in 250 mg/mL sulfobutylether-β-cyclo-dextrin, 60 h continuous intravenous infusion (30 μg/kg per h (0–4 h); 60 μg/kg per h (4–24 h); 90 μg/kg per h (24–52 h); 60 μg/kg per h (52–56 h); 30 μg/kg per h (56–60 h). | Women with PPD (Study 1 n = 120; Study 2 n = 100), 18–45 y.o. | Brexanolone was generally well tolerated in PPD patients. Common adverse effects included somnolence, dizziness and sedation in 30% of patients. Improvement in depressive symptoms (HAM-D total scores, HAM-D remission and CGI-I response) was observed up to 30 days after brexanolone treatment. No changes in GAD-7, EPDS or PHQ scores were noted. | Meltzer-Brody et al., 2018 [62] |
| Postpartum Depression | Brexanolone 5 mg/mL in 250 mg/mL sulfobutylether-β-cyclo-dextrin, 60 h continuous intravenous infusion (30 μg/kg per h (0–4 h); 60 μg/kg per h (4–24 h); 90 μg/kg per h (24–52 h); 60 μg/kg per h (52–56 h); 30 μg/kg per h (56–60 h). | Women with PPD (n = 16), 18–45 y.o. | Brexanolone was well tolerated in PPD patients. Improvement in depressive symptoms (HAM-D scores) was observed up to 16 months after brexanolone treatment. | Patterson et al., 2022 [65] |
| Postpartum Depression | Zuranolone 30 mg, administered orally each evening for 2 weeks. | Women with PPD (n = 150), 18–45 y.o. | Zuranolone was well tolerated in PPD patients; however (one patient experienced a serious adverse effect (confusional state) and abandoned the trial. Improvement in depressive symptoms (HAM-D, MADRS and HRSA scores) up to 45 days after Zuranolone treatment. | Deligiannidis et al., 2021 [359] |
| Major Depressive Disorder | Zuranolone 30 mg, administered orally each evening for 2 weeks. | Men and women with MDD (n = 89), 18–65 y.o. | Zuranolone was well tolerated in MDD patients. Improvement in depressive symptoms (HAM-D and CGI-I scores) at day 15. | Gunduz-Bruce et al., 2019 [360] |
| Major Depressive Disorder | Testosterone 50–1000 mg/day, administered for 4–144 weeks | Men with MDD (n = 1890), 27–80 y.o. | Testosterone was well tolerated in patients with MDD. Reduction in depressive symptoms (HDRS, BDI-I/BDI-II, MADRS, PHQ-9, GDS, BRMS or/and HADS-D scores) was observed. | Walther et al., 2019 (Meta-analysis) [362] |
| Bipolar Depression | Pregnenolone titrated to 500 mg/day, oral administration (100 mg/day for 1 week, 150 mg/day for 3 weeks, 500 mg/day for 8 weeks). | Men and women with BPD (n = 80), 18–75 y.o. | Pregnanolone was well tolerated in BPD patients. Improvement in depressive symptoms (HRSD scores) was observed, but not in anxiety symptoms (HRSA scores) or manic behavior (YMRS scores). A significant depression remission rate in the IDS-SR. | Brown et al., 2014 [43] |
| Unipolar and Bipolar Depression | Pregnenolone titrated to 100 mg/day for 8 weeks. | Men and women with BPD I or II with episodes of MDD and history of SUD (n = 70), 17–70 y.o. | Pregnenolone was well tolerated in BPD patients. A trend toward significance favoring pregnanolone was detected in depressive (HRSD scores), and manic symptoms (YMRS scores) at the end of the treatment (week 8). No significant effect was observed on the cognitive assessment (RAVLT scores, TMT and Stroop Test). | Osuji et al., 2010 [45] |
| Cocaine Use Disorder | Progesterone 400 mg/day, oral administration for 7 days. | Men and women with CUD (n = 46), 35–50 y.o. | Progesterone was found to be safe and well tolerated in CUD patients. Increases in allopregnanolone and pregnanolone plasma levels were observed in all patients. No effects on the levels of pregnenolone, testosterone, androstanediol, or DHEA; however, testosterone and androstanediol levels were higher in men than in women. | Milivojevic et al., 2019 [59] |
| Cocaine Use Disorder | AEF0117, an unmetabolized derivative of pregnenolone: 0.06 mg/day (Cohort I), 1 mg/day (Cohort II), oral administration for 6 days. | Men and women with CUD (n = 29), 21–60 y.o. | AEF0117 (1 mg/day) reduced the subjective effects of cannabis, as measured by the ‘Intoxication’ subscale and the ‘Felt Good Cannabis Effect’ item. AEF0117 also decreased cannabis self-administration, with the 1 mg/day dose showing a greater effect compared to the 0.06 mg/day dose. The sequence of AEF0117 administration impacted outcomes, with AEF0117 maintaining its effects even after a washout period of ≥14 days, likely due to its long elimination half-life. AEF0117 was safe and well tolerated, with no significant treatment-related SAEs. Any observed AEs were similar between the AEF0117 and placebo groups, except for one unrelated severe AE. There was no evidence to suggest that AEF0117 precipitated symptoms of cannabis withdrawal. AEF0117 did not produce significant changes in endocannabinoid levels compared to placebo, except for a slight increase in AEA levels with the lower dose (0.06 mg/day), which was likely not caused by AEF0117 administration. There were minor effects on certain mood ratings, but these did not seem to be clinically relevant and were likely influenced by individual sensitivity to AEF0117’s effects. | Haney et al., 2023 [66] |
