Afamelanotide for Treatment of the Protoporphyrias: Impact on Quality of Life and Laboratory Parameters in a US Cohort
Abstract
1. Introduction
2. Methods
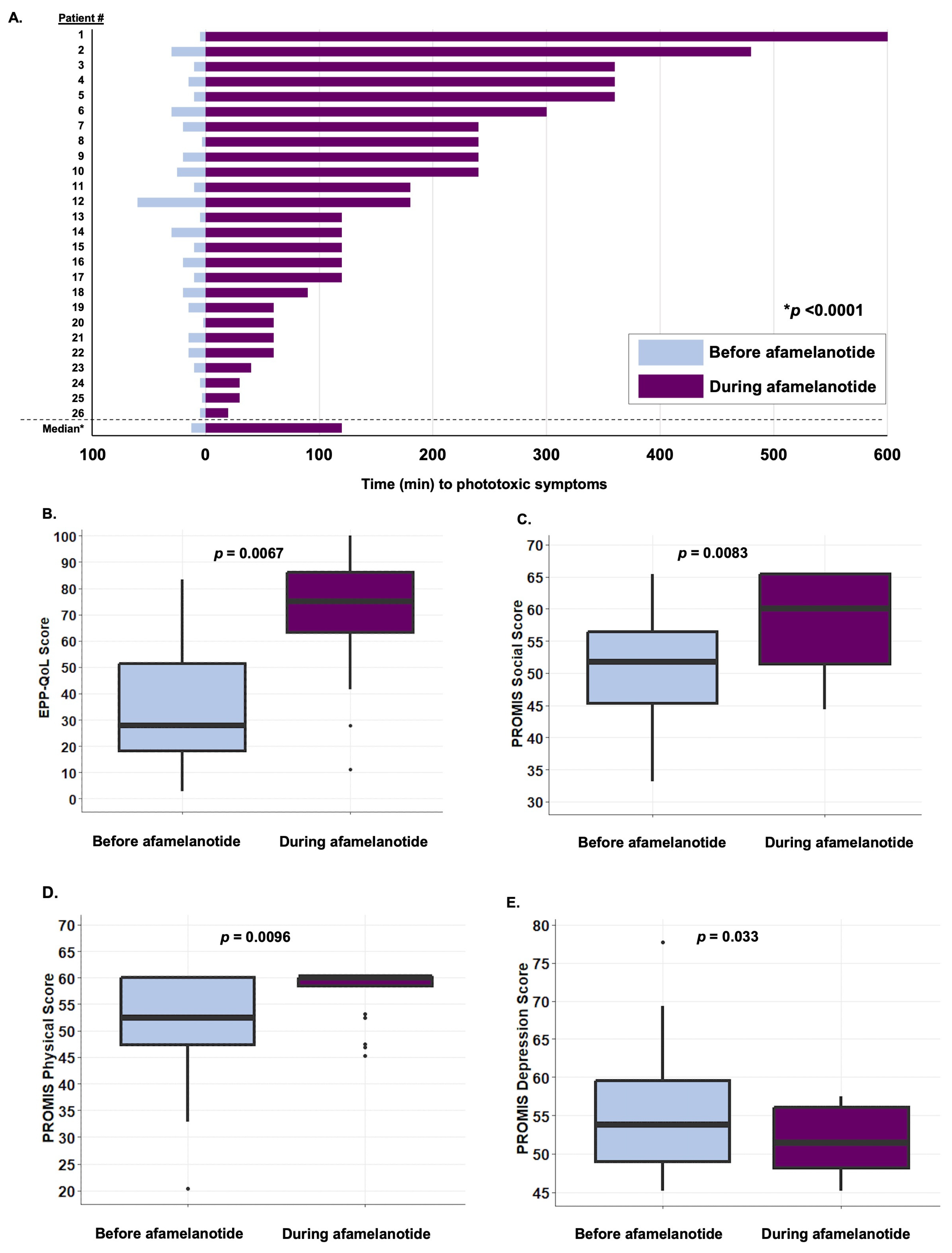
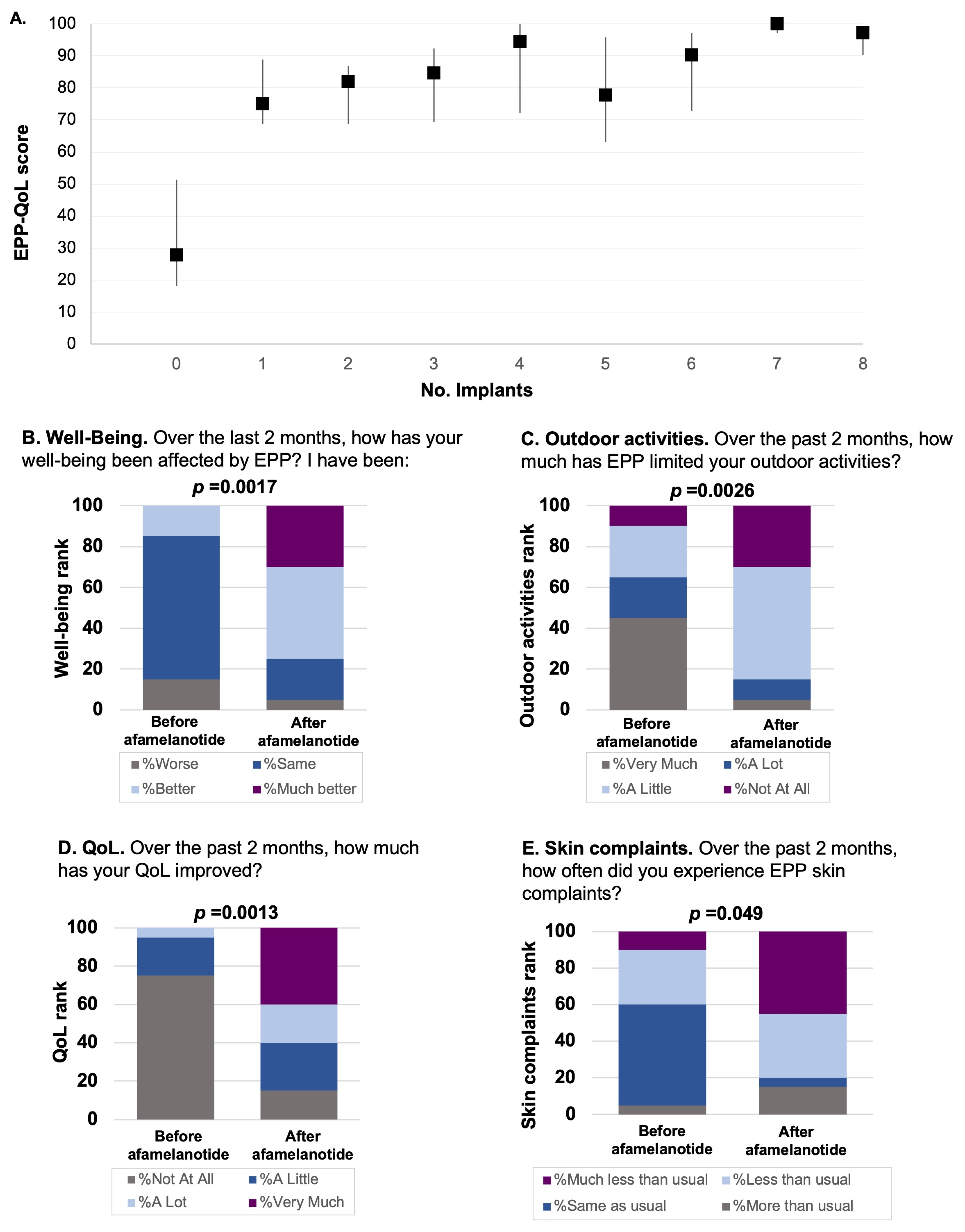
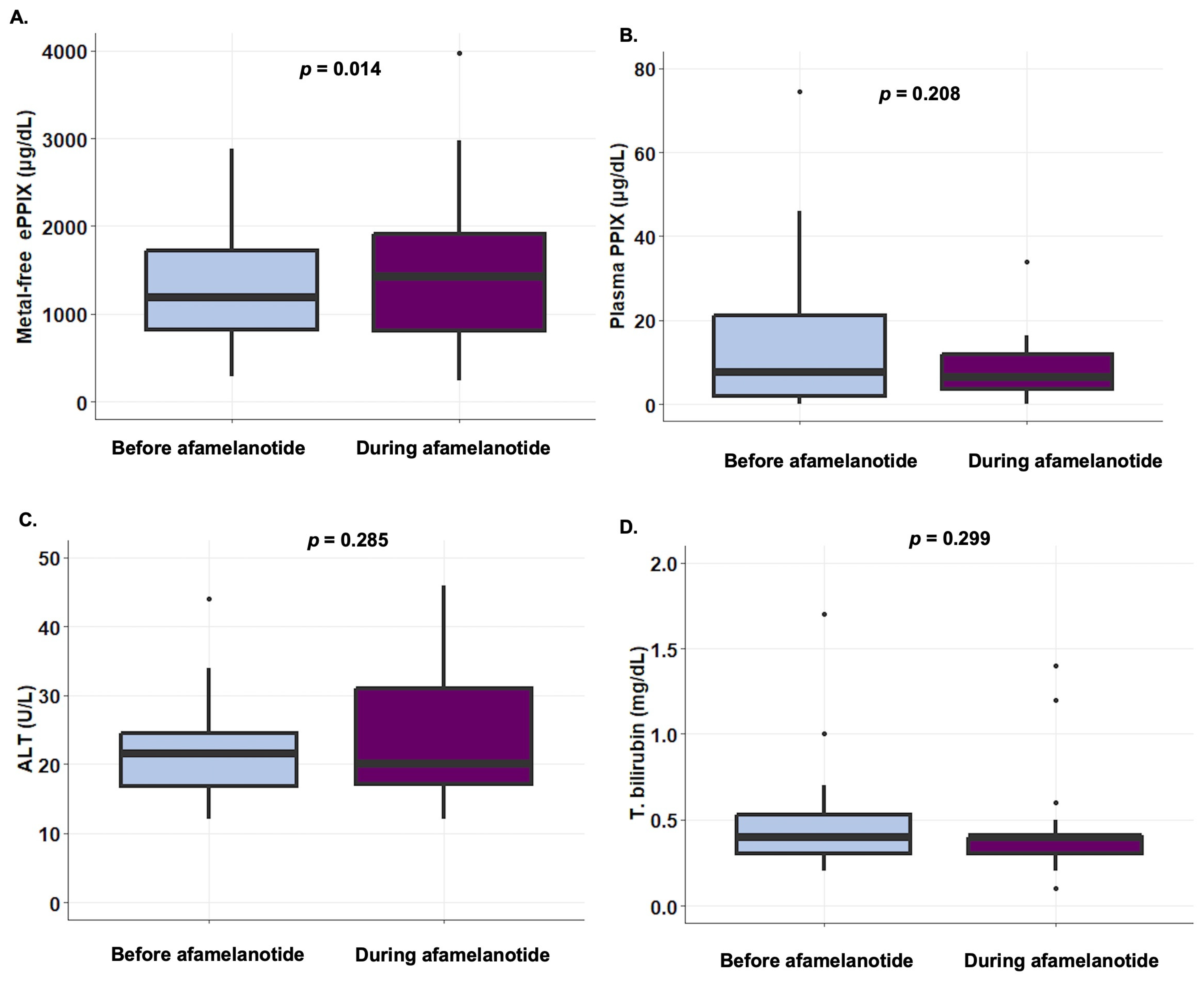
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeLeo, V.A.; Poh-Fitzpatrick, M.; Mathews-Roth, M.; Harber, L.C. Erythropoietic protoporphyria. 10 years experience. Am. J. Med. 1976, 60, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Leaf, R.K.; Dickey, A.K. How I treat erythropoietic protoporphyria and X-linked protoporphyria. Blood 2023, 141, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Langendonk, J.G.; Balwani, M.; Anderson, K.E.; Bonkovsky, H.L.; Anstey, A.V.; Bissell, D.M.; Bloomer, J.; Edwards, C.; Neumann, N.J.; Parker, C.; et al. Afamelanotide for Erythropoietic Protoporphyria. N. Engl. J. Med. 2015, 373, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.F.; Jensen, S.E.; Schalet, B.D.; Beaumont, J.L.; Amtmann, D.; Czajkowski, S.; Dewalt, D.A.; Fries, J.F.; Pilkonis, P.A.; Reeve, B.B.; et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J. Clin. Epidemiol. 2016, 73, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Biolcati, G.; Hanneken, S.; Minder, E.I.; Neumann, N.J.; Wilson, J.H.P.; Wolgen, P.J.; Wright, D.J.; Lloyd, A.J. Validation of a novel patient reported tool to assess the impact of treatment in erythropoietic protoporphyria: The EPP-QoL. J. Patient-Rep. Outcomes 2021, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Biolcati, G.; Marchesini, E.; Sorge, F.; Barbieri, L.; Schneider-Yin, X.; Minder, E.I. