The Clinical Impact of Access Site Selection for Successful Thrombolysis and Intervention in Acute Critical Lower Limb Ischaemia (RAD-ALI Registry)
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedural Endpoints
2.2. Inclusion and Exclusion Criteria
2.3. Antithrombotic and Thrombolytic Regimen
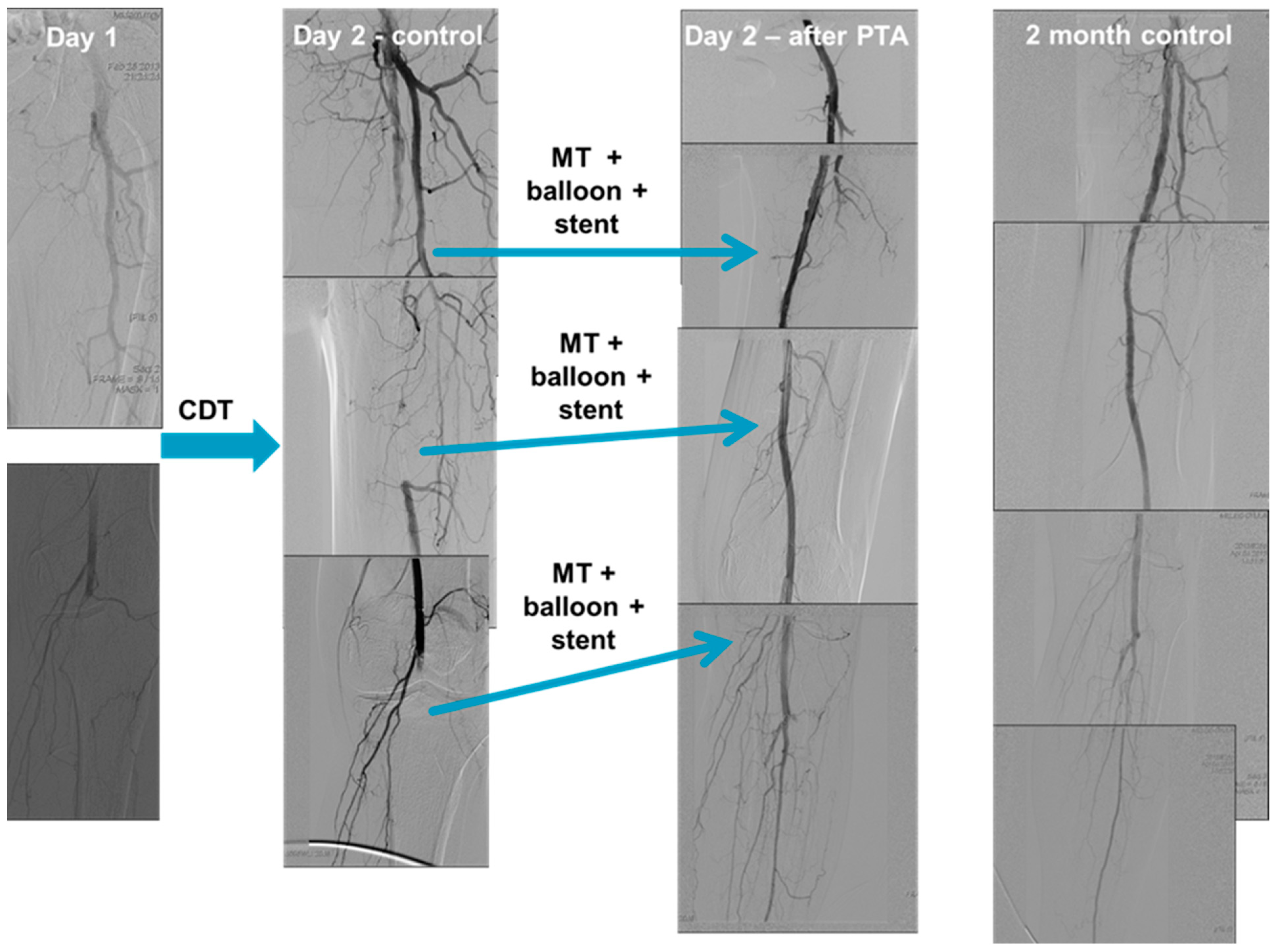
2.4. Catheter Directed Thrombolysis
2.5. Definitions
2.5.1. Major Adverse Event
2.5.2. Major Adverse Limb Event
2.5.3. Vascular Complication
2.5.4. Technical Success
2.5.5. Clinical Success
2.5.6. Access Site Crossover
2.6. Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Angiographic and Procedural Data
3.2. Procedural Complications and 1-Year Follow-Up
3.3. MAE and MALE Predictors
4. Discussion
4.1. Acute and Long-Term Results of CDT
4.2. Complications of CDT
4.3. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| ACC | American College of Cardiology |
| ALI | acute limb ischemia |
| BA | brachial artery |
| CDT | catheter-directed thrombolysis |
| CI | confidence interval |
| FA | femoral artery |
| HR | hazard ratio |
| MAE | major adverse event |
| MALE | major adverse limb event |
| MT | mechanical thrombectomy |
| PTA | percutaneous transluminal angioplasty |
| RA | radial artery |
| STILE | Surgery or Thrombolysis in Lower Extremity Ischemia |
| TOPAS | Thrombolysis Or Peripheral Artery Surgery |
References
- Shammas, N.W.; Radaideh, Q. A Combined Radial and Pedal Access to Treat a Flush Chronic Total Occlusion of the Superficial Femoral Artery in a Critical Limb Ischemia Patient. Open J. Cardiovasc. Surg. 2019, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Fischbach, R.; Brochhagen, H.G.; Heindel, W.; Landwehr, P. Transbrachial thrombolysis, PTA and stenting in the lower extremities. Cardiovasc. Intervent. Radiol. 2003, 26, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.A.; Le, L.; Donovan, M.; Sidhom, T.; Smith, T.A.; Sternbergh, W.C., 3rd. Retrograde pedal access for patients with critical limb ischemia. J. Vasc. Surg. 2014, 60, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.W.; Shah, S.; Amoroso, N.; Diwan, R.; Makker, P.; Ratcliffe, J.A.; Lala, M.; Huang, Y.; Nanjundappa, A.; Daggubati, R.; et al. Feasibility and safety of routine transpedal arterial access for treatment of peripheral artery disease. J. Invasive Cardiol. 2015, 27, 327–330. [Google Scholar] [PubMed]