| Alcohol Use Disorder | Pregnenolone 300 mg/day or 500 mg/day, oral administration for 8 weeks. | Men and women with AUD (n = 43), 18–65 y.o. | Pregnenolone was well tolerated in AUD patients. Decreases in alcohol craving scores (stress- and cue-induced). Decrease in anxiety response to stress (VAS scores) in the 300 mg dose group. Normalization of the ACTH/cortisol ratio following stress or alcohol cue was observed at both doses. Normalization of heart rate (stress-induced), systolic blood pressure (stress- and cue-induced), and diastolic blood pressure was noted (cue-induced, only in the 300 mg dose group). | Milivojevic et al., 2023 [61] |
| Alzheimer’s disease | DHEA (50 mg per os) administered orally 2x/day for 6 months. | Men and postmenopausal women with AD ≥55 y.o. | Increased DHEA and DHEAS levels in serum. No improvements in cognitive performance (ADAS-Cog scores). | Wolkowitz et al., 2003 [488] |
| Alzheimer’s disease | Intravenous allopregnanolone 1x/week for 12 weeks (doses: 0.3–3 mL). | Men and women with AD or probable AD ≥55 y.o. | Allopregnanolone was safe and well tolerated in all participants, with no significant differences in adverse events between treatment arms. Allopregnanolone levels in plasma reached a Tmax at 30 min post-infusion (2, 4, and 6 mg doses) and returned to the lower limit of quantification 4 h post-infusion. No indicators of sedation were observed at lower doses of allopregnanolone (2 mg and 4 mg), but increased sedation was observed at higher doses (6–18 mg). After 12 weeks of treatment, there were no statistically significant differences among cohorts in cognitive assessments (ADAS-Cog score, MoCA total score, and Cogstate Brief Battery composite score). MRI imaging data showed no adverse outcomes of allopregnanolone treatment on hippocampal volume, with a trend suggesting decreased atrophy in allopregnanolone-treated participants. | Hernandez et al., 2020 [482] |
| Pain | Pregnenolone titrated up to 500 mg/day, oral administration (0 mg/day for 1 week, 50 mg twice a day for 1 week, 150 mg twice a day for 1 week, 250 mg twice a day for 2 weeks). | Iraq- and Afghanistan-era male veterans 18–65 years old with chronic lower back pain. | Pregnenolone was well tolerated, with minor adverse effects in subjects. Decreased lower back pain intensity and interference scores following 4 weeks of treatments. Increased levels of pregnenolone and allopregnanolone in serum. | Naylor et al., 2020 [417] |
| Autism Spectrum Disorder | Pregnenolone Titrated by weight: 20 to 45 kg, up to 2.5 mg/day (0.5 mg/day before sleep for 1 week. 0.5 mg weekly increases were used until 1 mg in the morning and 1.5 mg before sleep). >45 kg, up to 3.5 mg/day. A group received 100 mg twice a day. | 36 males and 23 females (11–17 y.o.). | Pregnenolone improved irritability, stereotypy, and hyperactivity in adolescents with ASD. No significant adverse effects were observed when comparing the different treatment groups to the placebo control. | Ayatollahi et al., 2020 [502] |
| Epilepsy | Ganaxolone 33–63 mg/kg or 900–1800 mg three times a day, oral suspension. | 88 patients (79.5% female) 2–19 y.o. with CDD. | Ganaxolone treatments were safe and well tolerated with mild adverse effects. Following 2 years of treatment, motor seizure intensity, duration, and frequency decreased. | Olson et al., 2024 [433] |
| Epilepsy | 40 mg progesterone daily in second half of the cycle from 15th to 25th day, oral administration. | 38 patients 18–45 y.o. women with catamenial epilepsy. | Treatments involving progesterone have shown promise in reducing seizure frequency in women with intractable catamenial epilepsy. | Najafi et al., 2013 [426] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, I.; Boero, G.; Chéry, S.L.; McFarland, M.H.; Lopez, A.G.; Morrow, A.L. Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders. Life 2024, 14, 582. https://doi.org/10.3390/life14050582
Balan I, Boero G, Chéry SL, McFarland MH, Lopez AG, Morrow AL. Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders. Life. 2024; 14(5):582. https://doi.org/10.3390/life14050582
Chicago/Turabian StyleBalan, Irina, Giorgia Boero, Samantha Lucenell Chéry, Minna H. McFarland, Alejandro G. Lopez, and A. Leslie Morrow. 2024. "Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders" Life 14, no. 5: 582. https://doi.org/10.3390/life14050582
APA StyleBalan, I., Boero, G., Chéry, S. L., McFarland, M. H., Lopez, A. G., & Morrow, A. L. (2024). Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders. Life, 14(5), 582. https://doi.org/10.3390/life14050582