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. Br. J. Dermatol. 2015, 172, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Wensink, D.; Wagenmakers, M.A.; Barman-Aksözen, J.; Friesema, E.C.; Wilson, J.P.; van Rosmalen, J.; Langendonk, J.G. Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice. JAMA Dermatol. 2020, 156, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Dechant, C.; Falchetto, R. Dersimelagon in Erythropoietic Protoporphyrias. N. Engl. J. Med. 2023, 388, 2491–2493. [Google Scholar] [CrossRef]
- Naik, H.; Shenbagam, S.; Go, A.M.; Balwani, M. Psychosocial issues in erythropoietic protoporphyria—The perspective of parents, children, and young adults: A qualitative study. Mol. Genet. Metab. 2019, 128, 314–319. [Google Scholar] [CrossRef]
- Dickey, A. Pitfalls and proposed solutions for patient communication about erythropoietic protoporphyria: A survey of parents and adult patients. J. Am. Acad. Dermatol. 2019, 81, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Ceresnie, M.S.; Mohney, L.; Ko, D.; Lim, H.W.; Mohammad, T.F. Association of quality of life measures with afamelanotide treatment in patients with erythropoietic protoporphyria and x-linked protoporphyria: A retrospective cohort study. J. Am. Acad. Dermatol. 2022, 88, 880–882. [Google Scholar] [CrossRef]