- Ruzsa, Z.; Nemes, B.; Bánsághi, Z.; Toth, K.; Kuti, F.; Kudrnova, S.; Berta, B.; Huettl, K.; Merkely, B. Transpedal access after failed anterograde recanalization of complex below-the-knee and femoropopliteal occlusions in critical limb ischemia. Catheter. Cardiovasc. Interv. 2014, 83, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.B.; Prout, D.L. J combined radial-pedal access strategy and radial-pedal rendezvous in the revascularization of complex total occlusions of the superficial femoral artery (the “No Femoral Access” Strategy). Endovasc. Ther. 2016, 23, 321. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Weaver, F.A.; Comerota, A.J.; Youngblood, M.; Froehlich, J.; Hosking, J.D.; Papanicolaou, G. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: Results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. J. Vasc. Surg. 1996, 24, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ouriel, K.; Veith, F.J.; Sasahara, A.A. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N. Engl. J. Med. 1998, 338, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Lyden, S.P. Endovascular treatment of acute limb ischemia: Review of current plasminogen activators and mechanical thrombectomy devices. Perspect. Vasc. Surg. Endovasc. Ther. 2010, 22, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Tostado, J.A.; Moise, M.A.; Bena, J.F.; Pavkov, M.L.; Greenberg, R.K.; Clair, D.G.; Kashyap, V.S. The brachial artery: A critical access for endovascular procedures. J. Vasc. Surg. 2009, 49, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Staniloae, C.S.; Korabathina, R.; Coppola, J.T. Transradial access for peripheral vascular interventions. Catheter. Cardiovasc. Interv. 2013, 81, 1194–1203. [Google Scholar] [CrossRef]
- Siracuse, J.J.; Farber, A.; Cheng, T.W.; Raulli, S.J.; Jones, D.W.; Kalish, J.A.; Smeds, M.R.; Rybin, D.; Schermerhorn, M.L.; Vascular Quality Initiative. Common femoral artery antegrade and retrograde approaches have similar access site complications. J. Vasc. Surg. 2019, 69, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Ruzsa, Z.; Bellavics, R.; Nemes, B.; Hüttl, A.; Nyerges, A.; Sótonyi, P.; Bertrand, O.F.; Hüttl, K.; Merkely, B. Combined transradial and transpedal approach for femoral artery interventions. JACC Cardiovasc. Interv. 2018, 11, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Campia, U.; Ota, H.; Didier, R.J.; Negi, S.I.; Kiramijyan, S.; Koifman, E.; Baker, N.C.; Magalhaes, M.A.; Lipinski, M.J.; et al. Vascular access in critical limb ischemia. Cardiovasc. Revasc. Med. 2016, 17, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.S.; Gilani, R.; Bena, J.F.; Bannazadeh, M.; Sarac, T.P. Endovascular therapy for acute limb ischemia. J. Vasc. Surg. 2011, 53, 340–346. [Google Scholar] [CrossRef]