- Resnik, S.R.; Targett, D.; Resnik, B.I.; Lim, H.W. Into the Light: Afamelanotide and the Treatment of Erythropoietic Protoporphyria in the United States. J. Drugs Dermatol. 2023, 22, 941–949. [Google Scholar] [CrossRef]
- Minder, A.E.; Schneider-Yin, X.; Zulewski, H.; Minder, C.E.; Minder, E.I. Afamelanotide Is Associated with Dose-Dependent Protective Effect from Liver Damage Related to Erythropoietic Protoporphyria. Life 2023, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Anstey, A.V.; Hift, R.J. Liver disease in erythropoietic protoporphyria: Insights and implications for management. Gut 2007, 56, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gou, E.; Weng, C.; Greene, T.; Anderson, K.E.; Phillips, J.D. Longitudinal Analysis of Erythrocyte and Plasma Protoporphyrin Levels in Patients with Protoporphyria. J. Appl. Lab. Med. 2018, 3, 213–221. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | N = 29 |
|---|---|
| Age (yr), median (IQR) | 40 (23–57) |
| Female sex, no. (%) | 22 (76%) |
| Erythrocyte PPIX (mcg/dL), median (IQR) | 1185 (815.0–1722.0) |
| Plasma PPIX (mcg/dL), median (IQR) | 7.5 (1.9–21.1) |
| Aspartate aminotransferase (U/L), median (IQR) | 20.50 (18.8–23.0) |
| Alanine aminotransferase (U/L), median (IQR) | 21.5 (16.8–24.5) |
| Total bilirubin (mg/dL), median (IQR) | 0.400 (0.30–0.53) |
| Time to symptom onset (min), median (IQR) | 12.5 (5.0–20.0) |