- Berridge, D.C.; Kessel, D.O.; Robertson, I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst. Rev. 2013, 6, CD002784. [Google Scholar] [CrossRef]
- Brener, M.I.; Bush, A.; Miller, J.M.; Hasan, R.K. Influence of radial versus femoral access site on coronary angiography and intervention outcomes: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2017, 90, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
| Variable | RA Group (n = 17) | BA Group (n = 9) | FA Group (n = 58) | p Value Overall | p Value RA vs. BA Groups | p Value RA vs. FA Groups | p Value BA vs. FA Groups |
|---|---|---|---|---|---|---|---|
| Age, median (IQR), years | 67.0 (59.0–69.0) | 60.0 (57.0–63.0) | 64 (55.3–71.0) | 0.4558 | NA | NA | NA |
| Female | 5 (29.4%) | 2 (22.2%) | 12 (20.7%) | 0.8428 | NA | NA | NA |
| BMI, median (IQR), kg/m2 | 29.8 (23.3–30.9) | 23.1 (21.5–25.3) | 25.3 (22.5–29.4) | 0.1653 | NA | NA | NA |
| Hypertension | 16 (94.1%) | 8 (88.9%) | 48 (82.8%) | 0.6326 | NA | NA | NA |
| Current smoker | 12 (70.6%) | 7 (77.8%) | 43 (74.1%) | 1.0 | NA | NA | NA |
| Diabetes mellitus | 4 (23.5%) | 3 (33.3%) | 14 (24.1%) | 0.8529 | NA | NA | NA |
| CAD | 2 (11.8%) | 2 (22.2%) | 9 (15.5%) | 0.7936 | NA | NA | NA |
| Previous PTA | 4 (23.5%) | 3 (33.3%) | 18 (31.0%) | 0.8683 | NA | NA | NA |
| Chronic renal failure | 1 (5.9%) | 2 (22.2%) | 7 (12.1%) | 0.5044 | NA | NA | NA |
| COPD | 2 (11.8%) | 5 (55.6%) | 9 (15.5%) | 0.0172 * | 0.0424 * | 1.0 | 0.0424 * |
| Clinical presentation | 0.5966 | NA | NA | NA | |||
| Rutherford stage I | 0 (0.0%) | 0 (0.0%) | 0 (0.0% | ||||
| Rutherford stage IIA | 13 (76.5%) | 6 (66.7%) | 47 (81.0%) | ||||
| Rutherford stage IIB | 4 (23.5%) | 3 (33.3%) | 11 (19.0%) | ||||
| Rutherford stage III | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Variable | RA Group (n = 17) | BA Group (n = 9) | FA Group (n = 58) | p Value Overall | p Value RA vs. BA Groups | p Value RA vs. FA Groups | p Value BA vs. FA Groups |
|---|---|---|---|---|---|---|---|
| Superficial femoral artery | |||||||
| Diameter stenosis, % | ND | ND | ND | ||||
| Lesion length, mm | ND | ND | ND | ||||
| Reference diameter, mm | ND | ND | ND | ||||
| Popliteal artery | |||||||
| Diameter stenosis, % | ND | ND | ND | ||||
| Lesion length, mm | ND | ND | ND | ||||
| Reference diameter, mm | ND | ND | ND | ||||
| Lesion type | 0.6932 | NA | NA | NA | |||
| TASC A | 0 (0.0%) | 0 (0.0%) | 0 (0.0% | ||||
| TASC B | 3 (17.6%) | 1 (11.1%) | 4 (6.9%) | ||||
| TASC C | 0 (0.0%) | 1 (11.1%) | 5 (8.6%) | ||||
| TASC D | 14 (82.4%) | 7 (77.8%) | 49 (84.5%) | ||||
| CTO | 1 (5.9%) | 0 (%) | 1 (1.7%) | 0.5256 | NA | NA | NA |
| Outcomes | RA Group (n = 17) | BA Group (n = 9) | FA Group (n = 58) | p Value Overall | p Value RA vs. BA Groups | p Value RA vs. FA Groups | p Value BA vs. FA Groups |
|---|---|---|---|---|---|---|---|
| Procedural success | ND | ND | ND | NA | NA | NA | NA |
| Clinical success | 14 (82.4%) | 8 (88.9%) | 52 (89.7%) | 0.8666 | NA | NA | NA |
| Access site complications | 0 (0.0%) | 3 (33.3%) | 18 (31.0%) | 0.0254 * | 0.0485 * | 0.0235 * | 1.0 |
| Major adverse events at 12 months | 7 (41.2%) | 6 (66.7%) | 29 (50.0%) | 0.4879 | NA | NA | NA |
| Crossover | 8 (47.1%) | 5 (55.6%) | 50 (86.2%) | 0.0021 * | 1.0 | 0.0054 * | 0.0708 |
| Additional thrombectomy | 3 (17.6%) | 2 (22.2%) | 12 (20.7%) | 1.0 | NA | NA | NA |
| Additional angioplasty/stent | 8 (47.1%) | 5 (55.6%) | 32 (55.2%) | 0.8883 | NA | NA | NA |
| Median procedural time(IQR), minutes | 40 (25.0–57.5) | 82.5 (76.3–91.3) | 45.0 (35.0–58.8) | 0.0218 * | 0.0135 * | 0.3229 | 0.0076 * |
| Median fluoroscopy time(IQR), minutes | 10.0 (6.4–19.5) | 23.8 (19.7–28.0) | 12.8 (8.4–18.4) | 0.1111 | NA | NA | NA |
| Median radiation dose(IQR), dyne | 19.9 (9.9–31.8) | 27.8 (19.7–37.3) | 12.6 (7.9–21.5) | 0.1246 | NA | NA | NA |
| Median contrast volume(IQR), mL | 120.0 (90.0–163.0) | 117.5 (95.0–165.0) | 120.0 (79.5–160.0) | 0.7656 | NA | NA | NA |
| Perioperative Complications | RA Group (n = 17) | BA Group (n = 9) | FA Group (n = 58) | All Patients (n = 84) |
|---|---|---|---|---|
| Access site complications | n (%) | n (%) | n (%) | n (%) |
| Major | 0 (0) | 1 (11.1) | 7 (12.1) | 8 (9.5) |
| Occlusion | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Haematoma | 0 (0) | 0 (0) | 4 (6.9) | 4 (4.8) |
| Bleeding | 0 (0) | 1 (11.1) | 1 (1.7) | 2 (2.4) |
| Pseudoaneurysm | 0 (0) | 0 (0) | 2 (3.4) | 2 (2.4) |
| Minor | 0 (0) | 2 (22.2) | 11 (18.9) | 13 (15.5) |
| Occlusion | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Haematoma | 0 (0) | 2 (22.2) | 11 (18.9) | 13 (15.5) |
| Bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Summary | 0 (0) | 3 (33.3) | 18 (31.0) | 21 (25) |
| MAE at 12 months | n(%) | n(%) | n(%) | n(%) |