| Number of implants, median (IQR) | 6 (2–8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leaf, R.K.; Naik, H.; Jiang, P.Y.; Elmariah, S.B.; Hodges, P.; Mead, J.; Trinidad, J.; Saberi, B.; Tran, B.; Valiante, S.; et al. Afamelanotide for Treatment of the Protoporphyrias: Impact on Quality of Life and Laboratory Parameters in a US Cohort. Life 2024, 14, 689. https://doi.org/10.3390/life14060689
Leaf RK, Naik H, Jiang PY, Elmariah SB, Hodges P, Mead J, Trinidad J, Saberi B, Tran B, Valiante S, et al. Afamelanotide for Treatment of the Protoporphyrias: Impact on Quality of Life and Laboratory Parameters in a US Cohort. Life. 2024; 14(6):689. https://doi.org/10.3390/life14060689
Chicago/Turabian StyleLeaf, Rebecca K., Hetanshi Naik, Paul Y. Jiang, Sarina B. Elmariah, Pamela Hodges, Jennifer Mead, John Trinidad, Behnam Saberi, Benny Tran, Sarah Valiante, and et al. 2024. "Afamelanotide for Treatment of the Protoporphyrias: Impact on Quality of Life and Laboratory Parameters in a US Cohort" Life 14, no. 6: 689. https://doi.org/10.3390/life14060689
APA StyleLeaf, R. K., Naik, H., Jiang, P. Y., Elmariah, S. B., Hodges, P., Mead, J., Trinidad, J., Saberi, B., Tran, B., Valiante, S., Mernick, F., Leaf, D. E., Anderson, K. E., & Dickey, A. K. (2024). Afamelanotide for Treatment of the Protoporphyrias: Impact on Quality of Life and Laboratory Parameters in a US Cohort. Life, 14(6), 689. https://doi.org/10.3390/life14060689