| Death | 3 (17.6) | 4 (44.4) | 5 (8.6) | 12 (14.3) |
| Major amputation | 4 (23.5) | 2 (22.2) | 13 (22.4) | 19 (22.6) |
| Re-PTA or bypass (TLR or TVR) | 2 (11.8) | 1 (11.1) | 12 (20.7) | 15 (17.8) |
| Myocardial infarction | 0 (0) | 0 (0) | 2 (3.4) | 2 (2.4) |
| Stroke | 2 (11.8) | 1 (11.1) | 5 (3.5) | 8 (9.5) |
| Summary (all events) | 11 (64.7) | 8 (88.9) | 37 (63.8) | 56 (66.7) |
| Summary (patients with events) | 7 (41.2) | 6 (66.7) | 29 (50) | 42 (50) |
| MALE at 12 months | n(%) | n(%) | n(%) | n(%) |
| Major amputation | 4 (23.5) | 2 (22.2) | 13 (22.4) | 19 (22.6) |
| Re-PTA or bypass (TLR or TVR) | 2 (11.8) | 1 (11.1) | 12 (20.7) | 15 (17.8) |
| Repeated ALI | 2 (11.8) | 2 (22.2) | 7 (12.1) | 11 (13.1) |
| Summary (all events) | 8 (47.1) | 5 (55.6) | 32 (55.2) | 45 (53.6) |
| Summary (patients with events) | 6 (35.3) | 3 (33.3) | 25 (43.1) | 34 (40.5) |
| Covariate | b | SE | Wald | P | HR | 95% CI of HR |
|---|---|---|---|---|---|---|
| Access site = ‘Brachial’ | 0.019559 | 0.55671 | 0.0012343 | 0.9720 | 1.01975 | 0.34246–3.03650 |
| Access site = ‘Radial’ | −1.31657 | 0.65040 | 4.09757 | * 0.0429 | 0.26805 | 0.07492–0.95908 |
| Rutherford stage = ‘IIB’ | 1.29269 | 0.42676 | 9.17549 | * 0.0025 | 3.64257 | 1.57814–8.40757 |
| Target vessel = ‘AA’ | 0.61379 | 1.10952 | 0.30603 | 0.5801 | 1.84742 | 0.20995–16.25564 |
| Target vessel = ‘BTK’ | −0.32042 | 1.06561 | 0.090418 | 0.7636 | 0.72584 | 0.08990–5.86011 |
| Target vessel = ‘CFA’ | 0.25333 | 0.75989 | 0.11114 | 0.7388 | 1.28831 | 0.29053–5.71286 |
| Target vessel = ‘CIA’ | 0.60224 | 0.68160 | 0.78068 | 0.3769 | 1.82620 | 0.48013–6.94610 |
| Target vessel = ‘EIA’ | 3.31489 | 1.15999 | 8.16636 | * 0.0043 | 27.51928 | 2.83291–267.32563 |
| Target vessel = ‘Graft’ | 0.18943 | 0.48137 | 0.15486 | 0.6939 | 1.20856 | 0.47045–3.10472 |
| Target vessel = ‘PA’ | 0.32409 | 0.52773 | 0.37715 | 0.5391 | 1.38277 | 0.49152–3.89013 |
| Additional procedure = ‘No’ | 0.38637 | 0.33879 | 1.30061 | 0.2541 | 1.47162 | 0.75756–2.85876 |
| Clinical success = ‘No’ | 2.04401 | 0.57854 | 12.48263 | * 0.0004 | 7.72154 | 2.48453–23.99742 |
| Diabetes mellitus = ‘Yes’ | 0.78023 | 0.39279 | 3.94574 | * 0.0470 | 2.18197 | 1.01042–4.71190 |
| Covariate | b | SE | Wald | P | HR | 95% CI of HR |
|---|---|---|---|---|---|---|
| Access site = ‘Brachial’ | −1.02310 | 0.62711 | 2.66162 | 0.1028 | 0.35948 | 0.10516–1.22880 |
| Access site = ‘Radial’ | −1.16539 | 0.64254 | 3.28957 | 0.0697 | 0.31180 | 0.08850–1.09855 |
| Rutherford stage = ‘IIB’ | 1.45839 | 0.39420 | 13.68746 | * 0.0002 | 4.29902 | 1.98529–9.30926 |
| Target vessel = ‘AA’ | 1.27547 | 1.06307 | 1.43952 | 0.2302 | 3.58040 | 0.44568–28.76313 |
| Target vessel = ‘BTK’ | −0.64437 | 1.05394 | 0.37381 | 0.5409 | 0.52499 | 0.06653–4.14268 |
| Target vessel = ‘CFA’ | 0.85106 | 0.71158 | 1.43045 | 0.2317 | 2.34213 | 0.58063–9.44764 |
| Target vessel = ‘CIA’ | 0.86280 | 0.59913 | 2.07387 | 0.1498 | 2.36979 | 0.73235–7.66830 |
| Target vessel = ‘EIA’ | 3.44271 | 1.12245 | 9.40739 | * 0.0022 | 31.27162 | 3.46500–282.22593 |
| Target vessel = ‘Graft’ | 0.34019 | 0.48305 | 0.49597 | 0.4813 | 1.40521 | 0.54521–3.62178 |
| Target vessel = ‘PA’ | −0.0017039 | 0.51949 | 0.000010758 | 0.9974 | 0.99830 | 0.36063–2.76347 |
| Additional procedure = ‘No’ | 0.38822 | 0.34302 | 1.28090 | 0.2577 | 1.47436 | 0.75269–2.88796 |
| Clinical success = ‘No’ | 0.71874 | 0.52552 | 1.87052 | 0.1714 | 2.05184 | 0.73251–5.74745 |
| Diabetes mellitus = ‘Yes’ | 0.26969 | 0.39667 | 0.46223 | 0.4966 | 1.30955 | 0.60183–2.84954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csavajda, A.; Toth, K.; Kovacs, N.; Rona, S.; Vamosi, Z.; Berta, B.; Kulcsar, F.Z.; Bertrand, O.F.; Hizoh, I.; Ruzsa, Z. The Clinical Impact of Access Site Selection for Successful Thrombolysis and Intervention in Acute Critical Lower Limb Ischaemia (RAD-ALI Registry). Life 2024, 14, 666. https://doi.org/10.3390/life14060666
Csavajda A, Toth K, Kovacs N, Rona S, Vamosi Z, Berta B, Kulcsar FZ, Bertrand OF, Hizoh I, Ruzsa Z. The Clinical Impact of Access Site Selection for Successful Thrombolysis and Intervention in Acute Critical Lower Limb Ischaemia (RAD-ALI Registry). Life. 2024; 14(6):666. https://doi.org/10.3390/life14060666
Chicago/Turabian StyleCsavajda, Adam, Karoly Toth, Nandor Kovacs, Szilard Rona, Zoltan Vamosi, Balazs Berta, Flora Zsofia Kulcsar, Olivier F. Bertrand, Istvan Hizoh, and Zoltan Ruzsa. 2024. "The Clinical Impact of Access Site Selection for Successful Thrombolysis and Intervention in Acute Critical Lower Limb Ischaemia (RAD-ALI Registry)" Life 14, no. 6: 666. https://doi.org/10.3390/life14060666
APA StyleCsavajda, A., Toth, K., Kovacs, N., Rona, S., Vamosi, Z., Berta, B., Kulcsar, F. Z., Bertrand, O. F., Hizoh, I., & Ruzsa, Z. (2024). The Clinical Impact of Access Site Selection for Successful Thrombolysis and Intervention in Acute Critical Lower Limb Ischaemia (RAD-ALI Registry). Life, 14(6), 666. https://doi.org/10.3390/life14060666






